Abstract
This study focuses on the influence of three operating parameters (gas flow rate, laser repetition rate, and fluence) on the number and size distributions of nanoparticles generated by laser ablation of acrylic paint. These particles, produced by gas-to-particle conversion of vapors generated by polymer vaporization, can have a spherical shape with a 16 nm diameter (called primary particles) but most of them are aggregated primary particles. The most critical parameter is the gas (air) flow rate in the ablation cell. Indeed, the total number of nanoparticles produced per shot increases with the air flow rate, whereas the aggregate size decreases. Indeed, the gas flow rate controls the transit time and the related aggregation duration, which decrease with increasing flow rates. The influence of the air flow rate on the nanoparticle total number produced per shot can be attributed to the evolution of the particle residence time in the setup with the flow rate. In order to validate this point, the setup has been modeled (model based on the Smoluchowski coagulation equations). The model has shown that the primary particle aggregation mainly takes place in a sphere of a few millimetres in diameter. This sphere varies in volume with the laser fluence but does not depend on the air flow rate in the cell. Moreover, the nanoparticle final number per shot does not depend on the primary particle initial number per shot but only on the size of the interaction volume, which is related to laser fluence.
1. INTRODUCTION
Nanosecond laser ablation of a material leads to nanoparticle formation. During the sample interaction with the laser beam, part of the matter is removed from the target as a vapor confined in a plume. After the laser pulse, the expanding plume cools down and the gaseous compounds generate some monomers (nuclei) by nucleation. Then these gaseous monomers are converted into nanoparticles by so-called nucleation and grow by condensation to form small particles with a quasi-spherical shape. Then particles can collide together due to Brownian motion and coalesce. Spherical particles, generally with a size of a few tens of nanometers, are then formed: they are called primary particles. Finally, when the plume temperature is no longer high enough for the particles to coalesce, they create chains to form aggregates of primary particles (Lushnikov Citation1996; Ullmann et al. Citation2002; Kuhn and Günther Citation2005; Liu Citation2005; Gonzalez et al. 2007a, 2007b).
Nanoparticles generated during laser ablation of various materials were widely studied in the literature over the past few years, for applications in nanotechnologies and nanomaterials (Heszler et al. Citation2002; Ullmann et al. Citation2002; Bereznai et al. Citation2006; Thareja and Shukla Citation2007; Mahfouz et al. Citation2008), thin film deposition (Lowndes et al. Citation1998; Craciun et al. Citation2002; Albert et al. Citation2003; Deno et al. Citation2008), chemical analysis (Liu et al. Citation2004; Kuhn et al. Citation2005; Hathorne et al. Citation2008; Koch et al. Citation2008) and surface cleaning or decontamination (Delaporte et al. Citation2003; Kusch et al. Citation2003; Lee and Cheng 2004a, 2004b; Mateo et al. Citation2009; Dewalle et al. Citation2010).
The influence of many parameters on the physico-chemical features of nanoparticles generated by a laser ablation process was also widely studied. These parameters include (Gonzalez et al. 2007) laser-related factors such as wavelength, pulse duration, repetition rate, laser fluence or irradiance, and beam diameter or energy (Boulaud Citation1993; Jeong et al. Citation1999; Kawakami et al. Citation1999; Camata et al. Citation2000; Ogawa et al. Citation2000; Seto et al. Citation2001; Heszler Citation2002; Marton et al. Citation2003; Landström et al. Citation2004; Lee and Cheng 2004a, 2004b; Liu Citation2005; Bereznai et al. Citation2006; Amoruso et al. Citation2007). There are also factors linked to the surrounding environment of the sample, such as the nature, pressure, flow rate, and temperature of the carrier gas (Boulaud Citation1993; Lowndes et al. Citation1998; Kawakami et al. Citation1999; Seol et al. Citation1999; Camata et al. Citation2000; Ogawa et al. Citation2000; Hirazawa et al. Citation2002; Liu Citation2005).
In a previous communication (Dewalle et al. Citation2010), characteristics of aerosols generated by nanosecond laser ablation of a green acrylic wall paint were described in detail. This paint is frequently used in nuclear facilities for the protection of surfaces as well as for the marking out of working areas, and laser ablation can be used for surface decontamination. Knowledge of particle formation mechanisms can help to optimize the industrial ablation processes involving aerosol retention and handling.
The study presented in this article focuses on the aggregation process of the nanoparticles produced during laser ablation of the acrylic paint. Three operating parameters were considered: gas flow rate, laser repetition rate, and laser fluence. Their influence on the number per shot and size distribution of the nanoparticles was investigated. The experimental trends were analyzed by means of a model of the whole system based on the Smoluchowski formulation of coagulation. Although the possibility of successive plumes overlapping is not taken into account (and thereby the influence of high laser repetition rates under low air flow rates), this model provides a good estimate of the place where aggregates are formed in the setup and confirms the predominant effect of residence time on the final number of nanoparticles per shot. More importantly, it shows that the plume volume in which primary particles interact directly governs the number of aggregated nanoparticles at the measurement point. Although this volume does not depend on the air flow regime, it has been found that laser fluence influences its size in such a way that primary particles keep a similar concentration inside. Finally, a semiquantitative estimate of the volume of this plume is deduced from comparison between the model and experimental results.
2. PREVIOUS STUDY
As mentioned in our previous communication (Dewalle et al. Citation2010), the nanoparticles generated during the ablation process come from the paint polymer (methacrylic resin) vaporization. These particles have an aggregated shape and their number size distribution is bimodal (), a first mode being situated around 10–20 nm and a second one around several tens of nanometers (electrical mobility diameter).
FIG. 1 Number size distribution and TEM micrograph of nanoparticles generated by laser ablation of a paint (the vertical axis is expressed in number of particles produced per laser shot per unit of ablated paint).
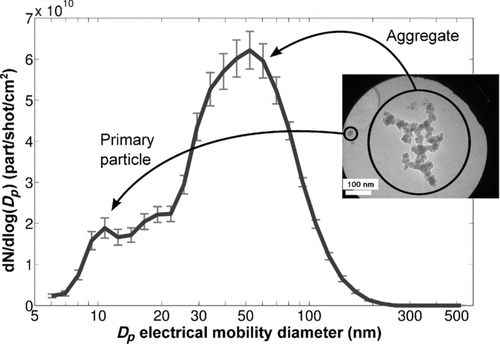
The first mode is composed by the spherical primary particles (mean diameter measured in transmission electron microscopy (TEM) micrographs: 16 ± 3.7 nm), and the second one comes from aggregates of the primary particles.
The paint ablation also generates some large spherical particles of titanium dioxide. These particles will not be considered here.
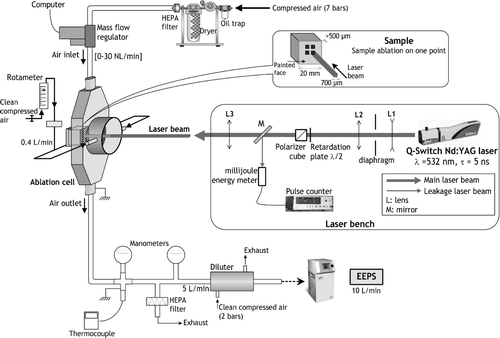
3. EXPERIMENTAL SETUP
The experimental bench was detailed in our previous publication (Dewalle et al. Citation2010). Therefore, only a brief description is included here. It is illustrated in .
Ablation is performed using a pulsed Nd:YAG Q-Switch laser (“Brilliant,” Quantel) with a 532-nm wavelength and a 5-ns pulse duration. The available repetition rate ranges from 0.1 to 10 Hz. The laser beam area on the sample has a square shape (700 × 700 μm2) with uniform energy distribution within (“flat top”). The laser beam energy can be varied by means of a half-wave retardation plate associated with a polarizer cube. By this way, the available fluence on the sample ranges from 0.1 to 11 J/cm2. The laser fluence is defined as the quantity of energy deposited by the laser beam during one laser shot per unit area of sample surface.
Laser–matter interaction occurs into a 68-cm3 stainless steel cell containing the target sample, under ambient pressure and temperature conditions. A sample consists of a small cube of aluminium (2 × 1 × 1 cm3) on which a thick layer of paint was deposited. This layer is always more than 500-μm thick (so that the substrate is not ablated). The paint is mainly composed of an organic binder (methacrylic resine) and a mineral load (titanium dioxide particles). It should be noted that in our experimental conditions, neither the repetition rate nor the paint layer thickness has an influence on the ablation process (Brygo et al. Citation2006). The cell is fed with a controlled clean air flow (compressed, oil-free, dried, and filtered air, flowing between 0 and 30 NL/min).
This cell was specially designed so that the air flow is laminar inside (homogeneous speed field) and turbulent at its output so as to get homogeneous aerosols outside the cell.
Nanoparticle number and their number size distributions are measured with an electrostatic aerosol analyzer with built-in charger and electrometers, namely the Engine Exhaust Particle Sizer (EEPS 3090) from TSI. The reported diameter corresponds to an electrical mobility diameter, for which the measurement range goes from 5.6 to 560 nm. A diluter (DEKATI DI-1000 L7) is placed downstream of the cell in order to operate in the EEPS optimal range (remaining below its maximal operating concentration). In order to make sure that the diluter does not impact the final size distribution of the aggregates (for instance, due to postcell condensation induced by fast cooling at this point), some EEPS measurements have also been carried out without diluter (with low-concentration aerosol), and it has been found that particle size distribution was not modified. Particle losses in the whole system were kept to a minimum by grounding of all metallic elements and using as short antistatic tubes as possible (limiting electrostatic losses by self-repulsion of unipolar aerosol), as well as by avoiding bends. Although grounding of metallic elements raises the concern of electrostatic losses by image force, this would be negligible in our sampling system that avoids metal walls as much as possible (Alonso et al. Citation2007). Possible losses due to thermophoresis can be neglected as well since the temperature of the carrier gas, which has been monitored with a thermocouple (), was always at ambient temperature. Finally, theoretical loss calculations were carried out for diffusion losses with the AeroCalc software (Baron and Willeke Citation2001). The results have shown that nanoparticle losses in the whole system were less than 5% for all the operating conditions of this study (ambient temperature, particle residence times ranging from 600 ms to 1 s between the ablation point and the measurement device).
In this article, particle numbers N are expressed in part/shot/cm2, i.e., each measured concentration profile (part/cm3) is integrated over the measurement time interval and corrected from concentration noise, then converted into a total number of particles produced by each laser shot on the paint by taking into account the air flow rate and the number of laser shots during the considered time interval, and eventually normalized to one square centimeter of ablated paint:
4. RESULTS
4.1. Influence of Laser Fluence on Nanoparticle Size Distributions
The number per shot and per surface unit of nanoparticles generated during the paint laser ablation increases with fluence, mainly between 0.2 and 1 J/cm2, after which a saturation effect occurs (Dewalle et al. Citation2010). Independent ablated mass measurements show the same variation with this parameter (Dewalle et al. Citation2010). This evolution is only related to the ablation process, which, as mentioned in Section 3, is independent of our operating conditions, including the laser shot number at a given place on the sample (as long as the paint thickness remains above a few tens of micrometers as demonstrated by Brygo et al. Citation2006).
FIG. 3 Nanoparticle number size distributions for various laser fluences (repetition rate = 1 Hz, cell air flow rate = 11.5 L/min).
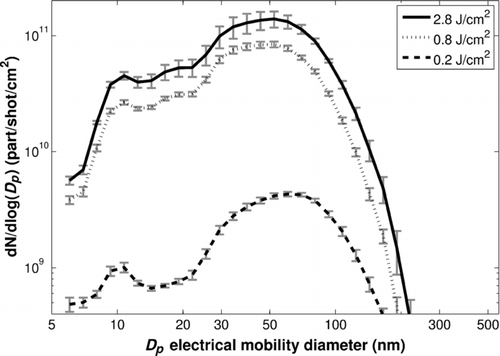
However, whatever the fluence is, the particle size distributions are similar. Indeed, shows that this parameter does not influence the primary particle size (first mode, 10–15 nm) and only slightly changes the aggregate size (second mode, 50–60 nm in this example) despite the very different numbers of particles generated for the different fluences. Indeed, from 0.2 to 2.8 J/cm2, the total number of particles per shot per surface unit has been multiplied by more than 30, while the modal diameter has only decreased from 60.4 to 52.3 nm (which are actually the sizes of the 16th and 17th EEPS channel, respectively). In comparison, other parameters such as the air flow rate in the cell have a much stronger influence, as the next subsection will show. The results from the model proposed in Section 6 will provide an explanation for these phenomena.
The laser–paint interaction is evidently strongly dependent on laser fluence. This is not the case for the two other operating parameters considered in this study. Indeed, the particle transport flow rate does not influence the paint ablation. It is the same for the range of repetition rate studied here (Brygo et al. Citation2006).
4.2. Influence of Air Flow Rate in the Cell on Nanoparticle Size Distributions
The nanoparticle total number per shot and per surface unit increases by a factor of about 4 when the air flow rate in the cell goes from 7 to 33 L/min ().
FIG. 4 Nanoparticle number per shot and per surface unit as a function of air flow rate in the cell (fluence = 0.8 J/cm2, repetition rate = 10 Hz).
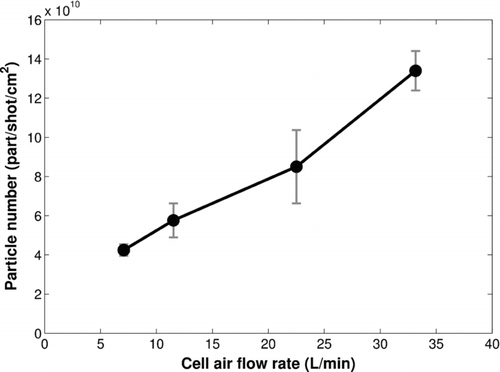
Moreover, a significant change in the particle size distributions with the air flow rate is observed (). Although an increase of the air flow rate in the cell does not modify the primary particle size (first mode, 10–15 nm), it strongly alters the aggregate size (second mode).
FIG. 5 Normalized nanoparticle number size distributions for various air flow rates in the cell (fluence = 0.8 J/cm2, repetition rate = 10 Hz).
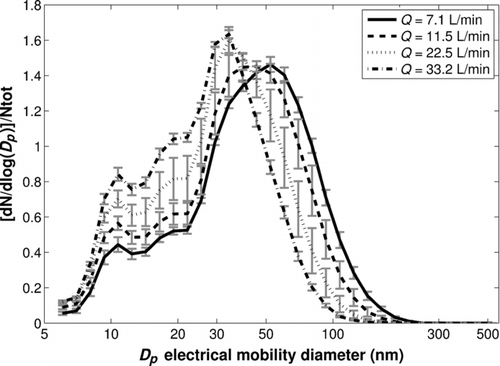
Furthermore, clearly shows that the numerical proportion of primary particles (first mode) increases with the air flow rate, whereas the proportion of larger aggregates (Dp > 60 nm) decreases. Thus, the increase of the nanoparticle total number (/shot/cm2) with the air flow rate () is due to the increase of the primary particle number and the formation of aggregates with a smaller size.
4.3. Influence of Repetition Rate on Nanoparticle Size Distributions
When the air flow rate into the cell is low (7 L/min), nanoparticle number (/shot/cm2) at a 10-Hz repetition rate is a bit smaller than at a 1-Hz repetition rate (). However, for higher flow rates (33 L/min), their number does not decrease between 1 and 10 Hz ().
TABLE 1 Nanoparticle number per shot and per surface unit at repetition rates of 1 and 10 Hz for air flow rates into the cell of 7 and 33 L/min (fluence = 3 J/cm2)
Moreover, there is a slight impact of this operating parameter on the nanoparticle size distributions for the 7-L/min air flow rate. Indeed, aggregate size (second mode) increases slightly (∼10 nm) between 1 and 10 Hz (). This trend is not observed at 33 L/min. However, whatever the air flow rate in the cell is, an increase of repetition rate does not change the size of the primary particles (first mode, around 10–15 nm).
FIG. 6 Normalized nanoparticle number size distributions for various repetition rates and air flow rates in the cell (fluence = 3 J/cm2).
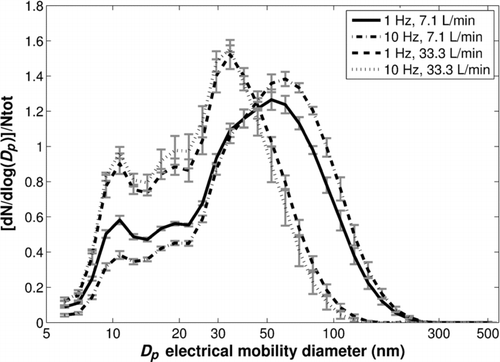
also shows that the numerical proportion of primary particles (first mode, 10–15 nm) decreases slightly with the repetition rate at 7 L/min whereas the proportion of larger aggregates (Dp > 60 nm) increases slightly. These trends with the repetition rate are not observed for a 33-L/min air flow rate in the cell (). Thus, the evolution observed with the lowest air flow rate suggests a possible increase of collision probability between primary particles to form bigger aggregates, most probably due to postproduction overlapping of successive plumes. This point will be further discussed in Section 6.5.
5. DISCUSSION
Results that have been presented above show that the air flow rate into the cell and the repetition rate have an influence on the numerical proportion of the primary particles and on the aggregate size. Although these two parameters have no influence on paint ablation, they do act on the aggregate formation, i.e., on the primary particle aggregation. Indeed, the aggregation process is favored by low air flow rates and by high repetition rates.
The air flow rate in the cell is the parameter that produces the most significant changes both in particle number (/shot/cm2) and size distribution. The observed influences can be explained by the particle residence time in the cell and the downstream tubes up to the measurement devices that decreases when the gas flow increases. Thereby, the time during which particles are able to collide and form aggregates is reduced. As a consequence, the particle number per shot and per surface unit could increase due to smaller resulting aggregates. This effect is well described in most literature studies that focus on the influence of the gas flow rate on final size distribution of nanoparticles produced by laser ablation (Boulaud Citation1993; Liu Citation2005) as well as by plasma surface interaction (Borra Citation2006, 2008).
TABLE 2 K according to particle diameter (P = 101 kPa, T = 293.15 K)
In order to quantify the results on particle number per shot according to the flow rate, the air flow inside the whole sampling system including the ablation cell and the downstream tubes has been modeled by taking their geometry into account, and the Smoluchowski coagulation model has been applied to the particles traveling within. The latter is simple enough so that little parameterization effort is required and is thereby suited for providing a rough estimate of the localization of the coagulation process as well as of the time constants involved in it. The results that have been obtained from this model not only help to conclude about the influence of the air flow rate on the number per shot of the measured particles, but also provide some interesting thoughts regarding the relationship between the volume of the ablation plume and the resulting particle number per shot, that could explain the lack of influence of laser fluence on the particle size distributions.
6. PARTICLE TRANSPORT AND COAGULATION MODEL
6.1. Smoluchowski Model
The Smoluchowski model describes the simple monodisperse coagulation of particles considering Brownian coagulation. It models the time evolution of concentration and diameter for monodisperse particles that collide inside a given volume (Hinds Citation1999):
Since the nanoscale particles we measure range between about 10 nm and 80 nm diameter, and considering that pressure and temperature have stabilized to their ambient values after the primary particle formation, the coefficient K can be expressed as follows:
This coefficient K can be extracted from tables (Hinds Citation1999) of which an extract is presented in . In the range of this study for particle size, K has only little variation, therefore we can consider a constant value of 1.10−9 cm3/s and asses that the integral form of the Smoluchowski equation (Formula (4)) can be used in the model.
6.2. Application to the Different Parts of the Experimental Setup
For the coagulation model to be properly applied, the geometry of the whole sampling system must be taken into account and some hypotheses have to be formulated.
First, since there is a diluter between the ablation cell and the measurement device, it could be reasonable to assess that the reduced particle concentration downstream the diluter strongly disfavors further coagulation inside the tubes between the diluter and the measurement devices. In order to validate this hypothesis, we have measured size distributions, obtained both with and without the diluter, as stated in Section 3. Since no significant difference is observed on the location of the aggregate mode, we can consider that the coagulation process ends at the input of the diluter, which implies that the measurements performed by the EEPS are equivalent to those that would be obtained if the measurement had been carried out directly at the input of the diluter (except for the dilution ratio that will of course be taken into account for comparison of the model with the experimental results).
Therefore, to model only the upstream part of the system up to the diluter is sufficient to get an accurate picture of the places where coagulation is likely to occur. In this regard, represents the ablation cell starting from the laser–matter interaction point, including the subsequent tubes as well as an exhaust, up to the diluter.
FIG. 7 Schematization of the part of the experimental setup in which aggregation occurs (Re are the flow Reynolds numbers).
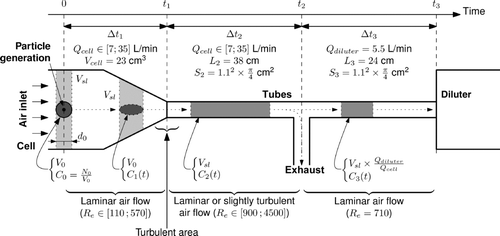
Second, we consider that, at each laser shot on the paint, an aerosol puff is formed as a mixture of air and particles within a small volume that will be referenced to as V 0. This puff is formed within the cell (at the sample location) at a time noted t 0. Since the flow is laminar and homogeneous in velocity in the cell, this volume V 0 should remain nearly constant in this part of the setup.
According to the various air flow rates in the cell and the tubes, we can distinguish three characteristic times (t 1, t 2, and t 3) that separate portions of the system in which the particles travel:
| • | t 1 is the time after which the puff reaches the output of the cell. Since the cell has been designed for the air flow to be laminar in this section, we can write | ||||
Two hypotheses are considered regarding the evolution of V 0 in this section.
First, the initial volume V 0 is supposed to remain constant during Δt 1 = t 1 − t 0 due to the laminar air flow. Initially (at time t 0), the primary particle concentration is C 0=N 0/V 0, where N 0 is the number per shot of primary particles. During Δt 1, according to Smoluchowski equation, the evolution of the concentration C 1(t) is given by
At the output of the cell (at time t 1), a turbulent zone homogenizes the particle puff and clean air within Vsl . Therefore, in the tube immediately outside the cell, we get an homogeneous volume Vsl in which the particle concentration is C 2(t 1)=C 1(t 1)·(V 0/Vsl ), which yields the following:
| • | t 2 is the time after which the Vsl volume reaches the junction between the exhaust tube and the tube to the diluter. In this part, the air flow is laminar again (possibly slightly turbulent when the air flow rate increases). Thus, the traveling of Vsl in this portion lasts Δt 2=t 2−t 1=(L 2·S 2)/Qcell , where L 2 is the length of the tube and S 2 is its section. For the air flow rates considered in this study, Δt2 varies between 60 and 310 ms. During Δt 2, the particle concentration in Vsl is C 2(t), | ||||
| • |
t
3 is the time after which the volume of remaining particles reaches the input of the diluter. As mentioned above, we consider that, from this point on, the particle concentration has reached a steady state. The time interval Δt3
lasts | ||||
Finally, we can write like above:
6.3. Model Equation of the Whole System
Up to now, in this study, all of the results have been expressed in terms of a number of particles generated by one laser shot on the paint and then normalized to one square centimeter of ablated paint [Equation (1)]. Since the goal is to model the evolution of the particle concentration due to one real laser shot on the paint (with an approximate crater area of 0.5 mm2 and responsible for the V 0 volume of the puff considered in the model), comparison with experimental results is best suited without normalization to 1 cm2 of ablated paint (which would only be reflected as an additional factor involving the crater area in the equations).
Therefore, subsequent experimental results will only be expressed as a number of particles generated by one laser shot on the paint. For comparison with the model, this boils down to determining N = C 3(t 3)·Vsl . The following general equation is thereby obtained:
According to the hypothesis proposed in Section 5 to explain the variation of particle number per shot with the air flow rate in the cell, we can assume, as a first approximation, that V 0 is not related to Qcell . The final number per shot of particles is only determined by the puff residence time in the setup (evolution of durations Δt 1 and Δt 2). This hypothesis can be considered because of the significant difference between the orders of magnitude of the gas speed in the cell (a few meters per second) and of the ablation plume speed (typically several hundreds of meters per second; Kokai et al. Citation1999; Hauer Citation2004; Liu Citation2005; Wen et al. Citation2007a, Citation2007b). This suggests that the puff occupies its volume V 0 quasi instantly whatever the air flow rate in the cell is. In order to also cover a possible relationship between V 0 and Qcell , a first order dependency can be introduced as V 0=Vi +α·Qcell , where Vi is the volume value when Qcell is null and α is a coefficient that is homogeneous to a time.
6.4. Model Fitting with Experimental Results
The experimental results provide several couples of measured number of particles per laser shot versus the air flow rate into the cell (Qcell , N). On the basis of those quantities, we have correlated the model and the experimental results for two fluences: 0.84 and 2.8 J/cm2, for which four air flow rates have been studied: 7.1, 11.5, 22.5, and 33.3 L/min. For each fluence, we search the model parameters (N 0 and V 0 or N 0, Vi , and α) that give the best correlation with the experimental results in terms of particle number per shot according to the flow rate.
In order to have at most two parameters to determine to match the model with the experimental results, the initial number per shot of primary particles N 0 has been estimated assuming that the total volume of primary particles [N 0·(1/6)·π·D 3 p , where Dp is the primary particle diameter (16 nm)] is equal to the total volume of particles measured by the particle sizer EEPS. Although this calculation overestimates the N 0 value due to the aggregated shape of nanoparticles, it will be shown in Section 6.5 that its exact value has little impact on the final results. recapitulates these data for the two studied fluences.
TABLE 3 Estimated values of the numbers of primary particles per shot N 0 for fluences of 0.84 and 2.8 J/cm2
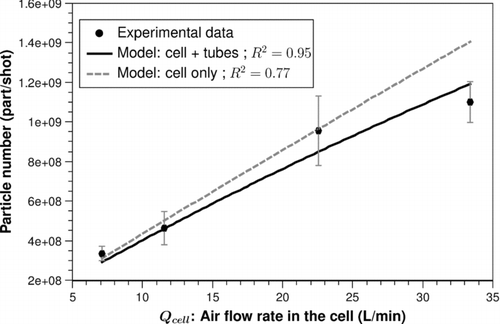
The fitting results are presented hereafter. In the case where V 0 is related to Qcell , the computed value for α is quasi null. We can conclude that the initial assumption is correct, that is, the initial volume of the puff V 0 is independent of the air flow rate in the cell. Thus, only V 0 remains to be determined. The fitting results (black curves) are presented in (0.84 J/cm2) and (2.8 J/cm2). The fit between the experimental results and the model gives a volume V 0 of about 40 mm3 at 0.84 J/cm2 and of about 60 mm3 at 2.8 J/cm2 with a good R 2 value (0.97 and 0.95, respectively).
FIG. 8 Fitting results between the experimental data and the model at 0.84 J/cm2 for a volume V0 of 39.1 mm3 (solid line: calculated number of particles per shot at the input of the diluter; dashed line: at the output of the cell).
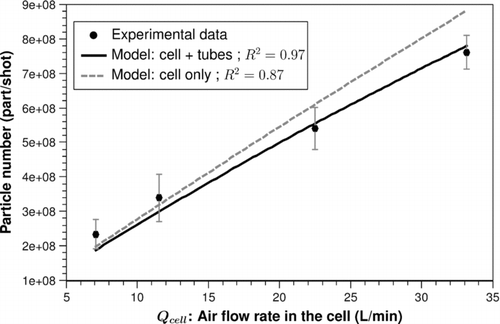
FIG. 9 Fitting results between the experimental data and the model at 2.8 J/cm2 for a volume V0 of 60.8 mm3 (solid line: calculated number of particles per shot at the input of the diluter; dashed line: at the output of the cell).
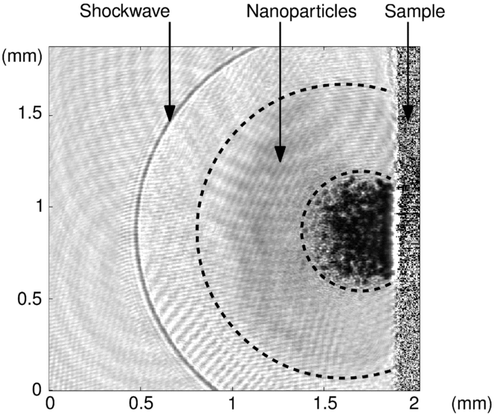
These two volumes correspond to spheres of respectively 4.2 and 4.9 mm diameter for the fluences of 0.84 and 2.8 J/cm2. These results can be compared with measured dimensions of the ablation plume that represents the initial particle puff. Indeed, we have realized an in-situ visualization of the plume produced by one laser shot on the paint (shadowgraphic imaging method). This optical characterization will be discussed in a future publication and is therefore not further described in this article, but a representative example () shows that the dimensions of the ablation plume are in accordance with the volume V 0 found by the model (about 4 to 5 mm in diameter). The nanoparticles are located in the grey area between the shockwave and a much darker zone that contains submicronic particles (Dewalle et al. Citation2010, which are out of scope of the present article). Since this camera shot is taken one microsecond after the laser shot on the sample, the sphere occupied by the nanoparticles (about 1.5 mm diameter at that time) will keep growing with a fast-reducing speed. Indeed, the measured growth of the nanoparticle sphere diameter is only of 0.2 mm per microsecond at the time of this camera shot, while it was more than 0.5 mm/μs only half a microsecond before. As a consequence, the nanoparticle sphere diameter will quickly stabilize by pressure equilibrium around a few millimeters, forming the expected V 0 volume that will travel in the cell during a much longer time (t 1, estimated at 40 to 200 milliseconds depending on the air flow rate in the cell).
6.5. Conclusion and Discussion from the Comparison Measurements/Model
Since the Smoluchowski model we applied was initially dedicated to monodisperse aerosol, the polydisperse size distributions obtained in this study call for a comment regarding the choice of coagulation coefficient K in Section 6.1. Indeed, this coefficient can be adapted for lognormal distributions with a given count median diameter (CMD) and geometric standard deviation (GSD; Hinds Citation1999). Although the corrected value of K for primary particles is nearly the same as the chosen value since they are only slighlty polydispersed (GSD = 1.27), this is no longer true for the aggregate mode (GSD = 1.7) for which the corrected K increases. Thus, V 0 should be greater than the values suggested by the model results. Still, taking this evolution of K into account would require step-by-step updating of K as a function of time and of the mode of the size distribution, thereby involving much more complexity. Therefore the proposed model maintains the value computed in Section 6.1 and the error due to polydispersity can be evaluated: with an estimated average K of 14 × 1010 cm3/s, the volume V 0 would be at most 40% higher, which would result in a puff diameter increased by only 12%.
Likewise, the nonspherical shape of aggregates tends to increase K by a similar amount (Hinds Citation1999). As a consequence, it could be reasonably considered that a more accurate value of the puff diameter should be about 20% higher than the results from the model.
Thus, despite its simplicity, our model gives semiquantitative estimates of the most critical parameters that govern the nanoparticle aggregation process in the setup:
| • | The air flow rate influence on the final nanoparticle number per shot is well described by the model. The primary particle aggregation process takes place in a small volume V 0, which varies with laser fluence (V 0 is larger for the highest fluence). Moreover, this volume is independent of the air flow rate, which controls the residence time from production to measurement tools and the subsequent related number of particles per shot. | ||||
| • | The hypothesis of constant puff volume V 0 in the cell is a simplifying assumption. In reality, the puff volume expands at least a little, probably by diffusion and convection due to the plume temperature. Thermophoresis effect is also a possible cause for evolution of V 0 inside the cell. Therefore, V 0 could be seen as an average volume in the cell. | ||||
| • | In and (dashed curves), we have also plotted the particle number per shot calculated at the cell outlet. Only slight differences can be observed with the final number per shot obtained at the input of the diluter ( and ; solid curves), especially at low air flow rates. For instance, at 7 L/min, the relative error between the dashed and the solid curves is only 5% and 7% for the two fluences, respectively. This suggests that most of the coagulation process occurs within the cell, where particle concentration into V 0 is the highest. Moreover, the relative gap between the two curves increases when the air flow rate increases. Indeed, the relative differences at 33 L/min rise to 13% and 18% for the two fluences, respectively. This can be explained by the shortening of Δt 1 duration inside the cell at higher flow rates, increasing the concentration C 2(t 1) at the output of the cell, and thereby favoring further coagulation in the tubes. | ||||
| • | The computed number of primary particles per shot N 0 is much greater than N, which suggests that the 1/N 0 term in Equation (12) is negligible. To support this assumption, the model fitting has also been performed with N 0 divided by two, and the resulting V 0 was only increased by less than 5%, showing that N 0 is of little significance. As a consequence, the final number of particles per shot at the output of the cell can be approximated as: | ||||
| • | These statements make it possible to propose an explanation for the results presented in Section 4.1 regarding the influence of laser fluence on particle number per shot and size distribution. Although fluence increases the total amount of ablated paint (Dewalle et al. Citation2010), the final number of particles per shot does not depend on this parameter (and thus, on N 0). Instead, it is only linked to the size of the interaction volume V 0. Furthermore, V 0 is very much likely to expand in similar proportions as N 0 with laser fluence, maintaining about the same initial concentration of primary particles C 0 no matter the value of N 0. Indeed, if V 0 did not depend on fluence, then C 0 would increase in the same proportions as N 0, resulting in bigger aggregates since their diameter is related to the cube root of concentration. Thus, the fact that the modal diameter does not increase with fluence () supports the assumption that V 0 expands in such a way that C 0 does not stronlgy evolve even though N 0 significantly changes. | ||||
The last parameter that has been discussed in Section 4 was the influence of the repetition rate on particle size distributions. However, the proposed model only focuses on the puff generated by one laser shot. Moreover, in the preceding results, the ratio between the air flow rate in the cell and the repetition rate was always superior to the volume of the slice of air Vsl associated with the computed value of V 0, which means that successive laser shots should not interact with each other. Therefore, the model is not able to reproduce the slight influence of the repetition rate that has been observed for the lowest air flow rates (Section 4.3). Still, it should be reminded that V 0 can be seen as an average volume along its route in the cell (from the ablation point to the output) and that its actual value likely increases a little bit during this time, which would make it easier for successive puffs to interact near the cell output. In any case, including this interaction into the model would require a good knowledge of V 0 and its evolution (in terms of geometry, homogeneity, etc.) that would call for further experimental investigations.
7. CONCLUSION
The influence of three operating parameters (air flow rate in the ablation cell, laser repetition rate, and fluence) on the primary particles and the aggregates produced by laser ablation of an acrylic paint has been studied in this article.
Their total number per shot and per surface unit increases significantly with the laser fluence and the air flow rate and decreases slightly with the laser repetition rate, if the air flow rate is low. Moreover, the numerical proportion of the primary particles increases with the air flow rate and decreases with the repetition rate (for low flow rates). The three operating parameters do not influence the primary particle size but the air flow rate and the repetition rate have an effect on the aggregate size. Their size decreases with the air flow rate and increases slightly with the repetition rate for low flow rates. Thereby, an increase of the air flow rate disfavors the primary particle aggregation to form aggregates and an increase of the repetition rate at low air flow rates favors it.
The most critical parameter is the air flow rate. The setup has been modeled (model based on the Smoluchowski coagulation description) in order to explain the particle number trend with this parameter. The modeling has shown that the observed behavior in particle number per shot is due to the evolution, with the flow rate, of the particle residence time in the setup and that most of the aggregation process occurs in the ablation cell and very little aggregation occurs in the tubes behind it. Moreover, the primary particle aggregation process takes place in a small sphere of a few millimeters in diameter, which increases in size with laser fluence but not with the air flow rate. Thereby, with this evolution of the sphere volume, the initial primary particle concentration remains constant and the aggregate have the same size whatever the fluence. Therefore, the final number of nanoparticles per shot is not governed by the initial primary particle number but only by the volume in which they interact to form aggregates.
Acknowledgments
This work was funded by IRSN (Institut de Radioprotection et de Sûreté Nucléaire), AREVA NC and CEA (Commissariat à l'Energie Atomique et aux Energies Alternatives). It was jointly conducted in two laboratories of IRSN and CEA: the Laboratory of Aerosol Physic and Metrology (IRSN/DSU/SERAC) and the Laboratory of Laser-Matter Interaction (CEA/DEN/DPC/SCP). The authors would like to thank the members of these two laboratories for their help and their technical assistance.
This work was funded by IRSN (Institut de Radioprotection et de Sûreté Nucléaire), AREVA NC, and CEA (Commissariat à l’Energie Atomique et aux Energies Alternatives). It was jointly conducted in two laboratories of IRSN and CEA: the Laboratory of Aerosol Physic and Metrology (IRSN/DSU/SERAC) and the Laboratory of Laser–Matter Interaction (CEA/DEN/DPC/SCP). The authors would like to thank the members of these two laboratories for their help and their technical assistance.
REFERENCES
- Albert , O. , Roger , S. , Glinec , Y. , Loulergue , J. C. , Etchepare , J. Boulmer-Leborgne , C. 2003 . Time-Resolved Spectroscopy Measurements of a Titanium Plasma Induced by Nanosecond and Femtosecond Lasers . Appl. Phys. A, Mater. Sci. Process , 76 : 319 – 323 .
- Alonso , M. , Alguacil , F. J. , Santos , J. P. , Jidenko , N. and Borra , J. P. 2007 . Deposition of Ultrafine Aerosol Particles on Wire Screens by Simultaneous Diffusion and Image Force . J. Aerosol Sci. , 38 ( 12 ) : 1230 – 1239 .
- Amoruso , S. , Ausanio , G. , Barone , A. C. , Bruzzese , R. , Campana , C. and Wang , X. 2007 . Nanoparticles Size Modifications During Femtosecond Laser Ablation of Nickel in Vacuum . Appl. Surf. Sci. , 254 : 1012 – 1016 .
- Baron , P. A. and Willeke , K. 2001 . Aerosol Measurement. Principles, Techniques and Applications , 2nd ed. , Wiley-Interscience, John Wiley, New York. . ISBN 0-471-35636-0
- Bereznai , M. , Heszler , P. , Toth , Z. , Wilhelmsson , O. and Boman , M. 2006 . Measurements of Nanoparticle Size Distribution Produced by Laser Ablation of Tungsten and Boron-Carbide in N2 Ambient . Appl. Surf. Sci. , 252 : 4368 – 4372 .
- Borra , J. P. 2006 . Nucleation and Aerosol Processing in Atmospheric Pressure Electrical Discharges: Powders Production, Coatings and Filtration . J. Phys. D Appl. Phys. , 39 : R19 – R54 .
- Borra , J. P. 2008 . Charging of Aerosol and Nucleation in Atmospheric Pressure Electrical Discharges . Plasma Phys. Contr. F. , 50 : 124036 ISSN: 0741-3335,
- Boulaud , D. 1993 . “ Aerosol Production by Laser Ablation ” . In Proceedings of the International Workshop on the Synthesis and Measurement of Ultrafine Particles. Synthesis and Measurement of Ultrafine Particles , Edited by: Marijnissen , J. C. M. and Pratsinis , S. 31 – 40 . Delft University Press . Amsterdam
- Brygo , F. , Dutouquet , Ch. , Le Guern , F. , Oltra , R. , Semerok , A. and Weulersse , J. M. 2006 . Laser Fluence, Repetion Rate and Pulse Duration Effects on Paint Ablation . Appl. Surf. Sci. , 252 : 2131 – 2138 .
- Camata , R. P. , Hirasawa , M. , Okuyama , K. and Takeuchi , K. 2000 . Observation of Aerosol Formation During Laser Ablation Using a Low-Pressure Differential Mobility Analyzer . J. Aerosol Sci. , 31 : 391 – 401 .
- Craciun , V. , Bassim , N. , Singh , R. K. , Craciun , D. , Hermann , J. and Boulmer-Leborgne , C. 2002 . Laser-Induced Explosive Boiling During Nanosecond Laser Ablation of Silicon . Appl. Surf. Sci. , 186 : 288 – 292 .
- Delaporte , Ph. , Gastaud , M. , Marine , W. , Sentis , M. , Uteza , O. Thouvenot , P. 2003 . Dry Excimer Laser Cleaning Applied to Nuclear Decontamination . Appl. Surf. Sci. , 208–209 : 298 – 308 .
- Deno , H. , Kamemoto , T. , Nemoto , S. , Koshio , A. and Kokai , F. 2008 . Formation of TiN-Ir Particle Films Using Pulsed-Laser Deposition and Their Electrolytic Properties in Producing Hypochlorous Acid . Appl. Surf. Sci. , 254 : 2776 – 2782 .
- Dewalle , P. , Vendel , J. , Weulersse , J.-M. , Hervé , P. and Decobert , G. 2010 . Characterization of Aerosols Generated by Nanosecond Laser Ablation of an Acrylic Wall Paint . Aerosp. Sci. Technol. , 44 ( 10 ) : 902 – 915 .
- Gonzalez , J. J. , Liu , C. , Wen , S. B. , Mao , X. and Russo , R. E. 2007a . Metal Particles Produced by Laser Ablation for ICP-MS Measurements . Talanta, , 73 : 567 – 576 .
- Gonzalez , J. J. , Liu , C. , Wen , S. B. , Mao , X. and Russo , R. E. 2007b . Glass Particles Produced by Laser Ablation for ICP-MS Measurements . Talanta , 73 : 577 – 582 .
- Hathorne , E. C. , James , R. H. , Savage , P. and Alard , O. 2008 . Physical and Chemical Characteristics of Particles Produced by Laser Ablation of Biogenic Calcium Carbonate . J. Anal. At. Spectrom. , 23 : 240 – 243 .
- Hauer , R. H. 2004 . Laser Ablation of Polymers Studied by Time Resolved Methods. Ph.D. thesis , Zurich, , Switzerland : Swiss Federal Institute of Technology .
- Heszler , P. 2002 . Emission Spectroscopy and Size Distribution of Gas Phase Nanoparticles Generated by Laser-Based Methods . Appl. Surf. Sci. , 186 : 538 – 545 .
- Heszler , P. , Elihn , K. , Landström , L. and Boman , M. 2002 . Formation and Emission Spectroscopy of Laser-Generated Nanoparticles . Smart Mater. Struct. , 11 : 631 – 639 .
- Hinds , W. C. 1999 . Aerosol Technology. Properties, Behavior, and Measurement of Airborne Particles , 2nd ed. , Wiley-Interscience, John Wiley . New York. ISBN 978-0-471-19410-1
- Hirazawa , M. , Seto , T. , Orii , T. , Aya , N. and Shimura , H. 2002 . Synthesis of Size-Selected TiOx Nanoparticles . Appl. Surf. Sci. , 197–198 : 661 – 665 .
- Jeong , S. H. , Borisov , O. V. , Yoo , J. H. , Mao , X. L. and Russo , R. E. 1999 . Effects of Particle Size Distribution on Inductively Coupled Plasma Mass Spectrometry Signal Intensity During Laser Ablation of Glass Samples . Anal. Chem. , 71 ( 22 ) : 5123 – 5130 .
- Kawakami , Y. , Seto , T. and Ozawa , E. 1999 . Characteristics of Ultrafine Tungsten Particles Produced by Nd:YAG Laser Irradiation . Appl. Phys. A , 69 ( Suppl. ) : S249 – S252 .
- Koch , J. , Wälle , M. , Dietiker , R. and Günther , D. 2008 . Analysis of Laser-Produced Aerosols by Inductively Coupled Plasma Mass Spectrometry: Transport Phenomena and Elemental Fractionation . Anal. Chem. , 80 ( 4 ) : 915 – 921 .
- Kokai , K. , Takahashi , K. , Shimizu , K. , Yudasaka , M. and Iijima , S. 1999 . Shadowgraphic and Emission Imaging Spectroscopic Studies of the Laser Ablation of Graphite in an Ar Gas Atmosphere . Appl. Phys. A, Mater. Sci. Process. , 69 : S223 – S227 .
- Kuhn , H.-R. and Günther , D. 2005 . The Agglomeration State of Nanosecond Laser-Generated Aerosol Particles Entering the ICP . Anal. Bioanal. Chem. , 383 : 434 – 441 .
- Kuhn , H. R. , Koch , J. , Hergenröder , R. , Niemax , K. , Kalberer , M. and Günther , D. 2005 . Evaluation of Different Techniques for Particle Size Distribution Measurements on Laser-Generated Aerosols . J. Anal. At. Spectrom. , 20 : 894 – 900 .
- Kusch , H.-G. , Heinze , T. and Wiedemann , G. 2003 . Hazardous Emissions and Health Risk During Laser Cleaning of Natural Stones . J. Cult. Herit. , 4 38s–44s
- Landström , L. , Marton , Z. S. , Boman , M. and Heszler , P. 2004 . Monitoring Nanoparticle Formation During Laser Ablation of Graphite in an Atmospheric-Pressure Ambient . Appl. Phys. A, Mater. Sci. Process. , 79 : 537 – 542 .
- Lee , D. W. and Cheng , M.-D. 2004a . Investigations of Nanoparticle Generation During Surface Decontamination by Laser Ablation at Low Fluence . J. Aerosol Sci. , 35 : 1513 – 1526 .
- Lee , D. W. and Cheng , M.-D. 2004b . Particle Generation by Laser Ablation During Surface Decontamination . J. Aerosol Sci. , 35 : 1527 – 1540 .
- Liu , C. , Mao , X. L. , Mao , S. S. , Zeng , X. , Greif , R. and Russo , R. E. 2004 . Nanosecond and Femtosecond Laser Ablation of Brass: Particulate and ICPMS Measurements . Anal. Chem. , 76 : 379 – 383 .
- Liu , C. L. 2005 . A Study of Particle Generation During Laser Ablation with Applications. Ph.D. thesis , Berkeley, , USA : University of California .
- Lowndes , D. H. , Rouleau , C. M. , Thundat , T. , Duscher , G. , Kenik , E. A. and Pennycook , S. J. 1998 . Silicon and Zinc Telluride Nanoparticles Synthesized by Pulsed Laser Ablation: Size Distributions and Nanoscale Structure . Appl. Surf. Sci. , 127–129 : 355 – 361 .
- Lushnikov , A. A. 1996 . Laser Induced Aerosols . J. Aerosol Sci. , 27 ( 1 ) : S377 – S378 .
- Mahfouz , R. , Cadete Santos Aires , F. J. , Brenier , A. , Jacquier , B. and Bertolini , J. C. 2008 . Synthesis and Physico-Chemical Characteristics of Nanosized Particles Produced by Laser Ablation of a Nickel Target in Water . Appl. Surf. Sci. , 254 : 5181 – 5190 .
- Marton , Zs. , Landström , L. , Boman , M. and Heszler , P. 2003 . A Comparative Study of Size Distribution of Nanoparticles Generated by Laser Ablation of Graphite and Tungsten . Mater. Sci. Eng. C , 23 : 225 – 228 .
- Mateo , M. P. , Ctvrtnickova , T. , Fernandez , E. , Ramos , J. A. , Yanez , A. and Nicolas , G. 2009 . Laser Cleaning of Varnishes and Contaminants on Brass . Appl. Surf. Sci. , 255 ( 10 ) : 5579 – 5583 .
- Ogawa , K. , Vogt , T. , Ullmann , M. , Johnson , S. and Friedlander , S. K. 2000 . Elastic Properties of Nanoparticles Chain Aggregates of TiO2, Al2O3, and Fe2O3 Generated by Laser Ablation . J. Appl. Phys. , 87 ( 1 ) : 63 – 73 .
- Seol , K. S. , Camata , R. P. and Takeuchi , K. 1999 . Study on the Formation of Silicon Nanoparticles During Laser Ablation Using a Low-Pressure Differential Mobility Analyzer . J. Aerosol Sci. , 30 ( Suppl. 1 ) S467–S468
- Seto , T. , Kawakami , Y. , Suzuki , N. , Hirazawa , M. , Kano , S. Aya , N. 2001 . Evaluation of Morphology and Size Distribution of Silicon and Titanium Oxide Nanoparticules Generated by Laser Ablation . J. Nanopart. Res. , 3 : 185 – 191 .
- Thareja , R. K. and Shukla , S. 2007 . Synthesis and Characterization of Zinc Oxide Nanoparticles by Laser Ablation of Zinc in Liquid . Appl. Surf. Sci. , 253 : 8889 – 8895 .
- Ullmann , M. , Friedlander , S. K. and Schmidt-Ott , A. 2002 . Nanoparticle Formation by Laser Ablation . J. Nano. Res. , 4 : 499 – 509 .
- Wen , S.-B. , Mao , X. , Greif , R. and Russo , R. E. 2007a . Experimental and Theoretical Studies of Particle Generation After Laser Ablation of Copper with a Background Gas at Atmospheric Pressure . J. Appl. Phys. , 101 ( 123105 ) : 1 – 15 .
- Wen , S.-B. , Mao , X. , Greif , R. and Russo , R. E. 2007b . Laser Ablation Induced Vapour Plume Expansion into a Background Gas. II. Experimental Analysis . J. Appl. Phys. , 101 ( 023115 ) : 1 – 13 .