Abstract
Purpose and patients. During the period from January 1990 to January 1996 a total of 953 patients with lymph node negative primary breast cancer were randomised to oral pamidronate (n=460) 150 mg twice daily for 4 years or no adjuvant pamidronate (n=493) in order to investigate whether oral pamidronate can prevent the occurrence of bone metastases and fractures. The patients received adjuvant chemotherapy, loco-regional radiation therapy, but no endocrine treatment. Results. During the follow-up period the number of patients with pure bone metastases was 35 in the control group and 31 in the pamidronate group. The number of patients with a combination of bone and other distant metastases were 22 in the control group and 20 in the pamidronate group. The hazard rate ratio for recurrence in bone in the pamidronate group compared to the control group was 1.03 (95% confidence interval 0.75–1.40) and p=0.86. No effect was observed on overall survival. In a small subgroup of 27 patients from the study, 12 of whom were treated with pamidronate a significant bone preserving effect was observed on bone mineral density in the lumbar spine, but not in the proximal femur. Conclusion. The results from the trial do not support a beneficial effect of oral pamidronate on the occurrence of bone metastases or fractures in patients with primary breast cancer receiving adjuvant chemotherapy.
The skeleton is the most frequent site of recurrence in women with breast cancer and 70–80% of the patients with metastatic disease develop bone metastases in the course of their remaining life time Citation[1]. The use of adjuvant therapy continues to improve survival in breast cancer patients Citation[2] and several clinical trials have shown that the bisphosphonates delay and reduce the occurrence of hypercalcaemia, pathologic fractures, and spinal cord compression in breast cancer patients with bone metastases Citation[3], Citation[4]. Oral clodronate was the first bisphosphonate to be used in an adjuvant setting in primary breast cancer, but the results from three randomised trials until now are conflicting Citation[5–7]. However, very preliminary data reported from a small study with intravenous pamidronate seem encouraging Citation[8]. Here we report on the results from a randomised, open study with oral pamidronate in patients with primary breast cancer.
Material and methods
The present trial constituted the second part of a trial designed to compare two chemotherapy regimens and pamidronate treatment with no bisphosphonate treatment in the adjuvant situation. The Danish Breast Cancer Cooperative Group (DBCG) prepared the protocol and the trial was conducted as an open-label, randomised phase 3 multicentre trial recruiting patients from oncology centres all over Denmark, from two centres in Sweden, and one centre in Iceland. The trial was approved by ethical committees in the participating regions and countries and it was conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983. All patients were informed verbally and in writing and they gave informed consent before randomisation. The participating centres collected and transferred data to The DBCG Registry. Before the final analysis the DBCG Data Centre conducted an update with the results from a data enquiry to all participating centres.
Patients
The trial included women with resectable adenocarcinoma of the breast and without signs of distant metastases according to an initial physical examination, x-ray examination of the chest, and axial x-ray examination of the skeleton or a whole-body bone scintigraphy confirmed by x-ray examination if suspect for bone metastases. Due to ongoing trials in patients with oestrogen and/or progesterone receptor positive tumours, patients for the trial were recruited from the following three groups: A) premenopausal women without lymph node metastases but with grade 2 or 3 malignancy and a primary tumour ≤5 cm in diameter independent of hormone receptor status, B) premenopausal women with negative or unknown hormone receptor status and with either axillary lymph node metastases or a primary tumour >5 cm in diameter, C) postmenopausal women with hormone receptor negative tumours and with either axillary lymph node metastases or a primary tumour >5 cm in diameter. Tumour cells were classified as receptor positive as defined by the laboratories in each region. A woman was considered premenopausal if she had menostasis for less than two months, menostasis for less than 12 months and follicular stimulating hormone levels in the premenopausal range, or was younger than 51 years in case of hysterectomy.
Treatment
The patients received adjuvant chemotherapy i.e. cyclophosphamide, methotrexate, and 5-fluouracil (CMF) or cyclophosphamide, epirubicin, and 5-fluouracil (CEF) as has been described elsewhere Citation[2]. Loco-regional radiotherapy was given according to guidelines at the participating centres. The use of endocrine therapy was to be avoided.
Patients were randomised to either oral pamidronate 150 mg twice daily for 4 years or no adjuvant pamidronate. Each patient was recommended to take the capsules together with one glass of tap water while standing or sitting and at least ½ hour before a meal.
Follow-up
Every 12 weeks for the first year the following information was recorded: symptoms, side effects, and results from a clinical examination. Subsequently the interval was increased to every 6 months in the period 2 to 5 years and thereafter annually for another 5 years.
Before treatment serum alkaline phosphatases, ionised calcium, and creatinine were measured. During follow-up these quantities were measured after 24 and 48 weeks the first year and thereafter half-yearly for 3 years.
Every 6 months the patients had an x-ray examination of the spine and pelvis and every year bone scintigraphies were done for the period of 4 years. In case the bone scintigraphy showed changes suspect of metastases an x-ray examination of the relevant skeletal region was done for confirmation.
All fractures were registered as prospectively recorded in the patients’ hospital files. Systematic reviews of spinal x-rays for fractures were not done as the x-ray procedure was not standardized.
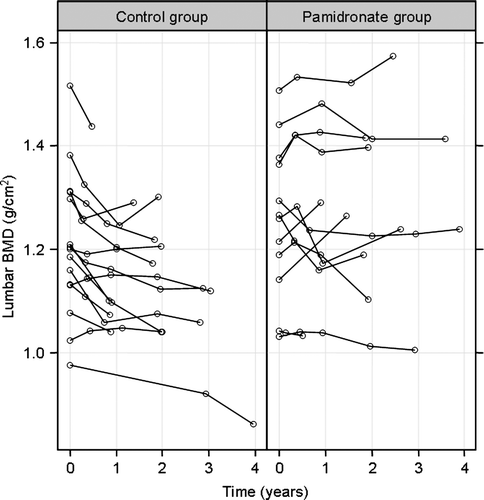
In a small Swedish subgroup of patients bone mineral density of the lumbar spine and the proximal femur was measured each year for a maximum period of 4 years. The same Lunar DPX-L X-ray Bone Densitometer, Version 1.35 was used for all scans in all patients. An estimated coefficient of variation was in the region of 0.7%.
Statistical analyses
Treatment groups were compared using the log-rank test, with stratification for centre and group. Multivariable analyses were done with Cox's proportional hazard regression model. Factors included in the multivariable analyses were group (A, B, C), age (<45, 45–49, 50–59, 60–69), tumour size (<20 mm, 21–50 mm, >50 mm), nodal status (0, 1–3, 4–10, >10 positive combined with 1–3, >3 examined), type of surgery, histologic type and grade (ductal grade I, ductal grade II, ductal grade III, ductal, but grade unknown, other histologic types), hormone receptor status (oestrogene or progesterone positive, at least one negative, both unknown), centre and treatment regimen. Interactions between treatment and the covariates group, age, positive lymph nodes, tumour size and hormone receptor status were investigated in categories and in separate models. Model assumptions were evaluated with log(-log) plots of the survival function, Schoenfeld residuals, and the inclusion of a time dependent covariate in the model. The hazard rate of histological type and grade as well as hormone receptor status was not proportional and stratification was used. Associations were tested with the χ2 test. Reported p-values are two-sided. All the statistical survival analyses were done using the SAS package, version 8.2. The longitudinal structure of the alkaline phosphatases and bone mineral density data was analysed using R, version 2.51 (R Foundation for Statistical Computing, Vienna, Austria) and the add-in packages lattice and nlme.
Results
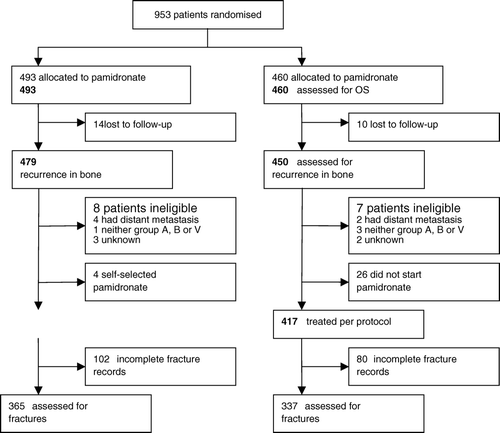
During the period from January 1990 to January 1996 a total of 953 patients were recruited into the trial. The course of the patients in the trial appears from , representing the Consolidation of Standards for Reporting Trials statement. Both analysis according to the intention-to-treat principle and a per-protocol analysis were done. There were no significant differences in relation to patient characteristics between the treatment groups (). Overall receptor status was balanced, however, there was a small imbalance with regard to oestrogen receptor status (p=0.04).
Table I. Base line characteristics.
Skeletal events
In the control group 88 of 479 patients had bone metastases compared to 93 of 450 patients in the pamidronate group (). In the multivariable model and analysed as per protocol the hazard rate ratio for recurrence in bone in the pamidronate group compared to the control group was 1.03 (95% confidence interval 0.75–1.40) and p = 0.86. The number of patients with fractures according to treatment and analysed as per protocol appears from and . shows slightly more fractures among patients treated with pamidronate, but the difference is not significant. shows the location of fractures in the skeleton with most of the fractures occurring in the spine. Four patients had more than one fracture, all in the pamidronate group.
Figure 2. Ten-year bone metastasis free survival in the pamidronate (light grey line) and control (black line) groups. Numbers below the X-axis show patients at risk.
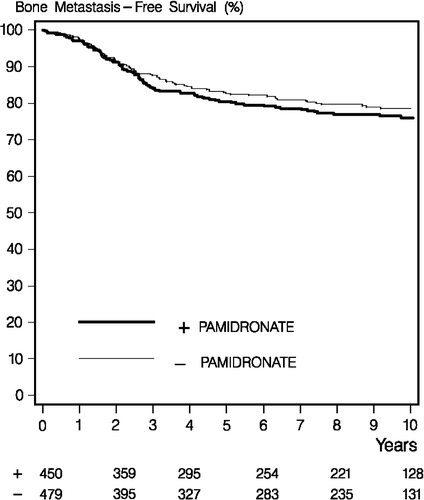
Table II. Number of patients with fractures according to questionnaire.
Table III. Fracture locations in the skeleton.
Pamidronate treatment
Information about duration of pamidronate treatment was available for the 417 per-protocol patients in the pamidronate arm. Median treatment duration was 1 082 days (range 2–2 836 days).
Side effects
Possible side effects related to pamidronate treatment are shown in . However, neither nausea, vomiting, stomatitis nor abdominal pain occurred more frequently among pamidronate treated patients than in control patients.
Table IV. Side effects possibly related to pamidronate treatment.
Bone mineral density, alkaline phosphatases, ionised calcium, and creatinine
Bone mineral density was measured in 15 control patients and in 12 pamidronate treated patients. The linear mixed models analyses showed a significant decrease in lumbar bone mineral density in the control group, p = 0.0001 () and a tendency towards a decrease in the femur, however non-significant at all measurement locations. Additionally there was a significant interaction between time and pamidronate treatment in the lumbar spine (p = 0.0092), but not in the femur. No significant differences between the treatment groups were found when analysing the biochemical parameters supposed to reflect bisphosphonate effects on bone metabolism and kidney function.
Discussion
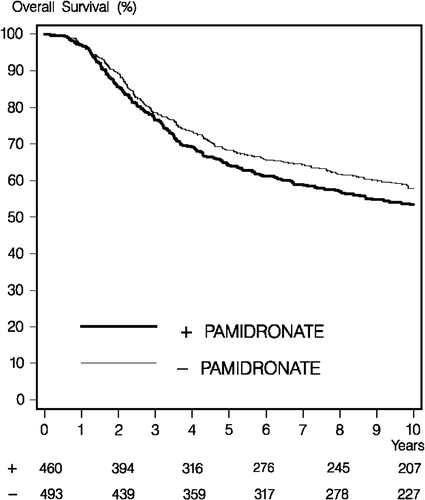
In this medium sized randomised trial, we found no support for a beneficial effect of oral pamidronate in patients with early breast cancer. Survival without bone metastases and overall survival was similar in the pamidronate and control groups. In addition, survival without fractures was similar in controls as compared to patients randomised to pamidronate.
Only 181 among the 953 patients randomised developed bone metastases despite more than 10 years of potential follow-up, and the low number of events reduced the statistical power of the current analysis. Patients randomised in our trial were not selected according to a high risk of bone metastasis Citation[10]. This may have contributed to a lower occurrence of bone related events albeit in both treatment groups.
We achieved a complete follow-up for overall survival and less than 3% were lost to follow-up regarding bone metastases. During the conduct of the trial, further development of oral pamidronate was stopped which affected the commitment of both patients and their physicians. To evaluate the possible influence of the non-optimal compliance, we performed a supplementary multivariable analysis of patients treated per protocol with adjustment for known risk factors. The results of the intention to treat and the adjusted per protocol analysis were identical, and it is unlikely that a closer adherence to the pamidronate regimen would have changed the outcome of this trial. The intestinal absorption of the bisphosphonates is poor and perhaps even more so with the aminobisphosphonates such as pamidronate Citation[11]. Poor adherence to the drug regimen resulting in a shorter duration of treatment adds to this problem. However, when estimated from the occurrence of gastrointestinal side effects pamidronate was well tolerated. Despite this side effects may have influenced the patient compliance to the drug in the long run. However, a highly significant effect of oral pamidronate in the BMD sub-study suggests an acceptable bioavailability of oral pamidronate. Furthermore the bisphosphonates have a wide variance in potency and there may be a link between the potency of bisphosphonates and the fracture risk reduction. The low effect of bisphosphonates on the risk of skeletal events in cancer suggests a difference in the calcium metabolic and skeletal signalling pathways affected by bone metastases compared to the pathways affected by the bisphosphonates. As a result development of new bone active drugs is focused on other signalling pathways in bone metabolism illustrated by the use of a human monoclonal antibody denosumab that binds a factor (RANKL) essential for osteoclast differentiation, activation, and survival Citation[12].
However, the very preliminary results from the small Japanese study with intravenous administration of pamidronate to patients with primary breast cancer suggest that intravenous administration is the best way to give bisphosphonates as it ensures a correct delivery and avoids the stringent administration instructions required for oral bisphosphonates Citation[8].
A protective effect was not established of oral pamidronate concerning bone fractures. A long-term follow-up was however only completed in approximately 75% of the randomized patients, and this might have confounded the analysis. Risk factors for fractures were not recorded systematically and we are therefore unable to perform an adjusted analysis for fractures.
Only three randomised clinical trials with adjuvant bisphosphonate in early breast cancer have been published. In these trials a first generation bisphosphonate viz. clodronate was used and the drug was given in an oral formulation. The results however are conflicting.
In the first trial 302 patients with primary breast cancer and histochemically detected breast cancer cells in the bone marrow were randomised to clodronate or no bisphosphonate with a treatment period of 2 years Citation[5]. After almost 5 years of follow-up the recurrence rate of bone metastases was significantly reduced, there was a trend towards reduced occurrence of visceral metastases, and a significant increase in overall survival Citation[9]. In the second study 299 women with primary breast cancer and axillary lymph node metastases were randomised to clodronate or control for 3 years. After 5 years bone metastases were equally frequent in both groups, non-skeletal metastases were significantly more frequent in the clodronate group, and overall survival was significantly lower in the clodronate group Citation[6]. The third trial included 1 069 patients with primary breast cancer. After 2 years of treatment with clodronate there was a significant increase in overall survival compared to the control group. After 2 years of treatment the occurrence of bone metastases in the clodronate group was significantly lower, but the difference had disappeared after 5 years of follow-up. The effect on overall survival remained after 5 years Citation[7].
However, even if the bisphosphonates fail in the oncological connection a long-term beneficial effect on bone metabolism as regards osteoporosis may be present and could be utmost relevant due to the increasing use of aromatase inhibitors in the adjuvant setting Citation[13]. The subgroup results of the bone mineral density measurements are in accordance with observations from large bisphosphonate osteoporosis studies Citation[14] and they support a bone mineral preserving effect of pamidronate in this small sample of patients from the trial. Interestingly a recent randomised trial has shown that once-yearly infusion of the bisphosphonate zoledronic acid reduces the occurrence of vertebral, hip, and nonvertebral fractures in postmenopausal women Citation[15].
In conclusion, data from our trial do not support a beneficial effect of oral pamidronate on the occurrence of bone metastases and fractures in patients with primary breast cancer receiving adjuvant chemotherapy. Due to the intriguing but contradictory results of the trials with adjuvant oral bisphosphonate the ability of these drugs to reduce the incidence of bone metastases in patients with primary breast cancer remains unsolved and the results of ongoing trials are awaited. If the bisphosphonates prove to have antimetastatic effects it remains to be established which drugs are optimal, which dose and schedule should be used, and how long the duration of treatment should be. Optimally these questions should be studied in breast cancer patients with axillary lymph node metastases and in patients with local or regional recurrence or recurrence in soft tissue Citation[10].
Acknowledgements
Supported in part by grants-in-aid from Pharmacia (now Pfizer) and Ciba-Giegy (now Novartis).
References
- Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer 1987; 55: 61–6
- Ejlertsen B, Mouridsen HT, Jensen M-B, Andersen J, Jensen BB, Cold S, et al. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007; 43: 877–84
- Pavlakis N, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev 1: CD003474; 2002.
- Ross JR, Saunders Y, Edmonds PM, Patel S, Broadley KE, Johnston SR. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 2003; 30: 469
- Diel IJ, Solomayer E-F, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med 1998; 339: 357–63
- Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-negative breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol 2001; 19: 10–7
- Powles T, Paterson S, Kanis JA, McCloskey E, Ashley S, Tidy A, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol 2002; 20: 3219–24
- Kokufu I, Kohno N, Takao M, Yamamoto M, Miyashita M, Kohno S, et al Adjuvant pamidronate therapy for the prevention of bone metastasis in breast cancer patients with four or more positive nodes. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004;22(14S):530.
- Jaschke A, Bastert G, Solomayer EF, Costa S, Schuetz F, Diel IJ. Adjuvant clodronate treatment improves the overall survival of primary breast cancer patients with micrometastases to bone marrow: A longtime follow-up. Proc Am Soc Clin Oncol 2004; 23, abstract 529
- Colleoni M, O'Neill A, Goldhirsch A, Gelber RD, Bonetti M, Thürliman B, et al. Identifying breast cancer patients at high risk for bone metastases. J Clin Oncol 2000; 18: 3925–35
- Fleich H. Bisphosphonates in bone disease. From the laboratory to the patient4th edn. Academic Press, San Diego 2000
- McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354: 821–31
- Brufsky A, Harker WG, Beck JT, Carroll R, Tan-Chiu E, Seidler C, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol 2007; 25: 829–36
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: Result from the Fracture Intervention Trial. JAMA 1998; 280: 2077–82
- Black DM, Delmas P, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–22