Abstract
Introduction. A cohort of premenopausal patients with primary hormone receptor positive breast cancer was prospectively identified to be eligible for the DBCG 89B trial. We perform a long-term follow-up and evaluate the external validity of the trial. Material and methods. Following registration in a population-based registry, patients were invited to be randomized to ovarian ablation (OA) versus nine courses of three-weekly cyclophosphamide, methotrexate and 5-fluorouracil (CMF). The same procedures were used in all patients, including report forms, central review, querying, and analysis of data. Multivariate analysis was used to adjust for differences in base-line characteristics. Results. Participation in the randomization varied according to center and time period. One thousand six hundred and twenty eight eligible patients were registered and 525 randomized in the DBCG 89B trial. Median estimated follow-up was 9.5 years for disease-free survival and 12.1 years for overall survival. Non-enrolled patients had a disease-free and overall survival similar to randomized patients. Within 5 years of surgery, results were similar following OA and CMF, but disease-free survival was significant inferior with OA more than five years after surgery, adjusted hazard ratio 1.38 (95% CI 1.03 to 1.85; p=0.03). This convened ten years after surgery to an inferior survival with OA, and the adjusted hazard ratio was 2.37 (95% CI 1.43 to 3.91; p<0.01). Discussion. This prospective cohort study indicates that eligible patients not participating in the DBCG 89B trial had a similar disease-free and overall survival as participants. Survival was similar after OA and CMF in the first ten years, but became inferior in the OA group 10 or more years after surgery.
The collaborative meta-analyses performed by the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) have demonstrated that ovarian ablation or suppression improves disease-free and long-term survival in premenopausal women with early and hormone receptor-positive breast cancer Citation[1]. Indirect comparisons in an early report from the EBCTCG suggested a similar efficacy to what was achieved with chemotherapy Citation[2]. Randomized trials have confirmed that ovarian ablation Citation[3], Citation[4] or luteinising hormone-releasing hormone agonists Citation[5] have an efficacy similar to chemotherapy, foremost cyclofosfamide, methotrexate and fluorouracil (CMF), in premenopausal women with early hormone receptor-positive breast cancer. Chemotherapy will introduce amenorrhea in a substantial proportion of premenopausal women and this formed the hypothesis that the efficacy of chemotherapy, at least in part, is mediated through ovarian suppression Citation[6]. Dissimilar risks and harms are anticipated following ovarian ablation and chemotherapy, and participation in randomized trials between these two treatments is likely to depend on information about toxicity. Loss of fertility, menopausal symptoms and side effects of chemotherapy are important quality of life issues Citation[7], and most certainly have influenced the decision of a number of patients whether to participate. Recruitment of a highly selected group of participants however may affect the external validity or generalizability of results from randomized trials Citation[8]. The prognostic profile of participants in randomized cancer trials have been demonstrated to differ from the profile of non-participants Citation[9], Citation[10], and the large differences in targets, application and setting between ovarian ablation and chemotherapy could make these interventions particular prone to patient characteristics.
We have previously reported results from a randomized trial comparing disease-free and overall survival following ovarian ablation and CMF in premenopausal women with hormone receptor positive breast cancer Citation[3]. Concern regarding the external validity of this trial was however substantiated by a considerable lower randomization rate compared to previous trials performed by the DBCG and by participation from centers outside Denmark. Here we report the nationwide results of participants in DBCG trial 89B compared to eligible patients registered prospectively but treated outside the trial according to the same protocol.
Methods
The DBCG 89 cohort
The DBCG 89 program prospectively identified a cohort fulfilling the following criteria: Women 18 to 74 years of age who had a completely excised unilateral invasive carcinoma of the breast by means of surgical procedure according to nationwide DBCG guidelines. Eligible patients had no signs of distant metastasis as determined by physical examination, biochemical tests and chest radiography. Other imaging examinations were done when indicated by symptoms or signs. Additional eligibility criteria were absence of previous or concomitant cancer, absence of serious or life-threatening medical conditions, absence of pregnancy and absence of previous chemotherapy or radiotherapy.
The randomized DBCG 89B trial
Patients eligible for the 89B trial belonged to the 89 cohort and were in addition premenopausal (amenorrhea for less than 2 months, amenorrhea for less than 12 months and FSH in the premenopausal range, or 50 years of age or younger in the case of hysterectomy), had hormone receptor positive tumors (estrogen (ER) and/or progesterone (PR) receptor positive) and were at high risk of relapse, defined as metastasis to at least one lymph node or a tumor exceeding 5 cm Citation[3]. Only patients from Denmark enrolled onto the randomised DBCG 89-B trial were included in this cohort study, while patients enrolled in Sweden and the Netherlands not were included. The DBCG 89B trial was conducted according to the Helsinki declaration and was approved by ethical committees with jurisdiction for the participating institutions.
Surgical and diagnostic procedures
Lower axillary clearance (level I and part of level II) in combination with breast-conserving surgery or mastectomy according to nationwide-implemented DBCG guidelines was required.
The pathological procedure included classification of histological type according to WHO, examination of tumor margins, invasion into skin or deep fascia, measurement of gross tumor size, number of metastatic and total number of lymph nodes identified. All invasive ductal carcinomas were graded for malignancy Citation[11]. ER and PR were analyzed using dextran-coated charcoal (DCC) assays in frozen tissue or immunohistochemical assays Citation[12]. Tumors were considered receptor positive if ER or PR content was 10 fmol receptor protein per mg cytosol protein or larger in the extraction assay or at least 10% of the epithelial cells stained positively for ER or PR.
Adjuvant Therapy
Ovarian ablation was performed by surgery or irradiation, and the requested limits of the pelvic portals used were from the inferior border of the fifth lumbar vertebra to the lowermost aspect of obturator foramen and 1–2 cm lateral of the inner pelvic sidewalls. The field arrangement involved the use of anterior and posterior fields against the minor pelvic region. The intended dose was a median absorbed dose in the target volume of 15 Gy, given in 5 fractions over a one-week period using a linear accelerator. Radiotherapy was recommended against regional nodes in node-positive disease (48 Gy), against the residual breast following lumpectomy (48 Gy + boost 10 Gy) and against the chest wall following mastectomy in patients with 4 or more positive nodes from 1990 through 1994, in all node positive patients and in patients with tumors larger than 5 cm (48 Gy) after 1994. In all cases 2 Gy in 5 fractions per week.
Patients not assigned to loco-regional radiotherapy received 9 cycles of cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2 and 5-fluorouracil 600 mg/m2 (CMF) given intravenously (IV) day one every third week, while patients assigned to radiotherapy received one or two cycles of CMF before radiotherapy and one or two cycles of single agent cyclophosphamide (850 mg/m2) concomitant with radiotherapy followed by CMF to a total of nine cycles of chemotherapy. The dose was described to be adjusted according to white cell and platelet counts (×109/l) on day 1 of the scheduled cycle as follows: platelets > 100 and WBC > 3, 100%; platelets 75–99 or WBC 2.0–2.9, 50% of both drugs. If platelets were < 75 or WBC were < 2.0, the treatment was delayed for 1 week.
Follow-up
All patients in the cohort, independent from enrollment in the randomized trial, were reported to DBCG registry using standardized forms, and data from all patients in the cohort was collected and accumulated centrally by the DBCG Registry. Symptoms, side-effects including amenorrhea, and findings on clinical examination were recorded every 12 weeks during the first year, every 6 months during the second through the fifth year, and thereafter annually to a total of 10 years. Hemoglobin, white blood cell count, and platelet count, were examined on day one of each CMF cycle. Additional biochemical tests and imaging examinations were done when indicated by symptoms or signs.
Statistical Analysis
Apart from randomization, the DBCG Data Center undertook the same procedures in all patients, including central review, querying, and analysis of data. Follow-up time was quantified in terms of a Kaplan-Meier estimate of potential follow-up Citation[13]. Overall survival (OS) was calculated as the time elapsed from definitive surgery until death, irrespective of cause of death. Disease-free survival (DFS) was defined as the duration of survival without loco-regional recurrence, distant metastases and contralateral breast cancer. Non-protocol anti-neoplastic treatments resulted in censoring on the first day of treatment. OS and DFS were analyzed unadjusted using Kaplan-Meier estimates and logrank test. For multivariate analysis the Cox proportional hazards regression model was applied to assess the adjusted relative risk of enrolled vs non-enrolled as well as treatment regimen, and to explore interactions. Factors included in the multivariate analysis were age (≤40, 41–45, 46–50, >50), tumor size (≤20 mm, 21–50 mm or unknown,>50 mm), nodal status (<10 lymph nodes examined, ≤10 lymph nodes examined as well as 0, 1–3, 4–5, 6–10, >10 positive lymph nodes), histologic type and grade (ductal grade I, ductal grade II, ductal grade III or unknown grade, lobular, other histologic type), hormone receptor status (both ER and PR positive, one positive and the other negative, one positive and the other unknown), radiotherapy (yes, no) as well as treatment regimen and participation in the randomized trial. The assumptions of proportional hazards were assessed by the use of test, log(-log) S plots and Schoenfeld residuals. The hazard rates of hormone receptor status were not proportional and therefore stratification was used. Associations between participation in the randomized trial and other characteristics were analyzed by chi-square test. P-values are two-tailed. Statistical analyses were done with the SAS 8.2 program package.
Results
From January 1990 to May 1998, 16 844 patients were registered in the DBCG 89 program ( Panel A) and hereof 1 628 patients fulfilled the criteria for randomization in DBCG trial 89B and 525 patients (32%) participated in the randomized trial ( Panel B). Patient, tumor and loco-regional treatment characteristics for the 1 463 patients treated per-protocol are presented in . The proportion of patients randomized yearly from 1992 to 1998 was 48%, 59%, 43%, 39%, 22%, 20%, 15% and 14% respectively. The proportion of patients randomized varied according to center from 10% to 75%. Eight patients, two randomized and six non-enrolled patients, received hormone replacement therapy. Tamoxifen was prescribed before relapse in eight non-enrolled patients.
Figure 1. Profile of the DBCG 89 program (Panel A) and the 89B cohort (Panel B). *To allow a direct comparison between randomized and non-enrolled patients seven randomized patients were excluded (four with distant metastasis, two with a previous malignancy and one who withdraw consent).
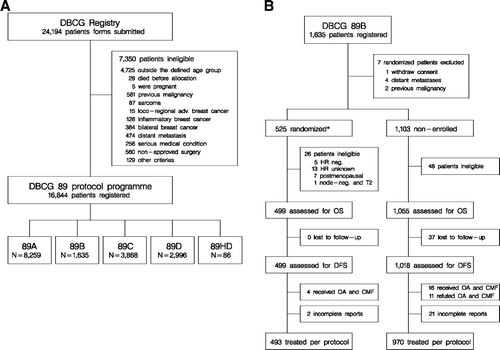
Table I. Base-line characteristics of the DBCG 89-B cohort.
Study outcome
For the 1 463 patients, median estimated potential follow-up was 9.5 years for DFS and 12.1 years for OS. The 10-year DFS rate was 47.2% (95% CI, 42.6% to 51.9%) in randomized patients and 48.9% (95% CI, 45.4% to 52.4%) in non-enrolled patients. shows the number of patients with DFS events and number of deaths according to randomization and treatment. Of the 708 first events reported, 255 occurred among randomized patients and 453 in the non-enrolled group (). DFS was similar among randomized and non-enrolled patients (A, p = 0.44) and the unadjusted hazard ratio for DFS among non-enrolled patients compared to randomized patients was 1.06 (95% CI, 0.91 to 1.24) (). Ten-year OS rates were 64.3% (95% CI, 60.0% to 68.5%) and 65.4% (95% CI, 62.3% to 68.4%) in randomized and non-enrolled patients, respectively (B). The unadjusted hazard ratio for death from any cause in the non-enrolled group compared to the randomized group was 1.06 (95% CI 0.90 to 1.26). Adjusting for prognostic factors (age, tumor size, nodal status, histological type and grade, hormone receptor status, and treatments) did not substantially affect these estimates ().
Figure 2. Disease-free survival (DFS) for randomized compared to non-enrolled patients (Panel A) and for CMF compared to ovarian ablation (Panel B). Overall survival (OS) for randomized compared to non-enrolled patients (Panel C) and for CMF compared to ovarian ablation (Panel D).
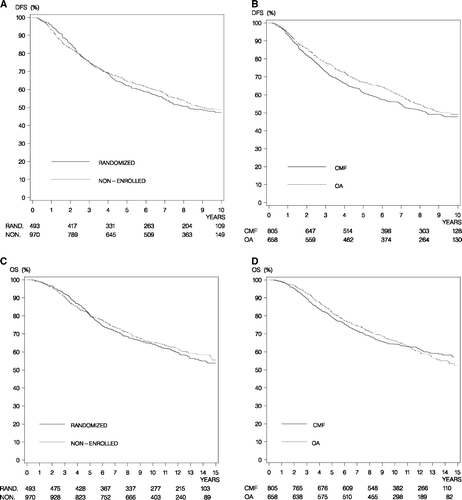
Table II. End-point events.
Table III. Outcomes in non-enrolled compared with randomized patients.
Of the 708 first events reported, 315 were in patients receiving OA and 393 were in patients receiving CMF. When patients in the OA group were compared to those in the CMF group the effect appeared to be modified by time (C). OA was statistically significant superior to CMF in the unadjusted analysis of DFS (p = 0.02 by the log-rank test) when time since surgery was below 5 years (Hazard ratio 0.82 (95% CI 0.69 to 0.98)). In contrast, no significant difference in DFS (p = 0.17) was observed between OA and CMF more than 5 years after surgery (Hazard ratio 1.22 (95% CI 0.92 to 1.62)). Significant differences were observed in patient characteristics, and adjustment for factors associated with DFS (age, tumor size, nodal status, histological type and grade, hormone receptor status, radiotherapy and participation in the randomization) changed the hazard ratio for DFS to 0.93 (95% CI 0.78 to 1.11; p = 0.43) when time since surgery was below 5 years and 1.37 (95% CI 1.02 to 1.83; p = 0.04) more than 5 years after surgery. The hazard ratio for OA versus CMF for DFS in the first 5 years was significantly different from the hazard ratio more than 5 years after surgery (p = 0.03). Distant recurrence-free survival (not including any deaths) was in an adjusted analysis inferior following OA more than 5 years after surgery (p < 0.01) but similar during the first 5 years after surgery (p = 0.18).
Causes of death are shown in , and the difference in overall survival (D) between the OA and CMF groups also seemed to depend on time since surgery. Overall survival was similar in the OA group compared to the CMF group in the first 10 years after surgery and the unadjusted analysis hazard ratio was 0.92 (95% CI 0.77 to 1.09; p = 0.32). After 10 years overall survival was significantly inferior following OA (Hazard ratio 1.98 (95% CI 1.22 to 3.23, p < 0.01)). Overall survival following OA and CMF remained similar (D) in the first 10 years after adjustment for prognostic factors and the adjusted hazard ratio was 1.01 (95% CI 0.85 to 1.21, p = 0.90). A significant difference in overall survival was observed after 10 years in the adjusted analysis (p < 0.01), and the adjusted hazard ratio was 2.29 (95% CI 1.39 to 3.75). For overall survival the hazard ratio below 10 years for OA versus CMF was significantly different from the hazard ratio more than 10 years after surgery (p < 0.01).
Toxicity
Treatment related deaths were not reported. Dose-limiting toxicities were rare among patients receiving CMF (), but depression of bone marrow function (WBC < 3.0×109/l) was observed in 57% among randomized and 59% of non-enrolled patients. The median relative cumulative dose (actual/planned mg/m2) of CMF was 0.97 in randomized as well as non-enrolled patients and the median relative dose intensity (actual/planned mg/m2 per time unit) of CMF was also identical in the two groups (0.91). Moderate or severe nausea and vomiting was significantly more often reported in randomized (36%) compared to non-enrolled (21%) patients receiving CMF (p < 0.01), while complete or severe alopecia was 5% in randomized and 6% in non-enrolled patients.
Table IV. Toxicity in the CMF groups.
A permanent cessation of menses was not attained in eight patients randomized to OA and in 19 patients in the non-enrolled group despite pelvic irradiation. Two and five patients, respectively, continued regular menses, four and seven patients respectively, continued to have irregular menstrual periods, and two and seven patients resumed menses after a suspension of at least one year. In patients randomized to CMF, 161 patients had regular menses at randomization, and 57 (35%) of these either continued regular menses (38 patients) or resumed regular menses (19 patients). A permanent cessation of menses was registered in 55 patients (34%) randomized to CMF, while the consequences were unclear in 49 patients (30%). In non-enrolled patients treated with CMF, 462 patients had regular menses at initiation of CMF, and 182 (39%) of these either continued regular menses (135 patients) or resumed regular menses (47 patients). A permanent cessation of menses was registered in 138 (30%) non-enrolled patients treated with CMF, while the consequences were unclear in 142 patients (31%).
Discussion
Only 32% of eligible patients were randomized in the 89B trial and the proportion of participating patients varied by center and over time during the conduct of this trial. Participation has been wider accepted in other DBCG trials Citation[14] but internationally less than 5% of cancer patients are included in randomized trials. Significant differences were observed between participants and non-participants in a number of base-line characteristics. Adjustment for possible prognostics factors did however not substantially affect the hazard ratios for disease-free and overall survival. The design of the study allowed us to gather complete information on outcome and potential known confounders but we cannot exclude residual confounding. Our results are in support of recent reviews concluding that there is insufficient evidence of a trial participation or inclusion effect in clinical trials Citation[15], Citation[16].
The strengths of the current design were primarily the population-based prospective identification and central registration of eligible patients. In addition the same procedures were used for all patients including assembling report forms, central review, and querying, pragmatic selection criteria were used in the trial, and a long-term follow-up was provided. To our knowledge, this is the first study of external validity that brings about these design elements.
The current design also has some limitations and we are unable to assess generalizability to other countries or health care systems Citation[8]. Eight patients (0.8%) in the non-trial group received tamoxifen, which was not recommended to pre-menopausal patients during the study period. Noncompliance with the guidelines was otherwise not observed. The three-weekly intravenous CMF regimen used in the 89B trial is inferior to regimens including taxanes and anthracyclins, especially in high-risk breast cancer patients Citation[17] and use of these therapies, now considered to be evidence-based standard, could have altered the results of the current analysis of chemotherapy versus ovarian ablation. An experimental treatment effect was not demonstrated and could not be anticipated in the current study Citation[18].
We found a similar short-term efficacy of ovarian ablation and CMF, but a significant difference in favour of CMF was seen in DFS more than five years and in overall survival more than 10 years after surgery. This difference was observed among randomized as well as non-enrolled patients. Compared to the published analysis of the randomized trial the current analysis has an additional two years of follow-up, which explains why the time-dependency not was found in our previous analysis of the randomized trial Citation[3]. An increase in distant recurrences more than five years after surgery in patients treated with ovarian ablation appear to be the primary explanation of the long-term superiority of CMF. Thus the main effect of chemotherapy could be a more complete eradication of sub-clinical disease compared to an impermanent growth arrest following ovarian ablation. This phenomenon has not been observed in trials comparing ovarian suppression to chemotherapy Citation[5], but few of these trials have sufficiently long follow-up. Patients accepting participation in the randomized trial more often reported toxicities than non-enrolled patients. We used the same procedures for reporting and registration of toxicities, and all patients received identical information material. Awareness of patients and their physicians might nevertheless have differentiated according to participation in the randomization.
We found no support for a beneficial or a harmful effect of participating in the randomization of the DBCG 89B trial. Disease-free and overall survival was similar in randomized and non-enrolled high-risk premenopausal node positive breast cancer patients with tumors expressing hormone receptors.
References
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–17.
- Early Breast Cancer Trialists’ Collaborative Group. Ovarian ablation in early breast cancer: Overview of the randomised trials. Lancet 1996;348:1189–96.
- Ejlertsen B, Mouridsen HT, Jensen MB, Bengtsson NO, Bergh J, Cold S, et al. Similar efficacy for ovarian ablation compared with cyclophosphamide, methotrexate, and fluorouracil: From a randomized comparison of premenopausal patients with node-positive, hormone receptor-positive breast cancer. J Clin Oncol 2006; 24: 4956–62
- Scottish Cancer Trials Breast Group and ICRF Breast Unit, Guy's Hospital, London. Adjuvant ovarian ablation versus CMF chemotherapy in premenopausal women with pathological stage II breast carcinoma: the Scottish trial. Lancet 1993;341:1293–8.
- Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: A meta-analysis of individual patient data from randomised adjuvant trials. Lancet 2007; 369: 1711–23
- Brincker H, Rose C, Rank F, Mouridsen HT, Jakobsen A, Dombernowsky P, et al. Evidence of a castration-mediated effect of adjuvant cytotoxic chemotherapy in premenopausal breast cancer. J Clin Oncol 1987; 5: 1771–8
- Groenvold M, Fayers PM, Petersen MA, Mouridsen HT. Chemotherapy versus ovarian ablation as adjuvant therapy for breast cancer: Impact on health-related quality of life in a randomized trial. Breast Cancer Res Treat 2006; 98: 275–84
- Rothwell PM. Treating individuals 2. Subgroup analysis in randomized controlled trials: Importance, indications, and interpretation. Lancet 2005; 365: 176–86
- Elting LS, Cooksley C, Bekele BN, Frumovitz M, Avritscher EB, Sun C, et al. Generalizability of cancer clinical trial results: Prognostic differences between participants and non participants. Cancer 2006; 106: 2452–8
- Steg PG, Lopez-Sendon J, Lopez de SE, Goodman SG, Gore JM, Anderson FA, et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med 2007; 167: 68–73
- Bloom J, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 1957; 11: 359–77
- Andersen J, Thorpe SM, King WJ, Rose C, Christensen I, Rasmussen BB, et al. The prognostic value of immunohistochemical estrogen receptor analysis in paraffin-embedded and frozen sections versus that of steroid-binding assays. Eur J Cancer 1990; 26: 442–9
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trial 1996; 17: 343–6
- Andersen KW, Mouridsen HT. Danish Breast Cancer Cooperative Group (DBCG). A description of the register of the nation-wide programme for primary breast cancer. Acta Oncol 1988; 27: 627–47
- Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: Conceptual framework and structured review. Lancet 2004; 363: 263–70
- Vist GE, Hagen KB, Devereaux PJ, Bryant D, Kristoffersen DT, Oxman AD. Systematic review to determine whether participation in a trial influences outcome. BMJ 2005; 330: 1175–81
- Goldhirsch A, Coates AS, Gelber RD, Glick JH, Thurlimann B, Senn HJ. First--select the target: Better choice of adjuvant treatments for breast cancer patients. Ann Oncol 2006; 17: 1772–6
- Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol 2001; 54: 217–24
Appendix A
B. Ejlertsen conceived and designed the study, interpreted the data, and drafted the manuscript. M.-B. Jensen did statistical analyses, and interpreted the data. H. T. Mouridsen drafted the manuscript and provided administrative support. All authors participated in the critical revision of the report.
The following institutions participated in the study: DBCG Registry, Copenhagen, Denmark: Susanne Möller, M.Sc; Aalborg Hospital, Aarhus University Hospital, Aalborg, Denmark: Marianne Ewertz, MD; Aarhus University Hospital, Aarhus, Denmark, Jørn Andersen, MD; Rigshospitalet, Copenhagen, Denmark: Henning T. Mouridsen, MD; Esbjerg County Hospital, Esbjerg, Denmark: Brita B. Jensen, MD; Herlev University Hospital, Herlev, Denmark: Claus Kamby, MD; Herning County Hospital, Herning, Denmark: Knud Aage Moller, MD; Naestved County Hospital, Naestved, Denmark: Preben Philip, MD; Odense University Hospital, Odense, Denmark: Soren Cold, MD; Roskilde County Hospital, Roskilde, Denmark: Peter Grundtvig, MD; Sonderborg County Hospital, Sonderborg, Denmark: Ebbe Lindegaard-Madsen, MD; Vejle County Hospital, Vejle, Denmark: Erik H. Jakobsen, MD; and Viborg County Hospital, Viborg, Denmark: Vera Haahr, MD.