Abstract
For some individuals, the degree of response to inhaled allergens or the efficacy of inhaled pharmaceuticals may be influenced by their lung morphometry. Fourteen healthy, nonsmoking subjects received a high-resolution computed tomography (HRCT) scan just prior to performance of a methacholine challenge. Following the methacholine challenge, subjects were given a bronchodilator and received a second HRCT scan. HRCT scans were reconstructed and measurements of airway length, diameter, branch angle, and inclination to gravity were made for the first six tracheobronchial airway generations. The average length (L) and diameter (d) of each airway generation were used to calculate the average airway generation circumference (πd) and surface area (πLd). The product of airway circumference and surface area was incorporated into an airway volume factor (Ld2 ), inversely proportional to the fractional decrease in airway circumference due to deposited methacholine. Ld2 was used to compare methacholine challenge responders with nonresponders. Six subjects did not respond to the methacholine challenge, while eight subjects responded with a greater than 3% decrease in their forced expiratory volume in 1 s. Compared with the nonresponders, these responders had smaller airway volume factors (from about the same in airway generation 1 to 36% smaller in airway generation 5). They also entered the study with less airway patency (the largest difference was in airway generation 5; 28% compared with 13% in the nonresponsive). Comparison of first and second (after bronchodilation) CT scans indicated that all subjects started the study with each airway generation having a unique degree of airway patency (2–33%).
Copyright 2013 American Association for Aerosol Research
INTRODUCTION
Deposition of inhaled particulate matter in the human respiratory tract primarily depends on physiological (i.e., breathing pattern and airway anatomy) and aerosol properties. If similar aerosols and breathing patterns are used, differences in inhaled particle deposition should be a function of anatomical differences. This project combines data from the methacholine challenge and the tracheobronchial anatomy to discern the influence airway patency has on individual response to inhaled allergens or on the efficacy of inhaled pharmaceuticals.
Preliminary studies with mice indicate that apparent differences in airway hyperactivity, as defined by the methacholine challenge, can be explained by tracheobronchial morphometry and the physiology of airway patency (Delorme and Moss Citation2002; Moss and Oldham Citation2006, Citation2011). These mouse data indicated that the methacholine challenge test reflects differences in relative airway size or normal degree of smooth muscle constriction (airway patency) and molecular or tissue-based bronchial hyperresponsiveness. Brown et al. (Citation2000) used high-resolution computed tomography (HRCT) to look at airway diameters in five nonresponsive human subjects exposed to methacholine during tidal breathing. They concluded that responses to methacholine were associated with conducting airway narrowing in a heterogeneous pattern, but also observed that “clearly, some airways both within and between subjects had a more vigorous response to Mch” (methacholine).
In this study, lung morphometry measurements derived from HRCT scans were used to investigate the extent to which a methacholine-challenge-based protocol may be influenced by lung morphometry and airway patency.
MATERIALS AND METHODS
Overview
For each subject, the methacholine challenge and lung scans were completed within 1 day: Upon arrival, HRCT images of the lungs were collected; this was followed by administration of the methacholine challenge. The change in pulmonary function was assessed by measuring the forced expiratory volume in 1 s (FEV1). Following administration of a bronchodilator, a second HRCT image was collected. For each set of HRCT scans, every airway in the first six airway generations was measured for airway gravity angle, branch angle, length, and diameter. These measurements were used to estimate airway-by-airway circumference (πd) and surface area (πLd). The product of airway circumference and surface area was incorporated into an airway volume factor (Ld2 ), inversely proportional to the fractional decrease in airway circumference due to the deposited methacholine (Moss and Oldham Citation2006, Citation2011).
Materials
The methacholine solutions used in this study were as follows: Methacholine (provocholine powder [C8H18ClNO2; m.w. 185.7 g/mole]; Methapharm, Coral Springs, FL, USA) in saline solution at concentrations of 0.0, 0.025, 0.25, 2.5, 10, and 25 mg/mL. For each methacholine exposure atmosphere, a Spraytec (Malvern Insterments, Worchestershire, UK) was used to measure, in real time, the particle/droplet size distribution. Aerodynamic diameters were estimated by assuming that the density of each particle/droplet was near 1 g/mL.
The bronchiodilator administered to the subjects was Albuterol (Salbutamol, inhaler): two inhalations (90 μg/ inhalation) from the inhaler.
Human Subjects
The human study protocol (protocol no. RJRCSM001) was approved by an Institutional Review Board (IRB). The IRB and all human subject procedures and measurements were conducted by Piedmont Medical (Winston-Salem, NC). A minimum of 10 and preferably 20 subjects were to be enrolled in the study based on the expected probability of seeing a population distribution due to tracheobronchial airway size in the first six airway generations (trachea = generation 1). All subjects were required to have an FEV1 > 80% predicted in a screening pulmonary function test.
Methacholine Challenge
Each methacholine challenge was delivered as an aerosol generated from a disposable nebulizer (PARI LC Plus, PARI, Midlothian, VA, USA; operated continuously at 12.5 psi for an output of 3.2 L/min). For each concentration introduced into the nebulizer, the subject followed a five-breath dosimeter protocol (American Thoracic Society Citation2000; American Association for Respiratory Care Citation2001). Subjects were asked to breathe normally (resting ventilation) and not to pause between inhalation and exhalation. Prior to the methacholine challenge, subjects performed a baseline FEV1 maneuver. Following the fifth inhalation of methacholine aerosol, the subject performed FEV1 maneuvers.
This five-breath methacholine challenge was repeated up to five times, wherein each subsequent challenge, the concentration of methacholine in the nebulized solution was increased by a factor of 2 or more (i.e., for concentrations of 0.0, 0.025, 0.25, 2.5, 10, and 25 mg/mL). In order to keep the cumulative effect of methacholine relatively constant, the time interval between the start of two subsequent concentrations was kept at 5 min. The challenges were repeated until the subject inhaled the aerosol generated from the solution with the highest methacholine concentration, until the subject's FEV1 decreased by more than 10%, or the subject refused to continue.
If at the highest exposure (25 mg/mL introduced into the nebulizer), there was less than 3% decrease in FEV1, the subject was identified as nonresponsive; otherwise, as responsive. Following the final methacholine challenge, the subject had two inhalations from an albuterol inhaler (90 μg/inhalation), waited 10 min prior to performing one or more FEV1 maneuvers. When FEV1 returned to at least baseline, the subject was immediately positioned for imaging. The average time between albuterol inhalation and imaging was 29 min.
TABLE 1 Subject demographics and response to methacholine challenge
Imaging
The lung of each subject was imaged in the supine position using HRCT (GE Lightspeed+; GE Healthcare, Chalfont St. Giles, UK). The acquisition parameters were 120 Kv, 1.03 ms exposure time, 0.0° of detector tilt, and 1.0 mm slice thickness. Two CT imaging sessions were scheduled: one before methacholine challenge and one after albuterol inhalation. A reproducible image immediately after the methacholine challenge was not possible due to the rapidity of recovery from the effects of methacholine. For each imaging session, a physical mark on the body was used to allow alignment of the images. Although a small difference exists between male and female in the average distance needed to collect data on the first six airway generations, the required data were generally obtained from CT images collected 35 mm above and 35 mm below the carina (first bifurcation of the trachea). The exception was for extremely tall subjects, where the distance above and below the carina was increased to cover the region of interest.
The scans from each HRCT imaging session were segmented and reconstructed (Clinkenbeard et al. Citation2002) in order to extract the characteristic branching, tree-like image of the first six tracheobronchial airway generations. The resulting three-dimensional image could be rotated and tilted.
Morphometry
Every airway in tracheobronchial airway generations 1–6 was measured. The reconstructed three-dimensional image of lung airways was used to obtain, from each individual airway, the following four values: airway length, diameter, branch angle, and gravity angle. Parallax was minimized by rotating and tilting the image. These measurements were used to construct a typical path lung model (TPLM) using the scheme of Weibel (Citation1963) for the first six tracheobronchial airway generations. The TPLM was constructed by averaging the dimensions of the airways of each airway generation. The tracheobronchial airway generations were classified consistent with Phalen et al. (Citation1978) as follows: generation 1 was defined as the trachea, the two tracheobronchial airways distal to the first bifurcation comprised generation 2, the four tracheobronchial airways distal to the next bifurcations comprised generation 3, and so on. For tracheobronchial airway generations 1–6, the following factors were also calculated: the average circumference (πd), the average surface area (πLd), and, based on the product of circumference and surface, the airway volume factor (Ld2 ). Statistical comparison of the airway volume were performed using the paired t-test (based upon previous CT literature O’Riordan et al. Citation1993) and the nonparametric Wilcoxon rank-sum test due to the low number of subjects (statistical significance for both tests p < 0.05).
RESULTS
Human Subjects
Fourteen healthy, nonsmoking subjects (6 males and 8 females) were recruited and completed the protocol (see for collected demographics).
Methacholine Challenge
The mass median diameter (MMD) for all solutions with 10 mg/mL or less of methacholine was 6.7 μm, with geometric standard deviation (GSD) of 2.2. For the 25 mg/mL methacholine concentration, the aerosol was bimodal, with 31% of the mass in a smaller distribution (MMD = 0.36; GSD = 2.1) and the remaining mass, 69%, in the larger distribution (MMD = 6.8; GSD = 1.9).
Response to the methacholine challenge separated subjects into two subpopulations (): responsive (8 subjects: 3 males and 5 females) and nonresponsive (6 subjects: 3 males and 3 females). The responsive subjects showed up to a 19% decrease in FEV1 at exposures below or equal to the highest methacholine challenge. And the nonresponsive subjects completed all exposures with less than 3% decrease in FEV1—there was no significant change even at the highest methacholine exposures.
TABLE 2 Individual airway surface area, πLd, and volume factor, Ld 2 (mean ± standard error), as a function of airway generation. Measurements from the HRCT scan taken just prior to the methacholine challenge
Imaging
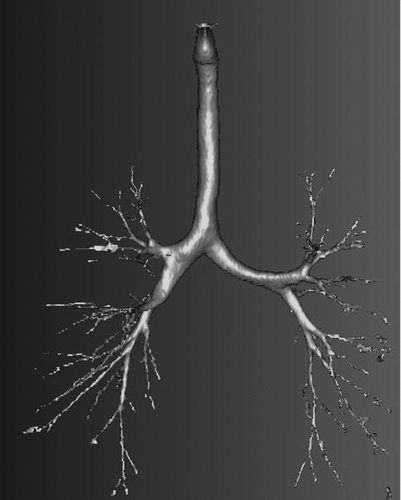
From the HRCT scans, a typical example of an extracted image of tracheobronchial airways is shown in . This subject (no. 8 in ) was one of the eight who were responsive to the methacholine challenge.
Morphometry
Among subjects having similar response to the methacholine challenge, the generation-by-generation airway volume factor, Ld2 , was averaged (). The largest difference in Ld2 was seen for tracheobronchial airway generation 5: for the nonresponsive group, as compared with the responsive group, the value of Ld2 was 1.4 times larger. For the other airway generations (1, 3, 4, and 6), the airway volume factor for the responsive group was approximately 1 to 20% less than the Ld2 for the nonresponsive group.
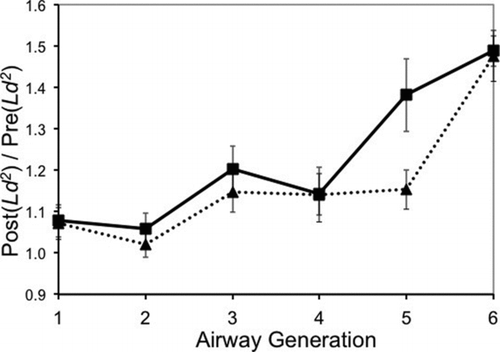
The morphometry measurements () also showed that for all response groups, the albuterol-induced increase in tracheobronchial airway size [as indicated by Post(Ld2 )/Pre(Ld2 )] was approximately the same for the trachea and tracheobronchial airway generation 2 (less than 10% increase in airway size following dilation), was similar between response groups but slightly higher for tracheobronchial airway generations 3 and 4 (12 to 20% increase), and even higher for tracheobronchial airway generation 6 (48 to 49% increase). The largest difference between the methacholine response groups was seen in tracheobronchial airway generation 5 (for responsive subjects, a 38% increase, and for nonresponsive subjects, a 15% increase in airway size).
FIG. 2 Impact of bronchodilation. For each subject, morphometric measurements were made from two HRCT scans. The second HRCT scan (the “Post” scan) was made after the methacholine challenge and following bronchodilation. The first HRCT scan (the “Pre” scan) was made prior to the methacholine challenge. Post/pre airway volume factors (Ld 2; mean ± standard error) are compared: nonresponsive (6 subjects, triangles connected with dotted line); responsive (8 subjects, squares connected with solid lines).
DISCUSSION
The scope of this research was to evaluate lung images from 14 subjects and to compare individual tracheobronchial airway measurements derived from these images to responses from a commonly used aerosol challenge, the methacholine challenge. It was expected that for normal healthy subjects, responses to methacholine would be correlated with tracheobronchial airway dimensions (i.e., baseline airway patency). A 3% decrease in FEV1 was chosen as the cutoff between nonresponsive and responsive individuals because it was the lower limit of our ability to detect differences in FEV1 for an individual subject, resulting in potential nonresponders with large airways being classified as responders. This would work against our above hypothesis. A 5% decrease in FEV1 cutoff would have resulted in the same conclusions since there appears to be a continuum of response from 0% to 9.5% decrease in FEV1 ().
Although the first HRCT scan was essential in calculating each subject's lung airway morphometry just prior to the methacholine challenge, the second HRCT scan provided an insight into airway patency {estimated as 1 – 1/[Post(Ld2 )/Pre(Ld2 )]}. All subjects came into the study with progressively increasing airway patency in tracheobronchial airway generations 4, 5, and 6. For all subjects, in airway generation 4, the difference between baseline airway patency and dilation was 12% [(1 – (1/1.14)], and in airway generation 6, it was 33%. This agreement between subjects was not the case for airway generation 5, where responsive subjects had a difference of 28% compared with nonresponsive subjects, where the difference was less than 13%.
The influence of airway size in depositing the inhaled material was confirmed by eight responsive subjects (57% of all subjects) who had slightly smaller airways () compared with the six nonresponsive subjects. Our results are consistent with those of Brown et al. (Citation2000) for nonresponsive individuals, including the identification of the responsive airway (generation 5). O’Riordan et al. (Citation1993) used a significantly smaller methacholine aerosol (MMAD = 1.85) and two-dimensional volume corrected C/P (central to peripheral airway) ratio to conclude that preferential deposition occurs in central airways of responsive and nonresponsive subjects (FEV1 decreased ≥ 20%) and does not significantly determine methacholine responsiveness. In our study, two subjects (nos. 1 and 2 in ) demonstrated a response (tissue sensitivity) that exceeded what could be explained by the airway anatomy. For the other responsive subjects, their response can be explained by the airway anatomy. Although our study was conducted in adults, if the airway anatomy has a significant impact on sensitivity to agents such as methacholine, then this should also apply to children. It is possible to imagine children who for their size have smaller airways, which might make them more sensitive. Because their airways are still growing, it is also possible that their airway growth could enable them to outgrow their sensitivity.
In the current study, tracheobronchial airway generation 5 was different (statistically significantly by the t-test but not by the Wilcoxon rank-sum test; stands out as a possible constriction point or respiratory isthmus). This observation is similar to that seen previously for Balb/c and B6C3F1 mice (Moss and Oldham Citation2006), where for airway generations 4, 5, and 6, the airway volume factor inversely reflected the response to methacholine. In the current study for tracheobronchial airway generations 1 through 6, the airway volume factor decreased with each successive airway generation (). The airway volume factor (Ld2 , cm3) was used to relate airway morphometry to the response following inhalation of methacholine.
There are several limitations to the current study, including a small number of subjects (n = 14), lack of rigid control of subjects’ breathing patterns, assumption that post-methacholine dose of albuterol caused fully patent airways, and the correlation between different positions used for the HRCT scans (supine) and methacholine challenge (erect). Lung volume decreases in the supine position compared with that in the erect position (Comroe et al. Citation1971; Ibanez and Raurich Citation1982; Tsai et al. Citation2008). Although differences in the diaphragm position and rib cage muscles have been noted between supine and erect positions (Druz and Sharp Citation1981; Tsai et al. Citation2008), it is not known which airways are specifically affected. However, it is least likely to affect the first six tracheobronchial generations due the cartilaginous rings and muscular support in these airways.
SUMMARY
This research addressed a basic question associated with any inhalation exposure: i.e., for identical inhalation exposures, what is the contribution of morphometry on between-subject variations in response?
From this study, we derived two main conclusions. First, all subjects started the study with airway patency 10% less dilated in generation 1 (the trachea) up to 33% at tracheobronchial airway generation 6 (). Only in tracheobronchial airway generation 5 did pre-exposure airway patency significantly differ () between responsive and nonresponsive subjects.
Second, those subjects with smaller upper tracheobronchial morphometry were more responsive to the methacholine challenge. For the methacholine challenge, there were six subjects who did not respond to the highest methacholine challenge, and eight subjects who did respond with a measured decrease in their forced expiratory volume in 1 s. Compared with the nonresponders, these responders had smaller airway volume factors (from about the same in airway generations 1 and 2 to 36% smaller in airway generation 5).
Acknowledgments
In regard to this specific research question and to the best of our knowledge, we assert no conflict of interests regarding the publication of this article.
This work was financially supported through an award to Owen R. Moss from the toxicology research program at RJR Tobacco (RJRT contract no. 06–770-029; Winston-Salem, NC). The authors would like to thank David T. Doolittle, PhD, DABT, ATS, and Cody Wilson, PhD, (both at RJR Tobacco Company, Winston-Salem, NC) for their vision in advocating funding for this basic research. The authors would also like to thank David Collins, MD, and staff, Cathryn Cole and Pat Snow (Piedmont Medical Group, Winston-Salem, NC), for their help in conducting the human protocol.
REFERENCES
- American Association for Respiratory Care. 2001 . AARC Clinical Practice Guideline—Methacholine Challenge Testing: 2001 Revision & Update . Respir. Care , 46 ( 5 ) : 523 – 530 .
- American Thoracic Society. 2000 . Guidelines for Methacholine and Exercise Challenge Testing—1999 . Am. J. Respir. Crit. Care Med. , 161 ( 1 ) : 309 – 329 .
- Brown , R. H. , Croisille , P. , Mudge , B. , Diemer , F. B. , Permutt , S. and Togias , A. 2000 . Airway Narrowing in Healthy Humans Inhaling Methacholine Without Deep Inspirations Demonstrated by HRCT . Am J. Respir. Crit. Care Med. , 161 : 1256 – 1263 .
- Clinkenbeard , R. E. , Johnson , D. L. , Parthasarathy , R. , Altan , C. , Tan , K. H. Crawford , R. H. 2002 . Replication of Human Tracheobronchial Hollow Airway Models Using a Selective Laser Sintering Rapid Prototyping Technique . Am. Ind. Hyg. J. , 63 : 141 – 150 .
- Comroe , J. H. , Forster , R. E. , Dubois , A. R. , Briscoe , W. A. and Carlesen , E. 1971 . The Lung: Clinical Physiology and Pulmonary Function Tests , 10 Chicago , IL : Year Book Medical Publishers .
- DeLorme , M. P. and Moss , O. R. 2002 . Pulmonary Function Assessment by Whole-Body Plethysmography in Restrained Versus Unrestrained Mice . J. Pharmacol. Toxicol. Methods , 47 ( 1 ) : 1 – 10 .
- Druz , W. S. and Sharp , J. T. 1981 . Activity of Respiratory Muscles in Upright and Recumbent Humans . J. Appl. Physiol. , 51 : 1552 – 1561 .
- Ibanez , J. and Raurich , J. M. 1982 . Normal Values of Functional Residual Capacity in the Sitting and Supine Positions . Intensive Care Med. , 8 : 173 – 177 .
- Moss , O. R. and Oldham , M. J. 2006 . Dosimetry Counts: Molecular Hypersensitivity May Not Drive Pulmonary Hyperresponsiveness . J. Aerosol Med. , 19 ( 4 ) : 555 – 564 .
- Moss , O. R. and Oldham , M. J. 2011 . Predicting Balb/c and B6C3F1 Mouse Sensitivity to Inhaled Methacholine: Impact of Calculating Lung-Airway Dimension and Airflow Distribution . Aerosol Sci. Technol. , 45 ( 7 ) : 821 – 826 .
- O’Riordan , T. G. , Walser , L. and Smaldone , G. C. 1993 . Changing Patterns of Aerosol Deposition During Methacholine Bronchoprovocation . Chest , 103 : 1385 – 1389 .
- Phalen , R. F. , Yeh , H. C. , Schum , G. M. and Raabe , O. G. 1978 . Application of an Idealized Model to Morphometry of the Mammalian Tracheobronchial Tree . Anat. Record , 190 : 167 – 176 .
- Tsai , L. L. , Mair , R. W. , Li , C.-H. , Rosen , M. S. , Patz , S. and Waisworth , R. L. 2008 . Posture-Dependent Human 3He Lung Imaging in an Open Access MRI System: Initial Results . Acad. Radiol. , 15 : 728 – 739 .
- Weibel , E. R. 1963 . Morphometry of the Human Lung , 136 – 140 . Berlin : Springer-Verlag .