Abstract
To fill the gap between microhabitat and landscape scale habitat models for freshwater fish, it is becoming increasingly common practice to adopt a continuous view of riverscapes, thus allowing a better understanding of the processes in place at the river management level (segments of 1–100 km). The aim of this study was to test the effects of the spatial structure of habitat on fish distribution at this scale. Inferred habitat relationships were generated using spatial metrics adapted from landscape ecology. These were calculated for two species of multi-habitat cyprinid fish in a 25-km long segment of the Seine River. A spatially continuous survey was then designed to acquire fish sampling data relating to the riverscape. Explanatory models were devised to quantify the extent to which environmental and spatial variables could describe fish distribution patterns. Spatially continuous sampling of feeding habitats at dawn and dusk provided greater understanding of the spatial distribution of common barbel (Barbus barbus, L.) and nase (Chondrostoma nasus, L.). Fish observations were aggregated longitudinally in neighboring feeding habitat patches, with the highest abundance found in patches with the best local conditions. The species were present for large feeding patches, as well as for a higher proximity index for those patches. This result emphasized the importance of the supplementation of feeding habitats. By quantifying spatial habitat relationships using spatial metrics, it was possible to identify the best suited configuration of functional habitats to the needs of shoals. At present, most conservation work focuses on restoring local habitats. There is also growing interest in large-scale fish management, which has been encouraged by the advent of metapopulation theory. This study highlights the need for greater work at a third, intermediate scale that is no less significant: restoring daily and seasonal movements between functional habitats.
Introduction
Patterns of environmental conditions in streams and rivers create a ‘mosaic’ of habitat patches used by fish for particular life functions such as feeding, resting, finding refuge, or spawning (Schlosser Citation1995). Identifying and protecting these patches is often a key criterion in conservation plans (Rosenfeld & Hatfield Citation2006). However, the (often overlooked) spatial structure of these functional habitat patches is also important, as it indicates whether fish with certain movement characteristics are likely to use the different habitats (Schlosser Citation1995). Rosenfeld (Citation2003) suggests using ‘optimal’ habitat ratios to identify limiting habitats for multi-habitat species. Dunning et al. (Citation1992) introduces the concepts of ‘complementary’ and ‘supplementary’ habitats. Two habitat patches are said to complement each other when they provide two essential resources or functions, such as feeding and refuge habitats. Supplementation occurs when they supply the same resources or function, such as is the case with adjacent feeding patches used successively as they deplete. For many fish species, especially those with complex life cycles, access to both complementary and supplementary habitats can be important considerations when assessing a river's overall habitat quality or carrying capacity (Kocik & Ferreri Citation1998; Fausch et al. Citation2002).
In terrestrial ecosystems, habitat complementation and supplementation have previously been identified as primary factors influencing the habitat use, movement, and density of several species (Pope et al. Citation2000; Choquenot & Ruscoe Citation2003; Ouin et al. Citation2004). In freshwater ecosystems, the extent to which the spatial structure of functional habitats affects fish abundance and distribution has yet to be examined in depth (with the exception of a handful of studies on salmonids). In a mostly conceptual paper, Kocik and Ferreri (Citation1998) used numerical simulation to show that the size and spatial structure (interspersion and juxtaposition metrics) of rearing and spawning habitats along a river could influence production and population resilience in Atlantic salmon (Salmo salar). Access to adjacent spawning and refuge habitats is crucial for Bonneville cutthroat trout (Onchoryncus clarkii), and illustrates the importance of habitat complementation, especially in cases of drought (White & Rahel Citation2008). In another study looking at steelhead trout (Oncorhynchus mykiss), the distribution of spawning sites was more easily predicted by examining their ‘ecological neighborhood’ (Falke et al. Citation2013). Elsewhere, the concept of complementation, while not explicitly mentioned, can be seen in studies where the importance of habitat configuration is recognized, especially in restoration strategy (Pedroli et al. Citation2002; Rosenfeld & Hatfield Citation2006). Another research project examined the role of the proximity of two different habitats for rosyside dace (Clinostomus funduloides, Freeman & Grossman Citation1993).
Although the importance of spatial relationships between different functional habitats is now more widely recognized, the concepts of complementation and supplementation are rarely evoked explicitly. Dealing with complex life histories and complementary resources has been identified as a challenge when incorporating the question of connectivity into riverine fish ecology (Fullerton et al. Citation2010). According to Rosenfeld and Hatfield (Citation2006), the most appropriate spatial metrics to examine functional habitat availability and connectivity have yet to be identified. They can change depending on species, life stage, season, and the characteristics of a given river. In addition to enhancing prediction of fish distributions, connectivity (through spatially explicit metrics) may help to improve the consistency and transferability of habitat models for various fish species (Benjamin et al. Citation2007; Isaak et al. Citation2007). Given the increasing availability of geographic information systems (GIS), spatial variables can provide a valuable contribution to habitat models used in wildlife conservation. This could prove especially useful in evaluating the level of access to complementary habitats necessary to ensure the long-term survival of endangered species (White & Rahel Citation2008). In one previous study, complementation and supplementation were quantified using metrics such as longitudinal distance to the nearest patch and habitat patch proximity index, modified for rivers from Gustafson and Parker (Citation1994) by Le Pichon et al. (Citation2009). More recently, Flitcroft et al. (Citation2012) performed an analysis of the proximity and connectivity of fish habitats using GIS to calculate network distances representing the spatial structure of juvenile coho salmon (Oncorhynchus kisutch).
In European rivers, fragmentation and habitat degradation combined with flow regulation have been identified as major causes of the decline of several ‘specialist’ species such as rheophilic cyprinids (Penczak & Kruk Citation2000). For the common barbel (Barbus barbus) and nase (Chondrostoma nasus), which move between their resting habitats and feeding habitats on a daily basis (Baras Citation1997), as well as move seasonally to upstream spawning habitats (Baras & Cherry Citation1990; Huber & Kirchhofer Citation1998; Ovidio & Philippart Citation2008), habitat complementation is an important consideration. Indeed, numbers of these gregarious species in human-impacted rivers are dwindling, due in no small part to insufficient and unreachable spawning and nursery habitats (Maier Citation1997; Boët et al. Citation1999; Penczak & Kruk Citation2000).
The objective of this study was to examine the influence of functional habitat spatial structure on the distribution of two rheophilic species in the Seine River. The hypothesis that barbel and nase distribution is partly explained by spatial relationships between functional habitats (i.e. feeding, resting, and spawning) was tested at two spatial scales (local (10–100 m) and segment (1–100 km)) in the Seine River. Habitat suitability and patch size were also taken into account as potential factors influencing fish distribution. Using a continuous multi-scale sampling technique (Fausch et al. Citation2002; Gresswell et al. Citation2006; Torgersen et al. Citation2006; White et al. Citation2014), this study attempts to contribute to a better understanding of fish–habitat relationships at varying spatial scales.
Methods
Study area and habitat mapping
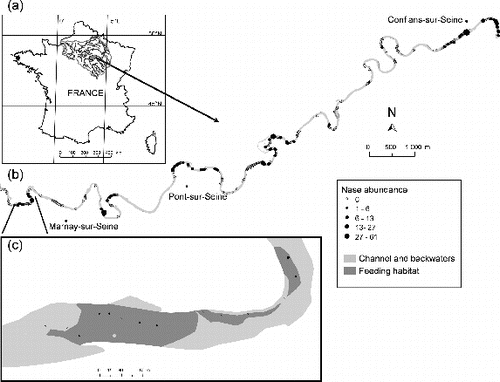
The study area is located in ‘La Bassée’, a fifth order (Strahler Citation1957) alluvial floodplain of the Seine River, 100 km upstream from Paris (). The specific area used for the study was a 25-km long and 50-m wide meandering reach, situated between two navigation weirs. There has been very little mechanical intervention in the area, since it is bypassed by a shipping canal. The area is dominated by agriculture, and there are many natural water bodies. Their connectivity to the main channel depends largely on seasonal variations in water levels.
Figure 1. Geographical location of study area. (a) The Seine river basin, France, and the ‘La Bassée’ alluvial floodplain; (b) study segment showing nase abundance for the 266 samples; and (c) a detail at the downstream limits of the segment.
Habitats were mapped using the riverscape approach described in Le Pichon, Gorges, Boët, et al. (Citation2006). The underwater environment was represented using two-dimensional GIS-based maps of habitat patches, defined according to activities (feeding, resting, and spawning). Channel boundaries, connected water bodies, depth < 1 m, bottom substrate, current velocities, and shelters were individually mapped for the dry five-year average flow (38 m3/s March–July, gauging station of Pont-sur-Seine, 1979–2004) using field surveys (2001, 2004) and digital orthophotographs (2000) available at this discharge. Few variations in the river morphology were observed between 2001 and 2004, because this lowland river is highly regulated by the presence of reservoirs protecting Paris from flooding. ArcGIS 9.3TM was used to combine favorable classes of each environmental variable based on the habitat preferences of both species and create GIS habitat maps for feeding, resting/refuge, and spawning. Feeding and spawning habitat patches were characterized by water depth (<1 m) and surface current velocities (0.2–1.5 m/s), feeding by sand and/or gravel, pebble, and cobble substrates ( and 1(c)) and spawning by gravel and gravel/sand substrates. Resting habitat was characterized by water depth (<1 m), surface current velocities (<0.5 m/s), and by the presence of shelters (block, woody debris and log jam, roots, natural water bodies).
Fish sampling and local environmental variables
To take into account the effects of spatial relationships between habitats, the study covers an area greater than that of the species' home ranges. A ‘focal patch study’ (Brennan et al. Citation2002) was conducted, focusing on feeding habitats–a key resource for the species under examination. Following the recommendations of Rushton (Citation2004), a spatially continuous and nested fish sampling scheme was used to model species distribution. Forty-eight feeding habitat patches were sampled over an 18-km long segment using a point abundance sampling method. Based on a large number of small samples (15 m2), this method provides insight into the spatial structure of populations (Persat & Copp Citation1990), especially in medium-sized rivers (Tomanova et al. Citation2013). Data were successively collected at dawn (22 patches) and dusk (26 patches) at expected foraging times (Baras Citation1997). Sampling began one hour before sunrise and sunset and lasted for two hours. Of the 266 samples (124 at dawn and 142 at dusk), 183 came from the 48 feeding patches and 83 came from other areas ( and 1(c)). The number of samples taken from each feeding patch was calculated as a compromise between area and shoreline length (Persat & Copp Citation1990). Thereby, no correlation exists between the number of samples and feeding patch area (). Sampling was conducted during a nine-day survey in the autumn of 2004 (discharge 40 m3/s, five-year dry return period), after the spawning period when adult fish are expected to reside in areas with complementary feeding and resting habitats with quite restricted movements (60–300 m, Baras Citation1992). This sampling period allows (1) similar discharges to those obtained using habitat mapping and (2) limits the possibility for individuals to migrate along the 18-km long study segment during the nine-day survey.
Fish were collected by electrofishing from a boat with a crew of five, using a generator-powered electroshocker (Heron DC, 300–330 V, 2–3 A). For each sample, a 4-m pole with a 30-cm anode ring was used. As the efficiency of electrofishing in non-wadeable water decreases as depth increases (Copp Citation1989), samples were distributed both in feeding patches (water depth < 1 m) and other habitats along the shoreline in water less than 2 m deep. Fish were collected with a fine-mesh dip net and were quickly identified, measured, and returned to the water. Each sample was located with 1 m accuracy using a global positioning system (GPS receiver, Trimble ProXRS). Several environmental variables were also recorded during fish sampling: current velocity at a depth of 20 cm using a flow meter, water depth, and visually estimated dominant bottom substratum ().
Table 1. Explanatory variables measured or calculated at the sampling unit scale, evaluated for association with the abundance of the species and explanatory variables calculated at the feeding habitat patch scale evaluated for association with the presence/absence of the species.
Spatial metrics
The effect of the spatial structure of habitat on the presence and abundance of fish was tested using different spatial metrics described in Le Pichon, Gorges, Boët, et al. (Citation2006) and Le Pichon et al. (Citation2009). For this study, a set of conceptually meaningful spatial metrics was selected: (1) longitudinal distances between patches or between samples and patches, (2) area-based proximity indices and distance-weighted metrics, and (3) proportions of habitat in a squared window centered on each sample.
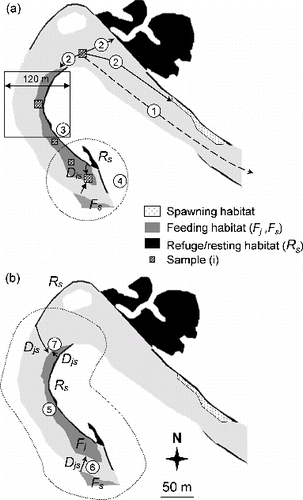
To calculate these spatial metrics, all samples were imported into ArcGIS 9.3TM with resting/feeding/spawning habitat maps. At the sample scale, the longitudinal distances from each sample to upstream weir (Distup) and to the nearest resting/feeding/spawning habitat patch (DistR, DistF, DistS) were computed using ArcGIS and Anaqualand 2.0 (Le Pichon, Gorges, Faure, & Boussard Citation2006), a freeware application for calculating oriented longitudinal distances along a river (). The proximity index of samples relative to resting/feeding habitat (PxSR, PxSF) was calculated using the formula in (). At feeding patch scale, the area (AreaF) and proximity index to neighboring feeding/resting habitats were calculated (PxFF, PxFR, ). The proximity index increases when sample/patch becomes less isolated (Gustafson & Parker Citation1994) and can reflect potential sources of dispersers located close to the sample/patch (Bender et al. Citation2003). Proximity index is calculated for a defined search distance, which should reflect the dispersal capacity of the organism (Brennan et al. Citation2002). A longitudinal search distance of 60 m was chosen (upstream and downstream: longitudinal range of 120 m), based on the species' capacity for daily movement (Baras Citation1997). This search distance was also used to define the window size of 120 m for habitat proportion calculations. The proportion of resting/feeding habitats in this 120 m square window, centered on each sample (PrSR, PrSF) was calculated using CHLOE 3.0, a software for multiple scale analysis (Baudry et al. Citation2005) ().
Figure 2. Spatial variables calculated at two scales. (a) At the sample (i) scale: 1– distance to upstream weir (Distup), 2– distance to the nearest patch of spawning/resting/feeding habitat (DistS, DistR, DistF), 3– proportion of resting (or feeding) habitat in a 120 m square window (PrSR, PrSF) around the sample, 4– proximity index to patches of resting (or feeding) habitat (PxSR, PxSF), Dis is the minimal distance between sample (i) and the patch of resting (or feeding) habitat. (b) At the feeding habitat patch (Fj) scale: 5– patch area (AreaF), 6– proximity index to patches of feeding habitat (PxFF, search distance = 60 m), 7– proximity index to patches of resting habitat (PxFR, search distance = 60 m), Djs is the edge-to-edge longitudinal distance between habitat patches. See for formula.
Data analysis
Young-of-the-year fish (0+) were distinguished from others (>0+) by length–frequency distributions or reference literature. The following thresholds were used as minimum size for >0+: barbel > 65 mm and nase > 70 mm. Only >0+ fish were selected for the following analysis and most individuals of both species belonged to the 1+ and 2+ age groups.
The hypothesis that species prefer feeding patches was tested against the null hypothesis that there is no difference in densities between sample for feeding patches and those belonging to other habitats. The Wilcoxon rank-sum (Mann–Whitney) test for two independent samples was used and the null hypothesis that the two distributions were the same was rejected at p-value < 0.05.
Generalized linear modeling (GLM) was used to explore the contribution and role of environmental variables and spatial metrics in explaining fish presence and abundance at two spatial scales, based on the framework suggested by Austin (Citation2002). GLM was used because it can deal with several families of probability distributions for ecological data (Guisan et al. Citation2002; Venables & Dichmont Citation2004). At the sample scale, the most probable abundance was modeled using a negative binomial distribution (266 samples). This family of distributions seemed appropriate in relation to the observed distribution of the abundance data (sample variance exceeds sample mean). Also, in the case of aggregated or over-dispersed data, this is more suitable than a Poisson distribution (Welsh et al. Citation1996). At feeding patch scale, logistic regression models were used to predict the probability of the studies species being present. Logistic regression has been used in previous studies to model fish–habitat relationships at different scales, from samples to habitat patch, sites, and streams (Dunham & Rieman Citation1999;Torgersen & Close Citation2004; Isaak et al. Citation2007). In particular, modeling the probability of use of a habitat patch, also called resource selection function, has previously been carried out using GLM (Boyce et al. Citation2002), with Akaike's information criterion (AIC) – which forms the basis of information-theoretical approaches – to identify the most parsimonious of a set of models. The main reason for this was that it includes a penalty for adding a new variable to the model (Rushton et al. Citation2004). Based on the approach used by Harig and Fausch (Citation2002) and Torgersen and Close (Citation2004), two sets of candidate models for each spatial scale were evaluated. The first set was composed of single-variable models, while the second was a combination of the ‘best’ models from the first set (models that ranked higher than the null model and containing non-correlated explanatory variables). In the combination set of ‘best’ single-variable models, only models containing non-correlated explanatory variables were tested. When correlation > |0.3|, the highest ranked variable was chosen. The models were ranked from the most plausible (low AIC) to least plausible (high AIC) and evaluated with ∆AIC (the difference between AIC of a given model and the most parsimonious one) and AIC weight, which is the weight of evidence in favor of a given model being the actual ‘best’ model (Burnham & Anderson Citation2002). AICc, a corrected version of AIC adjusted for small sample size (Burnham & Anderson Citation2002), was used at the patch scale. All these statistical analyses were performed using R packages (R Development Core Team Citation2005).
The spatial aggregation of fish presence in neighboring feeding patches was analyzed with non-parametric tests for multiple groups (MG, O'Brien Citation1976). For each species, the 48 feeding patches were ranked longitudinally, and presence/absence was converted in a 0/1 series. The observed variance estimator of the length of 0 series between two 1 was calculated for each series (MGobs). The presence of aggregations was verified using a permutation test (MGsim). The frequency (f) of MGsim higher than MGobs was calculated. The null hypothesis that the presence aggregations were randomly distributed was rejected at f < 0.1.
Results
Distribution and abundance of the species at the sample scale
Of the 266 samples sampled, 230 samples contained at least one fish. A total of 2840 individual fish were caught, representing 23 species. The community was dominated by eight cyprinids: the most abundant were roach (Rutilus rutilus), gudgeon (Gobio gobio), and bleak (Alburnus alburnus; 16%–22% relative abundance). The relative abundance was 13% for nase and 6% for barbel and both species were present in 30% of the samples and one of the two in 9% of the samples.
Before aggregating dawn and dusk samples, the homogeneity of fish densities and the occurrence in samples at dawn and dusk were tested using the Wilcoxon rank test. Densities in all samples ranged from 0.07 to 0.74 individuals/m2 for barbel and from 0.07 to 4.06 individuals/m2 for nase with a large proportion of low densities. The distribution of densities for >0+ fish of both species was significantly different between the sample located in feeding habitat patches and other habitats (p < 0.05), with a higher mean density observed in the patches of feeding habitat.
Based on the AIC values, distance to a patch of spawning habitat (DistS) was the highest ranked variable in the first set of single-variable models predicting the most probable abundance of barbel and nase (). The weight of evidence for DistS as the single ‘best’ predictor of the most probable abundance was lower for nase (0.25) than for barbel (0.97). For nase, other significant variables that ranked higher than the null model and with ΔAIC < 2 were current velocity (V), distance to a patch of resting habitat (DistR), proportion of resting habitat (PrSR), and water depth (De, ). The longitudinal position of the sample (Distup) ranked lower than the null model for barbel and was not significant for nase, indicating that no longitudinal trend occurs. There was strong evidence (AIC weight = 0.86) that the model containing current velocity (V), water depth (De), distance to a patch of spawning habitat (DistS), and distance to a patch of resting habitat (DistR) was more plausible for predicting the most probable abundance of nase (). For barbel, the ΔAIC indicated that there was substantial support for the first two models, containing distance to a patch of spawning habitat (DistS), current velocity (V), and proximity index to patches of resting habitat (PxSR) (). Parameter estimates from the best models suggested that the most probable abundance of nase was associated with shallow water depth, high current velocity, with short distance from a patch of spawning habitat and increasing distance to a patch of resting habitat (). For barbel, parameter estimates from the best model indicated that the most probable abundance was associated with high current velocities and short distance to a patch of spawning habitat ().
Table 2. Results of the AIC-based selection for models of single variables predicting the most probable abundance of C. nasus and B. barbus from the 266 samples collected at dawn and dusk. Models are ranked from the most plausible (low AIC) to least plausible; only those models that ranked higher than the null model are presented.
Table 3. Results of the AIC-based selection for models that are a combination of the ‘best’ single-variable models predicting the most probable abundance of C. nasus and B. barbus from the 266 samples collected at dawn and dusk. Only models containing non-correlated explanatory variables (Kendall correlation < |0.3|) are proposed.
Table 4. Parameter estimates and significance levels for the best models predicting the most probable abundance of C. nasus and B. barbus from the 266 samples collected at dawn and dusk.
Spatial distribution of the species at feeding patch scale
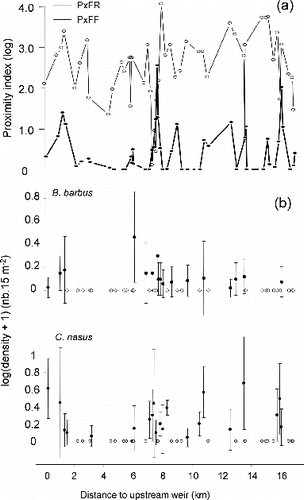
The 48 feeding patches made up 16.2% of the wet area in the 18-km long study segment. Their spatial distribution reflected its heterogeneity, with areas ranging from 63 to 17,800 m2 (median area = 1500 m2). High values of PxFF indicated segment sectors where spatial supplementation occurs (), while high values of PxFR indicated segment sectors where spatial complementation between feeding and resting habitats occurs (). Almost all feeding patches belonged to a complex of resting patches (no null value of PxFR, ) and periodic peaks of these proximity indexes could be related to the presence of large connected backwaters, used as resting habitat.
Figure 3. (a) Proximity index to patches of feeding habitat (PxFF) and resting habitat (PxFR), plotted versus their minimal longitudinal distance to upstream weir. (b) Longitudinal distribution of the mean densities (±SD) of >0+ barbel and nase in the 48 patches of feeding habitat, plotted versus their minimal longitudinal distance to upstream weir. White dots indicate patches with null density.
Among the 183 samples belonging to the 48 patches of feeding habitat, 158 samples contained at least one fish. A total of 1884 individual fish were caught, representing 21 species collected. The community was dominated by four cyprinids: the most abundant were gudgeon (21% relative abundance), nase (17%), roach and bleak (15%). The relative abundance of barbel, dace (Leuciscus leuciscus), and spirlin (Alburnoides biponctatus) was 7%–8%.
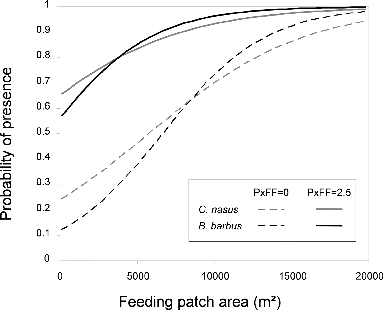
The spatial distribution of both species' abundances in feeding habitat patches, ranked longitudinally, emphasized the difference between location of occupied patches and empty patches (). Among the patches, 54% are occupied by barbel or nase and 46% of these occupied patches are shared by both species. Presence in neighboring patches was significantly aggregated for barbel (MG test, f = 0.01) and nase (MG test, f = 0.07). Complexes of occupied patches were located at 0–1.5 and 7–8 km for both species, at 13–14 km for barbel and at 10–11, 16 km for nase (, mean nearest neighbor distance between occupied patches of feeding habitat = 196 m). The results suggest that the best overall model contained feeding patch area (AreaF) and proximity index to patches of feeding habitat (PxFF) for both species, with an AICc weight of 0.5 and 0.63, respectively, for nase and barbel (). Parameter estimates from the best models suggested that the probability of occurrence for both species was positively and strongly associated with AreaF and positively with PxFF (). These models were used to plot response curves for the probability of fish occurrence across the observed range of feeding patch area (AreaF) and for the observed minimal and maximal values of PxFF (). The probability of occurrence increased with increasing patch area with a quite similar response curve for barbel and nase. For the smallest feeding habitat patches (<5000 m2), the probability of occurrence was higher when the proximity index was high. For the largest feeding patches (>15,000 m2), high values of PxFF only resulted in a small increase of the probability of occurrence.
Table 5. Results of the AIC-based selection for models predicting the presence at dawn and dusk of C. nasus and B. barbus within feeding habitat patches (48 patches). Models are ranked from the most plausible (low AIC) to least plausible; only those models that ranked higher than the null model are presented.
Table 6. Parameter estimates and significance levels for the best models predicting the presence at dawn and dusk of C. nasus and B. barbus within feeding habitat patches (48 patches).
Discussion
This study emphasizes how GIS-based habitat mapping can be a useful tool in riverscape analysis. Using this kind of approach, it is possible to: (1) design a sampling strategy for low abundant species and (2) test hypotheses about spatiotemporal use of habitats. The analysis of barbel and nase abundances validated the a-priori classification of habitats because abundances were higher in feeding habitats at dawn and dusk than in other habitats. It supported the mapping of functional habitats, based on existing data ( Le Pichon, Gorges, Boët, et al. Citation2006), in order to conduct stratified random sampling. For barbel and nase, considered low-abundance species, the use of a ‘focal patch study’ – sensu Brennan et al. (Citation2002) –with a sampling design based on a functional habitat (feeding patches), increased species detection and observed abundance. As pointed out by Angermeier et al. (Citation2002), low population densities could affect longitudinal discontinuity. Nevertheless, the proportion of occupied patches by barbel and nase (35% and 44%, respectively) versus their observed densities (0.07–1.0 individuals/m2) in the present study was close to the relationship observed between fish species in Virginia streams (Angermeier & Smogor Citation1995). In addition, the scope of the study allowed the detection of longitudinal discontinuities of presence, with only 54% of available feeding patches occupied at dawn and dusk. The importance of increasing the scope of the study has been highlighted in several previous works (Labbe & Fausch Citation2000; Ganio et al. Citation2005; Gresswell et al. Citation2006; Torgersen et al. Citation2006).
Patterns of species distribution
At sample scale, higher abundances were observed when samples came from feeding patches close to spawning patches, with high current velocities. As spawning habitats were more restricted than feeding habitats for substrate, this could indicate that species occupied mainly the gravel part of feeding patches, which is consistent with previously reported habitat preferences for both species during foraging times (Baras Citation1997; Huber & Kirchhofer Citation1997; Cowx & Welcomme Citation1998). The heterogeneous current velocities within feeding habitat patches induce a patchy distribution of drifting food supply and different opportunities to capture prey, which may explain the aggregation of individuals in feeding patch areas with good physical conditions (Baras Citation1997, Huber & Kirchhofer Citation1998). A process-based modeling approach has shown how heterogeneous current velocities affect invertebrate drift density and numbers of drift-feeding salmonids (Hayes et al. Citation2007). This was predicted by optimal foraging theory, where feeding patch choice is viewed as a function of optimal foraging where fish distribute themselves in order to maximize energy intake in high-quality patches (Matthews Citation1998).
A significant longitudinal clustering of species presence in some neighboring feeding habitat patches was observed. In addition, for a low abundance year (2005), the number of occupied patches decreased for barbel but the remaining patches were also occupied the year before (Le Pichon, unpublished data). This clustering in neighboring feeding patches is comparable with results from Baras (Citation1992) showing that the macro-distribution of barbel densities is related to the richness of feeding habitat patches. These results could indicate the attachment to particular core areas of activity (home site fidelity for feeding), as observed for some radio-tagged barbels in the River Ourthe, Belgium (Ovidio et al. Citation2007).The use of long-term residence areas provides a distinctive advantage for optimal foraging (Baras Citation1997; Huber & Kirchhofer Citation1997). Using six annual surveys Gresswell et al. (Citation2006) observed that while abundances of coastal cutthroat trout (Oncorhynchus clarkia clarkia) vary year on year, fish congregate in areas that exhibit high relative abundance each year in the same season. Flitcroft et al. (Citation2014) also found that, according to interannual adult abundances, juvenile coho salmon distribution expanded and contracted around stream sections that were continuously occupied. These observations are consistent with the concept of ideal free distribution, which predicts that fish will occupy the next ‘best’ patch of habitat if density increases in a high-quality patch (Schilling Citation2005). At segment scale, the use of feeding patches for these benthic mobile species could be influenced by spatial patchiness of food resources as shown for the longnose dace (Thompson et al. Citation2001). The spatial patchiness of lotic invertebrate densities is demonstrated at the riffles scale within a segment (Downes et al. Citation1993).
The observed pattern of fish distribution is valid for foraging times (dawn and dusk) for the dry five-year average flow, and may vary with the time of day, discharge, and season. In daytime, feeding habitat patches are not the predominant choice because both species are generally using resting habitats (Le Pichon, unpublished data). Feeding patch structure is different at median discharge (70 m3/s) with a decreasing number of patches and an increasing distance between patches (Le Pichon et al. Citation2009). Also, at seasonal scale, the spatial distribution of foragers could be strongly influenced by behavioral differences. Freeman and Grossman (Citation1993) found that rheophilic cyprinid foragers aggregate during July, October, and/or December, but spread out during other months.
Relevance of metrics of supplementation and complementation
The nested sampling scheme showed that spatial metrics were useful at both scales, both in explaining the presence of species inside a feeding patch, and in accounting for abundances in samples according to their context and patch quality (local environment). A conceptual model, summarizing the effects of local environment and context of feeding patch on the presence of studied species, is proposed in . The probability of presence increases with three nested variables: suitability of the local environment, size of feeding patches, and whether or not those feeding patches belong to a complex of other feeding patches. Both species are more likely to be present where local environment is suitable, feeding patches are larger, and the proximity index of feeding patches (PxFF) is higher.
Figure 5. Conceptual model describing the role of local environment and the spatial habitat relationships in explaining the probability of presence of studied species.
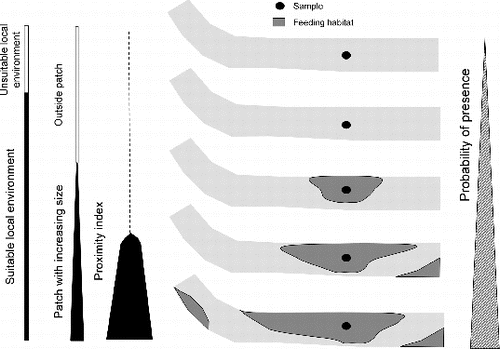
Adult barbel and nase feed in monospecific shoals of 20–300 individuals (Baras Citation1997; Kappus et al. Citation1997), but immature fish could form multi-specific shoals (Huber & Kirchhofer Citation1998). In this study, immature individuals of both species shared most of the occupied samples and feeding patches. Multi-specific shoals probably need high feeding resource quantities available either in one large patch or in several patches accessible within their daily range of movement. This supports the significance of supplementation of resources, a crucial spatial habitat relationship, and the usefulness of the proximity index as defined by Gustafson and Parker (Citation1994) to quantify it. This index is a biologically realistic metric because it considers areas that are potential sources of dispersers (Bender et al. Citation2003). Isaak et al. (Citation2007) observed a similar result using a connectivity index, adapted from Moilanen and Nieminen (Citation2002), that was the strongest predictor of Chinook salmon (Oncorhynchus tshawytscha) use of spawning patches and which becomes more important when populations are reduced. As individuals of barbel and nase were mostly immature fish, high densities in the proximity of spawning patches could indicate that they prefer to remain close to their birth area. Complementation between spawning and rearing/feeding patches is mentioned by Kocik and Ferreri (Citation1998). For another salmonid species, increasing density of juveniles was related to short distances to spawning and winter habitats, supporting the role of proximity between critical seasonal habitats (Flitcroft et al. Citation2012).
In the segment of the River Seine studied, the high values of PxFR (complementation between feeding and resting habitat) could explain why this spatial variable was not retained by the fish presence model. Indeed, small areas of resting habitat may be sufficient for these species. For instance, Huber and Kirchhofer (Citation1997) observed ∼2200 nase individuals resting in an area of 20 m2. This also corroborates an observation from Baras (Citation1997), showing that for adult barbel, the selection of feeding habitat overrides the selection of resting habitat. For nase, increasing distance to resting habitat was used in the model at the sample scale, indicating that fish at dawn and dusk are far from their resting area. This is consistent with the habitat use of radio-tagged fish in late autumn in the River Aare, Switzerland, which indicated movements of about 300 m between day and night habitats covered during dusk and dawn (Huber & Kirchhofer Citation1997). As pointed out by Bouchard and Boisclair (Citation2008), there are divergent results about the relative importance of the proximity of functional habitats in explaining fish densities, which could be related to the quantity and spatial arrangement of habitat patches in rivers.
Implications for conservation and restoration
Bond and Lake (Citation2003) alerted ecologists and managers to issues that could impact the effectiveness of local habitat restoration for stream biota, urging them to consider related ecological issues. They underlined the importance of the location of source populations and how the various habitats required should be arranged spatially. Using a continuous survey of a functional habitat (species grain) at the segment scale (104 m) (species extent), areas occupied by shoals were detected, providing useful information for barbel and nase conservation. Such spatially explicit and continuous studies, at a scale large enough to elucidate the effects of habitat structure on fish distribution, are relatively new, but nevertheless provide advances in managing restoration of habitats (Baxter Citation2002; Labbe & Fausch Citation2000; Tiffan et al. Citation2002; Torgersen & Close Citation2004). In these occupied areas, our results highlight the importance of supplementation for feeding habitat patches. The metrics used to quantify these spatial habitat relationships are useful in identifying the appropriate spatial configuration for functional habitats.
The results of this study suggest that an equivalent probability of presence could be reached for one large patch or several small patches, which provides guidance for habitat restoration. In an adjacent artificial segment of the same floodplain (same length), the total area of the feeding habitat was reduced to 2.6% (divided by eight) of the channel area (Le Pichon et al. Citation2009). In this artificial segment, managers will attempt to restore a minimal amount or a complex of feeding habitat patches by reducing the distance to reach a feeding habitat patch. Management policies should focus on the restoration of environmental conditions for local populations that provide feeding, resting, and spawning habitats, separated by distances consistent with the dispersal capacities of the fish. Between traditional local habitat restoration and a growing interest in large-scale management encouraged by metapopulation theory, there is an important intermediate scale of daily and seasonal movements between functional habitats. For spatially subdivided populations, focusing on key processes that influence the persistence of fish populations could be as important in designing restoration schemes as efforts to design minimal habitat reserves, as emphasized for the conservation of salmonids by Rieman and Dunham (Citation2000).
Acknowledgements
We are grateful to Pierre Mauricrace, Daniel Mira, Michaël Rabotin, and Pascal Roger for their invaluable assistance in carrying out fieldwork. We would also like to thank François Goreaud, Tom Nishida, and Michaël Ovidio for comments on an earlier version of this manuscript. This work was supported by the Environmental Service of Ile-de-France (DIREN) to restore the biological connectivity of the Seine River for fish and the CNRS scientific program ‘Piren-Seine’.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Angermeier PL, Krueger KL, Dolloff CA. 2002. Discontinuity in stream-fish distributions: implications for assessing and predicting species occurrences. In Scott JM, Heglund PJ, Morrison ML, Haufler JB, Raphael MG, Wall WA, Samson FB, editors. Predicting species occurrences: issues of accuracy and scale. Washington (DC): Island Press; p. 519–527.
- Angermeier PL, Smogor RA. 1995. Estimating number of species and relative abundances in stream-fish communities: effects of sampling effort and discontinuous spatial distributions. Can J Fish Aquat Sci. 52:936–949.
- Austin MP. 2002. Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol Model. 157:101–118.
- Baras E. 1992. A study of time and space utilisation strategies in the common barbel Barbus barbus (L.). Cahiers d'Ethologie. 12:125–442.
- Baras E. 1997. Environment determinants of residence area selection by Barbus barbus in the River Ourthe. Aquat Living Resour. 10:195–206.
- Baras E, Cherry B. 1990. Seasonal activities of female barbel Barbus barbus (L.) in the River Ourthe (southern Belgium), as revealed by radio tracking. Aquat Living Resour. 3:283–294.
- Baudry J, Boussard H, Schermann N. 2005. Chloe 3.1: freeware of multi-scales analyses on ASCII raster files. Rennes: INRA, SAD-Armorique.
- Baxter CV. 2002. Fish movement and assemblage dynamics in a pacific northwest riverscape [PhD dissertation]. Corvallis (OR): Oregon State University.
- Bender DJ, Tischendorf L, Fahrig L. 2003. Using patch isolation metrics to predict animal movement in binary landscapes. Landsc Ecol. 18:17–39.
- Benjamin JR, Dunham JB, Dare MR. 2007. Invasion by nonnative brook trout in panther creek, Idaho: roles of local habitat quality, biotic resistance, and connectivity to source habitats. Trans Am Fish Soc. 136:875–888.
- Boët P, Belliard J, Berrebi-dit-Thomas R, Tales E. 1999. Multiple human impacts by the City of Paris on fish communities in the Seine river basin, France. Hydrobiologia. 410:59–68.
- Bond NR, Lake PS. 2003. Local habitat restoration in streams: constraints on the effectiveness of restoration for stream biota. Ecol Manag Restor. 4:193–198.
- Bouchard J, Boisclair D. 2008. The relative importance of local, lateral, and longitudinal variables on the development of habitat quality models for a river. Can J Fish Aquat Sci. 65:61–73.
- Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA. 2002. Evaluating resource selection functions. Ecol Model. 157:281–300.
- Brennan JM, Bender DJ, Contreras TA, Fahrig L. 2002. Focal patch landscape studies for wildlife management: optimizing sampling effort across scales. In Liu J, Taylor WW, editors. Integrating landscape ecology into natural resource management. Cambridge: Cambridge University Press; p. 68–91.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inferences: a practical information-theoretic approach. 2nd ed. New York (NY): Springer.
- Choquenot D, Ruscoe WA. 2003. Landscape complementation and food limitation of large herbivores: habitat-related constraints on the foraging efficiency of wild pigs. J Anim Ecol. 72:14–26.
- Copp GH. 1989. Electrofishing for fish larvae and 0+ juveniles: equipment modifications for increased efficiency with short fishes. Aquac Fish Manag. 20:453–462.
- Cowx IG, Welcomme RL. 1998. Rehabilitation of rivers for fish. Oxford: Fishing News Books.
- Downes BJ, Lake PS, Schreiber ESG. 1993. Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization. Freshw Biol. 30:119–132.
- Dunham JB, Rieman BE. 1999. Metapopulation structure of bull trout: influences of physical, biotic, and geometrical landscape characteristics. Ecol Appl. 9:642–655.
- Dunning JB, Danielson BJ, Pulliam HR. 1992. Ecological processes that affect populations in complex landscapes. Oikos. 65:169–175.
- Falke JA, Dunham JB, Jordan CE, McNyset KM, Reeves GH. 2013. Spatial ecological processes and local factors predict the distribution and abundance of spawning by steelhead (Oncorhynchus mykiss) across a complex riverscape. PLoS One. 8:e79232.
- Fausch KD, Torgersen CE, Baxter CV, Li HW. 2002. Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. BioScience. 52:483–498.
- Flitcroft R, Burnett K, Snyder J, Reeves G, Ganio L. 2014. Riverscape patterns among years of juvenile coho salmon in midcoastal Oregon: implications for conservation. Trans Am Fish Soc. 143:26–38.
- Flitcroft RL, Burnett KM, Reeves GH, Ganio LM. 2012. Do network relationships matter? Comparing network and instream habitat variables to explain densities of juvenile coho salmon (Oncorhynchus kisutch) in mid-coastal Oregon, USA. Aquat Conserv. 22:288–302.
- Freeman MC, Grossman GD. 1993. Effects of habitat availability on dispersion of a stream cyprinid. Environ Biol Fish. 37:121–130.
- Fullerton AH, Burnett KM, Steel EA, Flitcroft RL, Pess GR, Feist BE, Torgersen CE, Miller DJ, Sanderson BL. 2010. Hydrological connectivity for riverine fish: measurement challenges and research opportunities. Freshw Biol. 55:2215–2237.
- Ganio LM, Torgersen CE, Gresswell RE. 2005. A geostatistical approach for describing spatial pattern in stream networks. Front Ecol Environ. 3:138–144.
- Gresswell RE, Torgersen CE, Bateman DS, Guy TJ, Hendricks SR, Wofford JEB. 2006. A spatially explicit approach for evaluating relationships among coastal cutthroat trout, habitat, and disturbance in small Oregon streams. In Hughes RM, Wang L, Seelbach PW, editors. Proceedings of the Symposium on Influences of Landscapes on Stream Habitats and Biological Assemblages. Bethesda (MD): American Fisheries Society; p. 457–471.
- Guisan A, Edwards TC, Hastie T. 2002. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model. 157:89–100.
- Gustafson EJ, Parker GR. 1994. Using an index of habitat patch proximity for landscape design. Landsc Urban Plan. 29:117–130.
- Harig AL, Fausch KD. 2002. Minimum habitat requirements for establishing translocated cutthroat trout populations. Ecol Appl. 12:535–551.
- Hayes JW, Hughes NF, Kelly LH. 2007. Process-based modelling of invertebrate drift transport, net energy intake and reach carrying capacity for drift-feeding salmonids. Ecol Model. 207:171–188.
- Huber M, Kirchhofer A. 1997. Habitat use of radiotagged adult nase (Chondrostoma nasus) in a regulated river. Folia Zool. 46:67–77.
- Huber M, Kirchhofer A. 1998. Radio telemetry as a tool to study habitat use of nase (Chondrostoma nasus L.) in medium-sized rivers. Hydrobiologia. 372:309–319.
- Isaak DJ, Thurow RF, Rieman BE, Dunham JB. 2007. Chinook salmon use of spawning patches: relative roles of habitat quality, size, and connectivity. Ecol Appl. 17:352–364.
- Kappus BM, Jansen W, Bohmer J, Rahmann H. 1997. Historical and present distribution and recent habitat use of nase, Chondrostoma nasus, in the lower Jagst River (Baden-Wurttemberg, Germany). Folia Zool. 46:51–60.
- Kocik JF, Ferreri CP. 1998. Juvenile production variation in salmonids: population dynamics, habitat, and the role of spatial relationships. Can J Fish Aquat Sci. 55:191–200.
- Labbe TR, Fausch KD. 2000. Dynamics of intermittent stream habitat regulate persistence of a threatened fish at multiple scales. Ecol Appl. 10:1774–1791.
- Le Pichon C, Gorges G, Baudry J, Goreaud F, Boët P. 2009. Spatial metrics and methods for riverscapes: quantifying variability in riverine fish habitat patterns. Environmetrics. 20:512–526.
- Le Pichon C, Gorges G, Boët P, Baudry J, Goreaud F, Faure T. 2006. A spatially explicit resource-based approach for managing stream fishes in riverscapes. Environ Manag. 37:322–335.
- Le Pichon C, Gorges G, Faure T, Boussard H. 2006. Anaqualand 2.0: freeware of distances calculations with frictions on a corridor. Version 2.0. Antony: Cemagref. Available from: https://www6.rennes.inra.fr/sad/Outils-Produits/Outils-informatiques/Anaqualand
- Maier K-J. 1997. On the nase, Chondrostoma nasus, spawning area situation in Switzerland. Folia Zool. 46:79–88.
- Matthews W. 1998. Patterns in freshwater ecology. New York (NY): Chapman & Hall.
- Moilanen A, Nieminen M. 2002. Simple connectivity measures in spatial ecology. Ecology. 83:1131–1145.
- O'Brien PC. 1976. A test for randomness. Biometrics. 32:391–401.
- Ouin A, Aviron S, Doverc J, Burel F. 2004. Complementation/supplementation of resources for butterflies in agricultural landscapes. Agric Ecosyst Environ. 103:473–479.
- Ovidio M, Parkinson D, Philippart JC, Baras E. 2007. Multiyear homing and fidelity to residence areas by individual barbel (Barbus barbus). Belg J Zool. 137:183–190.
- Ovidio M, Philippart JC. 2008. Movement patterns and spawning activity of individual nase (Chondrostoma nasus L.) in flow-regulated and weir-fragmented rivers. J Appl Ichthyol. 24:256–262.
- Pedroli B, de Blust G, van Looy K, van Rooij S. 2002. Setting targets in strategies for river restoration. Landsc Ecol. 17:5–18.
- Penczak T, Kruk A. 2000. Threatened obligatory riverine fishes in human-modified Polish rivers. Ecol Freshw Fish. 9:109–117.
- Persat H, Copp GH. 1990. Electric fishing and point abundance sampling for the ichthyology of large rivers. In Cowx IG, editor. Developments in electric fishing. Proceedings of an International Symposium on Fishing with Electricity. Oxford: Fishing News Books, Blackwell Scientific Publications; p. 197–210.
- Pope SE, Fahrig L, Merriam NG. 2000. Landscape complementation and metapopulation effects on leopard frog populations. Ecology. 81:2498–2508.
- R: a language and environment for statistical computing. 2005. Vienna: R Foundation for Statistical Computing.
- Rieman BE, Dunham JB. 2000. Metapopulations and salmonids: a synthesis of life history patterns and empirical observations. Ecol Freshw Fish. 9:51–64.
- Rosenfeld J. 2003. Assessing the habitat requirements of stream fishes: an overview and evaluation of different approaches. Trans Am Fish Soc. 132:953–968.
- Rosenfeld JS, Hatfield T. 2006. Information needs for assessing critical habitat of freshwater fish. Can J Fish Aquat Sci. 63:683–698.
- Rushton SP, Ormerod SJ, Kerby G. 2004. New paradigms for modelling species distributions? J Appl Ecol. 41:193–200.
- Schilling NS. 2005. Survival of the fittest: fish in patchy environments show ideal free distribution. Eukaryon. 1:11–16.
- Schlosser IJ. 1995. Critical landscape attributes that influence fish population dynamics in headwater streams. Hydrobiologia. 303:71–81.
- Strahler AN. 1957. Quantitative analysis of watershed geomorphology. Trans Am Geophys Union. 38:913–920.
- Thompson AR, Petty JT, Grossman GD. 2001. Multi-scale effects of resource patchiness on foraging behaviour and habitat use by longnose dace, Rhinichthys cataractae. Freshw Biol. 46:145–160.
- Tiffan KF, Garland RD, Rondorf DW. 2002. Quantifying flow-dependent changes in subyearling fall Chinook salmon rearing habitat using two-dimensional spatially explicit modelling. N Am J Fish Manag. 22:713–726.
- Tomanova S, Tedesco PA, Roset N, Thomas RBD, Belliard J. 2013. Systematic point sampling of fish communities in medium- and large-sized rivers: sampling procedure and effort. Fish Manag Ecol. 20:533–543.
- Torgersen CE, Baxter CV, Li HW, McIntosh BA. 2006. Landscape influences on longitudinal patterns of river fishes: spatially continuous analysis of fish-habitat relationships. In Hughes RM, Wang L, Seelbach PW, editors. Proceedings of the Symposium on Influences of Landscapes on Stream Habitats and Biological Assemblages. Bethesda (MD): American Fisheries Society; p. 473–492.
- Torgersen CE, Close DA. 2004. Influence of habitat heterogeneity on the distribution of larval Pacific lamprey (Lampetra tridentata) at two spatial scales. Freshw Biol. 49:614–630.
- Venables WN, Dichmont CM. 2004. GLMs, GAMs and GLMMs: an overview of theory for applications in fisheries research. Fish Res. 70:319–337.
- Welsh AH, Cunningham R, Donnelly C, Lindenmayer D. 1996. Modelling the abundance of rare species: statistical models for counts with extra zeros. Ecol Model. 88:297–308.
- White SM, Giannico G, Li H. 2014. A behaviorscape perspective on stream fish ecology and conservation: linking fish behavior to riverscapes. Wiley Interdiscip Rev. 1:385–400.
- White SM, Rahel FJ. 2008. Complementation of habitats for Bonneville cutthroat trout in watersheds influenced by beavers, livestock, and drought. Trans Am Fish Soc. 137:881–894.