Abstract
Introduction. This study was conducted to establish the maximum tolerated dose (MTD) of intravenous vinorelbine and on the determined dose to assess efficacy and safety in patients with metastatic breast cancer previously treated with epirubicin. Patients and methods. Patients had histologically proven breast cancer and had received a prior epirubicin based regimen either adjuvant or as first line therapy for advanced disease. Vinorelbine was administered intravenously day 1 and 8 in a 3 weeks’ schedule. Subsequently 48 additional patients were treated at one dose-level below MTD. Results. Fifty-five patients were included in the dose-escalation study, which defined 40 mg/m2 as the MTD. Neutropenia of short duration and autonomic neuropathy causing constipation were the most common dose-limiting toxicities. At the 35 mg/m2 dose-level 60 patients were included in total. Seven (12%; 95% CI 6 to 22) had a partial response and 16 additional patients had stable disease. 27% had stable disease (clinical benefit rate 38%, 95% CI 27 to 51). The median overall survival was 45 weeks and 39% of the patients were alive after one year. Discussion. The clinical benefit rate was 38% with an overall intention to treat response rate of only 12%. The results were, however, achieved with low subjective burdens of toxicity.
Metastatic breast carcinoma is basically incurable but the cause of the disease may be modified by current treatments. In first line treatment with taxane and anthracycline-containing regimens relatively high response rates can be achieved with overall response rates (ORR) of 55–82% Citation[1], Citation[2]. The efficacy of second and subsequent lines of palliative chemotherapy is relatively poor with an objective response rate in the range of 20% and median survival times of less than 12 months Citation[3].
Therefore, improvement or maintenance of quality of life is the main goal of second-line chemotherapy. Palliation therefore needs to be balanced against survival and toxicity.
Vinorelbine has established activity as a single-agent in the initial treatment of advanced breast cancer Citation[4] and has been used in second-line therapy Citation[5] with responses obtained in up to 30% of patients depending on their previous treatment history. The good tolerance profile of single-agent vinorelbine and its capacity to maintain an acceptable quality of life Citation[6] makes it an excellent candidate for evaluation in this palliative setting where disease control with minimal toxicity is the highest clinical priority for patients.
The aim of the present study was to evaluate the anti-tumor activity and tolerance of vinorelbine in patients with advanced breast cancer who had failed antracycline treatment.
Patients and methods
The Danish National Committee on Biomedical Research Ethics, the Danish Data Protection Agency and the Danish Medicines Agency approved the study before it was activated. The study was conducted in compliance with Good Clinical Practice guidelines, and written informed consent was required from all patients before participation.
Study design
Open, non-comparative, multicenter phase I-II study. In the first part patients were initially treated with 25 mg/m2 of vinorelbine and the dose was subsequently increased stepwise by 5 mg/m2 in every eight evaluable patients until MTD was reached. In the second part additional patients were included one dose-level below MTD.
Patients selection
Eligible patients were women aged 18–74 years with histologically verified and locally advanced or metastatic breast cancer either progressing during treatment or relapsing after an antracycline-containing regime administered either as first-line chemotherapy for metastatic disease or in the adjuvant setting. Patients were required to have WHO performance status ≤2 and at least one measurable or evaluable lesion, and adequate haematological parameters (WCC >3×109/, platelets >100×109/l) and biochemical measures of hepatic and renal function (ASAT/ALAT ≤120 iu/l, bilirubin ≤35 µmol/l, creatinine ≤140 µmol/l).
Exclusion criteria were prior or concomitant malignant disease except appropriately treated basal cell carcinoma of the skin or carcinoma in situ of the cervix, clinical symptoms suggesting peripheral neuropathy or brain metastases, prior irradiation of 25% or more of the red bone marrow, and more than one prior cytotoxic treatment for locally recurrent or metastatic disease.
Treatment protocol
Vinorelbine was administered on an outpatient basis via a 5–10 minute bolus into a free running N saline infusion on days 1 and 8 in a three weekly schedule. The MTD was defined as the dose at which four or more of the eight patients developed grade 3 or higher haematological toxicity or grade 4 leucopenia with fever or grade 4 thrombocytopenia on the first cycle. Dose modification was based on a haematological assessment on day 1 of the treatment cycle. Leucopenia <3.0×109/l or thrombocytopenia <75×109/l resulted in postponement of treatment by one week and the next cycle was given with a 20% dose-reduction for those patients experiencing grade 4 toxicity but no dose-reduction for lower grades. Grade 3 or higher neurotoxicity or other non-haematological toxicity, e.g. constipation, resulted in delay of treatment to recovery and then 20% dose reduction for subsequent cycles. Increase in bilirubine to higher than twice the upper limit of normal (2N) or ASAT/ALAT to more than 3N resulted in reduction of the dose by 50%.
Safety and efficacy
Patients were assessed for toxicity by WHO criteria before each cycle of therapy. Treatment was planned to be administered for at least three cycles after which efficacy was evaluated by WHO criteria unless patients had been withdrawn earlier because of progression (PD) or toxicity. Patients with stable disease (SD), partial response (PR) complete responses (CR) were to continue treatment for up to one year. Clinical monitoring consisted of physical examination, performance status, haematological and biochemical screen and documentation of treatment related toxicities or adverse events at the beginning of each cycle and repeat of imaging studies (e.g. chest x-ray, isotope bone scan, CT scan, skeletal survey, ultrasound investigation) every 9 weeks if any of these were the basis for documentation of active disease at study entry.
Response rate (PR and CR) was not assessed during the phase I-II part of the study but was the main criterion for response for the whole study population in the phase II part (intention to treat analysis). Survival, time to progression, and duration of response were calculated by the Kaplan-Meier method. Sample size for the phase II part of the study was calculated by the Fleming procedure requiring a response rate of >15% with a type I (α) error of 10% and a type II (β) error of 20%. This procedure utilised an interim analysis of 20 patients with continued accrual if >3 and <7 patients had obtained responses.
Results
Patients characteristics
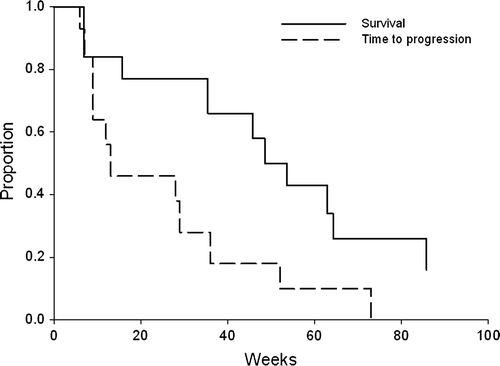
Patients were recruited in a total of ten centres in Denmark. The dose-escalation study involved 55 patients included between 2 November 1995 and 20 September 1996 and the phase II-trial recruited 48 patients between 27 September 1996 and 8 September 1997. Forty-seven of the 55 patients (85%) in the phase I part of the study were eligible and 45 of 48 patients (95%) in the phase II part of the study (). The characteristics of the patient population in the two parts of the study are shown in . Their prior treatments for breast cancer are shown in , and the evaluation of disease at inclusion time is listed in .
Table I. Distribution of patients
Table II. Patient characteristics
Table III. Prior treatment for breast cancer
Table IV. Evaluation of disease at inclusion time
Toxicity
The proportion of evaluable patients defined as having experienced dose-limiting toxicity (DLT) was 3/8 at 25 mg/m2, 4/15 at 30 mg/m2, 4/11 at 35 mg/m2, and 9/17 at 40 mg/m2.
At the last dose level the toxicities recorded were grade 3 constipation in three patients, grade 3 neuropathy in two patients, grade 3 alopecia in two patients and grade 3 infection and stomatitis in one patient respectively. 40 mg/m2 was therefore defined as the MTD and the dose for the phase II study was determined to be 35 mg/m2.
A total of 60 patients were treated at the 35 mg/m2 dose level. Of these 55 were eligible, 59 were assessable for toxicity (one patient died before receiving the first dose), and 54 were assessable for efficacy. The principal grade 3 and 4 toxicities experienced by patients treated at 35 mg/m2 were leucopenia (46% of patients, 15% of cycles), which was of short duration and not associated with significant infection, nausea at grade 3 (11% of patients, 4% of cycles), constipation at grade 3 (14% of patients, 4% of cycles), and alopecia at grade 3 (19% of patients). No other grade 3 and 4 toxicity was recorded as affecting more than 2% of patients.
Response to treatment
In the intention to treat population seven (12%, 95% CI 6 to 22) achieved a partial response and 16 (27%, 95% CI 17 to 39) had stable disease. The median time to progression was 13 weeks, median progression-free survival was 13 weeks, and median overall survival was 45 weeks with 39% of patients alive at one year. Of the 52 eligible patients who received 35 mg/m2 44 were evaluated for response after having received 3 cycles. Overall survival and time to progression are shown in .
Discussion
MTD for single agent vinorelbine given intravenously day 1 and 8 every 3 weeks was in the current dose finding study established at 40 mg/m2. The preceding dose level of 35 mg/m2 was subsequently used in the phase II part of the study. The initial phase I study explored weekly administration of vinorelbine Citation[7] and proposed 30 mg/m2 for subsequent phase II trials. Administration of the day 15 dose of vinorelbine was however often omitted in following phase II trials due to transient neutropenia and as a consequence we explored a three-weekly schedule administering the drug on days 1 and 8. Others have used 30 or even 25 mg/m2 days 1 and 8 every week without direct randomised comparisons of safety and efficacy of the different regimens Citation[8]. In the current study 35 mg/m2 of vinorelbine days 1 and 8 every 3 weeks was feasible with high treatment intensity and a low-level of side-effects.
None of the patients in the current study achieved a complete response and the overall response rate was only 12%. Our result is comparable to a 16% response rate in the largest phase II of pre-treated patients Citation[9] but clearly below the response rate of 35 to 59% in previously untreated patients Citation[8]. The majority of these trials used a lower dose-intensity as compared to the current trial and patient selection is the most plausible explanation. Publication bias is another possible explanation that cannot completely be ruled out.
Acknowledgements
This study was supported by Institut de Recherche Pierre Fabre, Boulogne France.
References
- Hayes DF, Henderson IC. CAF in metastatic breast cancer: Standard therapy or another effective regimen?. J Clin Oncol 1987; 5: 1497–9
- Chan S, Friedrichs K, Noel D, Pinter T, Van Belle S, Vorobiof D, et al. Prospective randomized trial of docetacel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 1999; 17: 2341–54
- Cardoso F, Leo AD, Lohrisch C, Bernard C, Ferreira F, Piccart MJ. Second and subsequent lines of chemotherapy for metastatic breast cancer: What did we learn in the last two decades?. Ann Oncol 2002; 13: 197–207
- Fumoleau P, Delgado FM, Delozier T, Monnier A, Gil Delgado MA, Kerbrat P, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol 1993; 11: 1245–52
- Gasparini G, Caffo O, Barni S, Frontini L, Testolin A, Guglielmi RB, et al. Vinorelbine is an active antiproliferative agent in pre-treated advanced breast cancer patients: A phase II study. J Clin Oncol 1994; 12: 2094–101
- Bertsch LA, Donaldson G. Quality of life analyses from vinorelbine (Navelbine): Clinical trials of women with metastatic breast cancer. Semin Oncol 1995; 22(Suppl 5)45–54
- Mathe G, Reizenstein P. Phase I pharmacological study of a new vinca alkaloid: Navelbine. Cancer Lett 1985; 27: 285–93
- Mano M. Vinorelbine in management of breast cancer: New perspectives, reviewed role in the era of targeted therapy. Cancer Treat Review 2006; 32: 106–118
- Degardin M, Bonneterre J, Hecquet B, Pion JM, Adenis A, Horner D, et al. Vinorelbine (navelbine) as a salvage treatment for advanced breast cancer. Ann Oncol 1994; 5: 423–6