Abstract
The improved survival and cure rate of breast cancer patients leads to increased diagnosis of later occurring side effects to therapy such as osteoporosis. Conventional chemotherapies such as CMF and CEF are known to induce premature menopause, which increases bone loss but these therapies have additional detrimental effects on bone. The loss in bone mass during chemotherapy is substantial and may lead to increased fracture risk. The influence of taxanes on bone is less well known. Whereas tamoxifen has a slight protective effect on bone loss the opposite is true for aromatase inhibitors. Adverse effect reportings show, that adjuvant treatment with aromatase inhibitors in postmenopausal women increases the risk of clinical fractures as compared to tamoxifen.
The Danish Bone Society suggests that all women with operable breast cancer have their fracture risk evaluated including a BMD measurement prior to initiation of adjuvant aromatase inhibitor therapy as a part of the standard examination program. If osteoporosis is diagnosed, anti-osteoporosis therapies should be considered. Moreover, all women undergoing adjuvant chemotherapy and endocrine therapy should be informed of the risk of bone loss and should receive life style advice of how to preserve bone. Adjuvant regimens in breast cancer patients improve survival and cure rates. Therefore it is preferable to use such therapies although they increase risk of side effects such as osteoporosis.
Whereas acute toxicity of anticancer therapies is routinely monitored and treated, later occurring side effects are less frequently observed and studied. It is evident that the improved survival and cure rate of operable breast cancer patients will lead to an increased need for diagnosing and treating side effects that occur late relative to intervention. Additionally, patients will need more information.
Bone is adversely influenced due to cytotoxicity and due to reduction of endogenous estrogen production both through premature induction of menopause and through inhibition of aromatase activity in postmenopausal women. As women with a breast cancer diagnosis survive longer, osteoporosis will be more frequently diagnosed. The population of breast cancer survivors with a need for diagnosis, treatment and monitoring of osteoporosis is new and needs attention.
Chemotherapy – cytotoxic drugs, bone metabolism, bone mass and fracture risk
Chemotherapy for breast cancer mainly consists of three principles: 1) Combination chemotherapy: CMF (cyclophosphamide, methotrexate, fluorouracil), CEF (cyclophosphamide, epirubicin, and fluorouracil) or other variations of these, 2) Taxanes (paclitaxel, docetaxel), and 3) Trastuzumab. The main detrimental effect on the skeleton by chemotherapy is mediated through loss of estrogen production induced by amenorrhoea in premenopausal women Citation[1]. The effects of chemotherapy in postmenopausal women are thus different from premenopausal women. Other detrimental mechanisms of chemotherapy are toxic effects of the drugs on the bone cells, lack of vitamin D, calcium and other nutrients following nausea and vomiting. Most studies on changes in bone turnover and density have been performed with the CMF regimen, which is no longer in use. Little data exists for the newer regimens, which is a matter of concern. To show the potential detrimental effects of chemotherapy on bone the effects of the CMF regimen have been presented to show the possible mechanisms of harm to the skeleton. Where available data have been presented for other regimens
Combination chemotherapy
Most available studies have addressed the CMF regimen, and have demonstrated that induction of amenorrhoea was the key factor in subsequent bone loss in premenopausal women. One study on adjuvant chemotherapy for premenopausal breast cancer and axillary lymph node metastases showed that 71% of 44 women were postmenopausal at the time of the study compared to 16% of the 44 matched control women (risk difference 55%), and that most of the patients developed amenorrhoea following initiation of chemotherapy Citation[2]. The patients in this study had received six cycles of CMF (dose not stated) Citation[2]. The mean lumbar spine BMD 3.5 years after treatment was 1.17 g/cm2 in patients compared to 1.29 g/cm2 in matched controls Citation[2] equalling a loss of around 1 Z-score or an expected relative risk of any fracture around 1.5 Citation[3]. In this study there was no difference in lumbar spine BMD in patients and controls (1.34 vs. 1.35 g/cm2, n = 11 in both groups) that were premenopausal at the time of the study, whereas patients who were postmenopausal at the time of the study had significantly lower lumbar spine BMD than controls who were premenopausal (1.17 vs. 1.29 g/cm2, n = 26 in both groups) Citation[2]. In the small group where both patients and controls were postmenopausal (n = 7 in both groups) the controls had a higher BMD than cases (1.30 vs. 1.2 g/cm2), but significance was not stated Citation[2].
All other studies have not used control groups but have reported longitudinal changes after initiation of CMF chemotherapy. A randomised controlled study in 96 patients with node positive early breast cancer compared two years with six cycles of CMF (n = 43, cyclophosphamide 500 mg/m2 i.v. on day 1 and 8 or 100 mg/m2 orally on day 1–14, methotrexate 40 mg/m2 i.v. on day 1 and 8, and fluorouracil 600 mg/m2 i.v. on day 1 and 8 with an interval of 28 days between each cycle) with goserelin (a GnRH analogue, n = 53, 3.6 mg s.c. with 28 days interval for two years, i.e. 26 injections) Citation[4]. After two years this study showed a considerable decrease in bone mineral in both the spine and hip with goserelin (−10.5% and −6.4%) and CMF (−6.5% and −4.5%) Citation[4]. However, the bone loss with CMF seemed to be irreversible in contrast to goserelin where a partial recovery was observed after three years Citation[4]. At two years 100% of women on goserelin were amenorrhoeic compared to 69% of women on CMF, but after three years only 27% of women on goserelin were amenorrhoeic compared to 77% of women treated with CMF. After discontinuation the BMD levels with goserelin only increased to the same level as with CMF so that the loss of BMD at three years was the same with goserelin and CMF Citation[4] probably due to the recovery of menses among the goserelin treated women. The loss of BMD was closely related to amenorrhoea Citation[4]: women in the CMF group who had amenorrhoea had a loss of 6.4% in lumbar spine BMD and 5.2% in hip BMD after one year compared to losses of 1.6 and 3.1% in the spine and hip after one year in patients who had menstruations Citation[4]. It should be noted that although women maintained their menstruations a considerable loss was still seen in BMD.
Vehmanen et al. also reported a large loss in BMD after CMF (cyclophosphamide 600 mg/m2 i.v. at day 1, methotrexate 40 mg/m2 i.v. at day 1, fluorouracil 600 mg/m2 i.v. on day 1 given in six cycles at an interval of three weeks) in amenorrhoeic patients (−10.4% in the lumbar spine and −5.8% in the femoral neck after 5 years) and a much smaller change in patients who maintained menstruations (−1.3% in the lumbar spine and −0.3% in the hip after 5 years) Citation[5]. These findings are in contrast to the findings of Fogelman et al. Citation[4] of a large loss despite maintenance of menstruations. However, some of the patients in the study by Vehmanen et al. had received treatment with the bisphosphonate clodronate Citation[5].
Saarto et al. conducted a comparable study on 148 premenopausal women with breast cancer without skeletal metastases treated with CMF Citation[6]. The patients were treated with six cycles of CMF (600 mg/m2 of cyclophosphamide, 400 mg/m2 of methotrexate, and 600 mg/m2 of fluorouracil i.v. on day one with an interval of three weeks) Citation[6]. This resulted in a loss of −9.5% in spine BMD and −3.9% in the femoral neck in patients with amenorrhoea vs. a loss of −0.3% in the spine and unchanged femoral neck BMD in patients with regular menstruations Citation[6]. Again these findings are in contrast to Fogelman et al. Citation[4].
For the CEF regimen one study exists using doxorubicin instead of epirubicin Citation[7]. Similar effects of menstrual and thus estrogen status were seen in this study with 6–10 cycles of cyclophosphamide (500 mg/m2), doxorubicin (50 mg/m2), and fluorouracil (500 mg/m2) Citation[7]. The study showed that women who became permanently amenorrheic had 14% lower BMD than women who maintained their menstrual cycles Citation[7]. In one study higher baseline BMD before CMF and larger decrease in serum estradiol were predictors of ovarian failure in breast cancer Citation[8].
Given that the average loss of BMD after natural menopause is around 1–2% per year Citation[9], the losses reported in these studies are large and gives rise to concern for fracture risk in long-term survivors although no fracture studies exist. The effects on BMD of adjuvant chemotherapy in women already postmenopausal are not known in detail and needs further evaluation. Finally, the influence on bone during regimens including taxans and trastuzumab is at present unknown. However, since cyclophosphamide and epirubicin are still components of adjuvant chemotherapy, it can be concluded that also adjuvant chemotherapy used at present represents a risk to bone.
Endocrine therapy: Bone metabolism, bone mass and fracture risk
Two principles of adjuvant endocrine therapy exist and both influences bone. 1) Estrogen receptor modulators such as tamoxifen and 2) aromatase inhibitors such as letrozole, anatrozole and exemestane.
Tamoxifen
Tamoxifen binds to the estrogen receptor and has estrogen agonist effects in some tissues (for example endometrium) or antagonist effects in other tissues (for example breast). In postmenopausal women tamoxifen has been shown to increase bone mass Citation[10].
Aromatase inhibitors
Whereas tamoxifen act by competitive antagonism (breast) or agonism (bone) of estrogen at its receptor site, aromatase inhibitors suppress plasma estrogen levels by inhibiting or inactivating aromatase, which is an enzyme that is responsible for the synthesis of estrogen from androstendion and testosterone. Estrogen production in postmenopausal women originates primarily from metabolism of androgens, and aromatase inhibition reduces circulating estrogens with >97%. Thus, the influence of aromatase inhibitors contrasts to the effects of tamoxifen on bone.
In the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial 5216 postmenopausal women with invasive breast cancer had completed surgery and chemotherapy. They were then randomized to anastrozole 1 mg or tamoxifen 20 mg daily Citation[11]. Their hormone receptor status was primarily positive and their age was on average 64 years at baseline. After 5 years therapy 11% of the women on anastrozole had sustained a clinical fracture versus 7.7% of the women on tamoxifen (p < 0.0001). With regard to the incidence of clinical vertebral fractures, the numbers were 1.5% on anastrozole versus 0.9% on tamoxifen (p = 0.03), whereas incident hip fractures were not significantly different between the two groups. In a subgroup of 240 participants BMD of the hip and spine was measured at baseline and after two years. Both groups experienced a bone loss but the loss was greater in women on anastrozole.
The combined ARNO 95/ABCSG 8 study (Arimidex-Nolvadex/Austrian Breast and Colorectal Cancer Study Group) study evaluated 3224 postmenopausal women with hormone receptor positive breast cancer who underwent surgery and radiotherapy Citation[12]. Initially the women, who were on average 62 years, received two years treatment with tamoxifen (20 or 30 mg). Thereafter they were randomized to therapy with anastrozole 1 mg or continuation with tamoxifen. After 28 months, there were 2% women with clinical fractures on anastrozole as compared to 1% with fractures on tamoxifen (p = 0.015).
In IES (Intergroup Exemestane Study) 4742 postmenopausal women (on average 64 years) had completed surgery and radiotherapy. Eighty-one percent of the women were hormone receptor positive and they received treatment with tamoxifen 20 mg for 2–3 years. Then they were randomized to continue tamoxifen or to be switched over to exemestane 25 mg Citation[13]. After 56 months 7% had experienced a clinical fracture in the exemestane group versus 4.9% in the tamoxifen group. This difference was statistically significant (p < 0.01). A 12 month BMD substudy showed that while bone was gained during tamoxifen therapy, it was lost during exemestane treatment and this difference was significant both in the lumbar spine and in the hip.
The BIG (Breast International Group) 1-98 trial randomized 4922 postmenopausal women with hormone receptor positive operable breast cancer to 5 years therapy with tamoxifen 20 mg or letrozole 2.5 mg daily. The clinical fracture incidence was 8.6% on letrozole which was significantly greater than the 5.8% incidence on tamoxifen (p < 0.001) Citation[14].
The MA.17 study recruited 5187 postmenopausal women who had been diagnosed with hormone receptor positive operable breast cancer and who had completed 4.5–6 years of tamoxifen adjuvant treatment Citation[15]. The women who were on average 62 years were randomized to letrozole 2.5 mg daily or placebo. After 5 years there were 5.3% with incident clinical fractures on letrozole as compared with 4.6% on placebo (p = 0.25). However, during the same time period osteoporosis was diagnosed in 8.1% of the women on letrozole as compared to 6.0% on placebo (p = 0.003). Moreover, in a 2-year substudy in 222 of the women, bone loss was significantly greater on letrozole than on placebo.
Although the studies are not completely comparable, the data show concurrently that all three aromatase inhibitors used in the adjuvant setting lead to an increased risk of fracture when compared with tamoxifen. The data also indicate that the aromatase inhibitors studied seemed quite similar when it comes to the effects on bone. That the difference to tamoxifen appears greater than the difference to placebo may be explained by the slight positive effect of tamoxifen on bone.
Due to improved efficacy in the breast endocrine adjuvant therapy is at present being shifted from tamoxifen to aromatase inhibitors. Moreover, survival and cure rate of breast cancer patients is improving. This will lead to an increased occurrence of fractures that physicians and women need to be aware of.
Strategies for therapeutic decisions regarding bone health in breast cancer patients
Evaluation and diagnosis
Women with a breast cancer diagnosis are at increased risk for developing osteoporosis and fracture Citation[16], Citation[17] even in the absence of metastatic bone disease. The goal is to detect bone loss early and intervene appropriately to reduce risk of fracture. It is necessary to develop strategies to determine the rate of bone loss, estimate the risk of fracture, and monitor treatment effects, and guidelines are available for the management of osteoporosis in otherwise healthy subjects Citation[18], Citation[19] and similar recommendations should be applied to women with breast cancer, especially women with suspected treatment-induced bone loss. The American Society of Clinical Oncology (ASCO) has published guidelines for the monitoring and treatment of bone loss associated with breast cancer treatment Citation[20]. The guidelines suggest that patient groups at high risk of osteoporosis and fracture are evaluated by dual-energy x-ray absorptimetry (DXA) bone scan to assess bone mineral density (BMD) and that patients with a T-score below −2.5 should be treated with a bisphosphonate.
The DXA scan is the gold standard of determining bone mass and it is a highly accurate x-ray technique. The preferred regions to measure are the hip and spine, which are also the common sites of osteoporotic fracture Citation[21]. Hip BMD is the best predictor of hip fractures and also predicts risk of fractures of other skeletal sites. Testing at the spine is the most sensitive to detect early signs of bone loss, but it can be falsely elevated because of degenerative changes (osteoarthritis) with increasing age Citation[21]. BMD is reported as grams per centimetre squared as well as T- and Z-scores, which characterize the difference between the patient's score and the reference norms. They are expressed in standard deviations above (positive values) or below (negative values) the mean Citation[21]. T-scores compare the patient's BMD with that of the mean for healthy young adults of the same sex, while Z-scores compare BMD with that of the mean for individuals of the same age and sex. The World Health Organization operationally defines osteoporosis as a bone density expressed as a T-score that is below −2.5. Osteopenia (low bone mass) is defined as a T-score of −1.0 to −2.5 below the normal score for young adult women Citation[22]. For every one standard deviation below normal, the relative risk of fracture is increased 1.5- to 3.0-fold Citation[22].
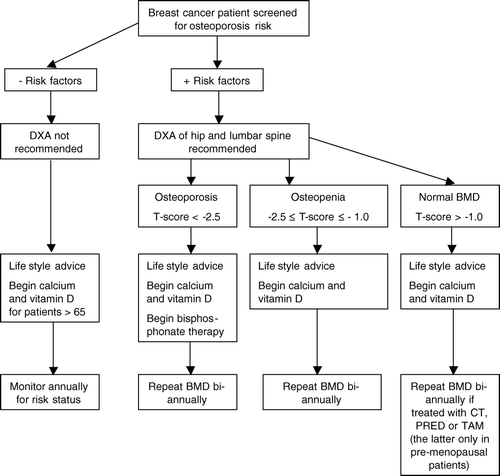
A number of risk factors for osteoporosis and the associated fractures have been identified, where low BMD and history of low-energy fracture are two of the strongest fracture risk factors. Others include genetic predisposition, low body weight (body mass index <19), immobilisation, insufficient intake of calcium and vitamin, late menarche, early menopause (<45 years of age), systemic glucocorticoid treatment (>5 mg prednisolone for 3 months or longer), smoking, significant alcohol intake, older age, diseases associated with osteoporosis (among others: anorexia nervosa, malabsorption, osteogenesis imperfecta, primary hyperparathyroidism, rheumatoid arthritis, multiple myeloma), and treatment with aromatase inhibitors Citation[18], Citation[20], Citation[23]. Breast cancer patients treated with either chemotherapy, tamoxifen (premenopausal women) or aromatase inhibitors are also at high risk of developing osteoporosis due to either ovarian ablation or low estrogen levels Citation[24]. Thus these patients should be considered at high risk of osteoporosis regardless of age, and should therefore be evaluated for osteoporosis by DXA scan, and preferably at the initiation of treatment (). As breast cancer patients are also frequently having other risk factors such as advanced age, insufficient calcium and vitamin D intake careful attention should be given to identify these individuals at an early stage. As in women without breast cancer, subsequent interventions are guided by BMD results. The ASCO guideline emphasises that oncology professionals, especially medical oncologists, need to take an expanded role in the routine and regular assessment of the osteoporosis risk in women with breast cancer Citation[20].
Figure 1. Recommended management strategy in Denmark for patients with diagnosed non-metastatic breast cancer who are eligible for surgery and adjuvant therapy. This management strategy is largely based on influence from results in non-breast cancer populations and from recommendations from the ASCO 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. BMD: bone mineral density, DXA: dual energy x-ray absorptiometry, CT: chemotherapy, PRED: prednisolone, TAM: Tamoxifen.
Monitoring
In otherwise healthy individuals treated for osteoporosis a 1–2 year interval between DXA measurements is usually recommended Citation[19]. However, breast cancer patients who develop chemotherapy-induced osteoporosis undergo accelerated and highly significant bone loss, most prominently in the spine Citation[24]. This bone loss is more pronounced than the bone loss at normal menopause. The ASCO guidelines recommend that all breast cancer patients at high risk of osteoporosis should have annual repeat DXA scans irrespective of the initial DXA scan result, and patients that are treated with anti-osteoporotic drugs along with untreated patients should be monitored at the same close intervals.
Therapeutic actions
This section includes considerations for therapeutic actions in breast cancer patients diagnosed with osteoporosis, excluding regimens for bone metastasis and hypercalcemia.
Breast cancer patients treated with chemotherapy or aromatase inhibitors and diagnosed with osteoporosis should initiate anti-osteoporotic treatment according to the following guidelines ().
In patients with no risk of hypercalcemia it is recommended to ensure a sufficient supply of calcium and vitamin D Citation[25], Citation[26]. The intestinal calcium absorption is stimulated by estrogen as well as vitamin D Citation[27–29]. Estrogen deprivation reduces the intestinal fractional calcium absorption and if a sufficient amount of calcium is not supplied in the diet, a negative calcium balance will result. This will lead to secondary hyperparathyroidism, increased bone turnover, a decrease in BMD and an increased risk of fracture. As the intestinal calcium absorption is also dependent on vitamin D, sufficient amounts of vitamin D should be provided as well. A daily intake of a 1000–1500 mg of calcium and 800–1000 IU of vitamin D is generally recommended for bone health. The supplement should be taken with a meal 2–3 times a day Citation[30–32].
Bisphosphonates are well-known potent anti-resorptive agents. They work by inhibiting osteoclastic bone resorption, thereby preserving bone tissue, and increasing bone mass and reduce fracture risk. Several generations of bisphosphonates are available; cyclic etidronate, risedronate, alendronate, ibandronate, pamidronate, zoledronic acid and clodronate. Bisphosphonates increase BMD and reduce the risk of fracture in postmenopausal patients with osteoporosis Citation[33–39]. Not all bisphosphonates have been tested in breast cancer patients with bone loss, but until further data are available all may potentially be recommended in patients with anti-cancer-treatment induced osteoporosis in standardized doses.
Regimens for administration and possible side effects may decide which agent is chosen for each individual. It may be advisable to evaluate treatment effect every second year in order to change treatment regimen in situations with continuous bone loss in the presence of osteoporosis. This could be initiation of an intravenous regime instead of an oral.
Few clinical studies are published evaluating the effect of treatment with bisphosphonates in women with anti-cancer-treatment induced bone loss and most studies exclude patients with osteoporosis. Delmas et al. investigated the effect of administration of cyclic risendronate in 53 postmenopausal women with breast cancer all non-osteoporotic Citation[40]. Study period was two years with one year follow-up. Intervention increased BMD significantly both in the spine and hip region and withdrawal of the bisphosphonate after two years resulted in a decline in BMD. Greenspan et al. published data from a study investigating the effect of weekly risedronate versus placebo for 12 months Citation[41]. The study population was 87 premenopausal women, where 2% had osteoporosis at inclusion. Patients were included after receiving chemotherapy. Intervention increased BMD both in the lumbar spine and hip region whereas a decline in BMD for both regions was observed for the placebo group. Fuleiham et al. investigated the effect of pamidronate IV every 3 months for one year in 40 premenopausal women, without bone metastasis and all non-osteoporotic Citation[42]. Treatment was preventive as pamidronate was initiated with start on the anti-cancer treatment. Intervention preserved bone during anti-cancer treatment in these patients whereas a decline in BMD was observed in the placebo group. Saarto et al. investigated the effect of daily oral clodronate in 61 postmenopausal women with breast cancer Citation[43]. Of these patients 6–7% had osteoporosis at the time of inclusion. Intervention was preventive as clodronate was initiated at the same time as the anti-hormone treatment. The study demonstrated a marginal increase in BMD compared to the placebo group.
The results of these studies demonstrate a beneficial effect of bisphosphonates on bone in breast cancer women with anti-cancer-treatment induced bone loss. The effect has been demonstrated both in a prophylactic as well as in a treatment regime.
Further studies are needed in breast cancer women with established osteoporosis as well as studies designed to compare the different bisphosphonates both in pre- and in post-menopausal women.
Several large, randomized, multicenter trials are underway to determine the effect of either upfront or delayed zoledronic acid treatment in patients with breast cancer treated with antiaromatase agents Citation[44], Citation[45].
Presently, it is recommended to initiate treatment with bisphosphonate in these women with established osteoporosis. Whether the treatment should be given prophylactic at the initiation of the anti-cancer-treatment needs to be investigated further in clinical trials Citation[46].
Usage of oral bisphosphonates may result in gastrointestinal side effects, which is an important consideration in cancer patients who may already experience nausea, vomiting or gastrointestinal difficulties from the anti-cancer-treatment. Osteonecrosis of the jaw, described as a painful refractory bone exposure in the jaws is a serious complication of bisphosphonate usage Citation[47]. It is seldom seen in relation to the usage of oral bisphosphonates. The osteonecrosis of the jaw is more prevalent in relation to the usage of IV bisphosphonates and especially in patients with cancer. Complete prevention of this complication is not possible, but pre-therapy dental care may reduce the incidence.
Conclusion
Breast cancer patients, who undergo chemotherapy and/or treatment with aromatase inhibitors are at increased risk of osteoporosis. As these patients survive longer and are more frequently cured, there is a need to evaluate the fracture risk – preferably at initiation of endocrine adjuvant therapy – give life style advice on how to counteract bone loss and consider antiosteoporosis intervention and monitoring if osteoporosis is diagnosed. Adjuvant regimens in breast cancer patients improve survival and cure rates. Therefore it is preferable to use such therapies although they increase risk of side effects such as osteoporosis. Breast cancer patients are followed up long term and it may be considered to evaluate the fracture risk of patients who have completed adjuvant therapy. Finally, patients need to be informed of the fracture risk.
References
- Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med 2001; 344: 1997–2008
- Bruning PF, Pit MJ, Jong-Bakker M, van den EA, Hart A, van Enk A. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer 1990; 61: 308–10
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312: 1254–9
- Fogelman I, Blake GM, Blamey R, Palmer M, Sauerbrei W, Schumacher M, et al. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporos Int 2003; 14: 1001–6
- Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer 2001; 37: 2373–8
- Saarto T, Blomqvist C, Valimaki M, Makela P, Sarna S, Elomaa I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: A randomized study in premenopausal breast cancer patients. J Clin Oncol 1997; 15: 1341–7
- Headley JA, Theriault RL, LeBlanc AD, Vassilopoulou-Sellin R, Hortobagyi GN. Pilot study of bone mineral density in breast cancer patients treated with adjuvant chemotherapy. Cancer Invest 1998; 16: 6–11
- Shapiro CL, Phillips G, Van Poznak CH, Jackson R, Leboff MS, Woodard S, et al. Baseline bone mineral density of the total lumbar spine may predict for chemotherapy-induced ovarian failure. Breast Cancer Res Treat 2005; 90: 41–6
- Alexeeva L, Burkhardt P, Christiansen C, Cooper C, Delmas P, Johnell O, et al Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Report of a WHO Study Group. 1st ed. Geneva: WHO Technical Report Series 843. 1994.
- Love RR, Barden HS, Mazess RB, Epstein S, Chappell RJ. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch Intern Med 1994; 154: 2585–8
- ATAC Trialists Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365:60–2.
- Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005; 366: 455–62
- Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. Randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004; 350: 1081–92
- Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol 2007; 25: 486–92
- Mann BS, Johnson JR, Kelly R, Sridhara R, Williams G, Pazdur R. Letrozole in the extended adjuvant treatment of postmenopausal women with history of early-stage breast cancer who have completed 5 years of adjuvant tamoxifen. Clin Cancer Res 2005; 11: 5671–7
- Theriault RL, Biermann JS, Brown E, Brufsky A, Demers L, Grewal RK, et al. NCCN Task Force Report: Bone Health and Cancer Care. J Natl Compr Cancer Netw 2006; 4(Suppl 2)S1–S20
- Winer EP, Hudis C, Burstein HJ, Bryant J, Chlebowski RT, Ingle JN, et al. American Society of Clinical Oncology technology assessment working group update: Use of aromatase inhibitors in the adjuvant setting. J Clin Oncol 2003; 21: 2597–9
- National Osteoporosis Foundation. NOF Physician's Guidelines. September 2005. Pharmacologic Options. http://www.nof.org/professionals/clinical.htm. 2005. 20-6-2007.
- U.S. Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: Recommendations and rationale. Ann Intern Med 2002;137:526–8.
- Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003; 21: 4042–57
- Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: Scientific review. JAMA 2002; 288: 1889–97
- World Health Organization. Prevention and management of osteoporosis: Report of a WHO Scientific Group. WHO Technical Report Series. http://whqlibdoc.who.int/trs/WHO_TRS_921.pdf. 2007. 20-6-2007.
- Seeman E. Pathogenesis of bone fragility in women and men. Lancet 2002; 359: 1841–50
- Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol 2001; 19: 3306–11
- NIH State-of-the-Science Conference Statement on Multivitamin/Mineral Supplements and Chronic Disease Prevention. NIH Concens State Sci Statements 2006;23:1–30.
- Heaney RP. Bone Health. Am J Clin Nutr 2007; 85: 300S–303S
- Ebeling PR, Sandgren ME, DiMagno EP, Lane AW, DeLuca HF, Riggs BL. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: Relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentration in normal women. J Clin Endocrinol Metab 1992; 1: 176–82
- Heaney RP, Recker RR, Saville PD. Menopausal changes in calcium balance performance. J Lab Clin Med 1978; 6: 953–63
- Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, et al. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D-receptor-independent mechanisms. J Bone Miner Res 2003; 10: 1725–36
- Bronner F. Mechanisms of intestinal calcium absorption. J Cell Biochem 2003; 88: 387–93
- Mosekilde L. Vitamin D and the elderly. Endocrinol (Oxf) 2005; 62: 265–81
- Guéguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr 2000; 19: 119S–136S
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–22
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy with Risendronate Therapy (VERT) Study Group. JAMA 1999; 282: 1344–52
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: Results from the Fracture Intervention Trial. JAMA 1998; 280: 2077–82
- Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 2006; 65: 654–61
- Shiota E, Tsuchiya K, Yamaoka K, Kawano O. Effect of intermittent cyclical treatment with etidronate disodium (HEBP) and calcium plus alphacalcidol in postmenopausal osteoporosis. J Orthop Sci 2001; 6: 133–6
- McCloskey EV, Beneton M, Charlesworth D, Kayan K, deTakats D, Dey A, et al. Clodronate reduces the incidence of fractures in community-dwelling elderly women unselected for osteoporosis; results of a double-blind, placebo-controlled randomized study. J Bone Miner Res 2007; 22: 135–41
- Vis M, Bultink IE, Dijkmans BA, Lems WF. The effect of intravenous pamidronate versus oral alendronate on bone mineral density in patients with osteoporosis. Osteoporosis Int 2005; 16: 1432–5
- Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: A double-blind, placebo-controlled study. J Clin Oncol 1997; 15: 955–62
- Greenspan SL, Bhattacharya RK, Sereika SM, Brufsky A, Vogel VG. Prevention of bone loss in survivors of breast cancer: A randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 2007; 92: 131–6
- Fuleihan Gel-H, Salamoun M, Mourad YA, Chehal A, Salem Z, Mahfoud Z, et al. Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: A randomized controlled trial. J Clin Endocrinol Metab 2005; 90: 3209–14
- Saarto T, Vehmanen L, Elomaa I, Välimäki M, Mäkelä P, Blomqvist C. The effect of clodronate and antioestrogens on bone loss associated with oestrogen withdrawal in postmenopausal women with breast cancer. Br J Cancer 2001; 84: 1047–51
- Aapro M. Improving bone health in patients with early breast cancer by adding bisphosphonates to letrozole: The Z-ZO-E-ZO-FAST program. Breast Feb 2006; 15(Suppl 1)S30–S40
- Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, Grampp S, Kaessmann H, Schmid M, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: A report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 2007; 25: 820–8
- Perez EA, Weilbaecher K. Aromatase inhibitors and bone loss. Oncology (Williston Park) 2006; 20: 1029–39
- Wilkinson GS, Kuo YF, Freeman JL, Goodwin JS. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: A population-based analysis. J Natl Cancer Inst 2007; 99: 1016–24