Abstract
A national study of BRCA1 and BRCA2 mutations in Danish HBOC (Hereditary Breast Ovarian Cancer) families revealed a total number of 322 mutation positive families, 206 (64%) BRCA1 and 116 (36%) BRCA2 positive families from a population of 5.5 million inhabitants. Seven hundred and twenty six mutation positive individuals were identified: 402 female BRCA1 carriers, 79 male BRCA1 carriers, 213 female BRCA2 carriers, and 32 male BRCA2 carriers by April 2006.
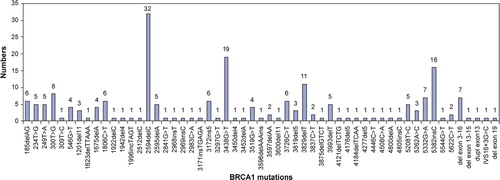
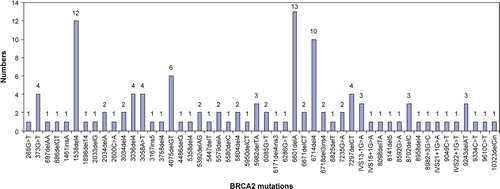
Most of the mutations were frame shift or nonsense mutations, while large genomic rearrangements were rare. Most mutations were only identified in one family. A few mutations were detected repeatedly. In BRCA1 the most common mutations were: 2594delC in 32 families (16%), 3438G>T in 19 families (9%), 5382insC in 16 families (8%), 3829delT in 11 families (5%). In BRCA2 the most common mutations were: 6601delA in 13 families (11%), 1538del4 in 12 families (10%), 6714del4 in 10 families (9%).
There was a tendency towards a higher frequency of BRCA2 mutations in West Denmark compared to East Denmark. The frequencies of specific BRCA1 and BRCA2 mutations were slightly different in the two regions. The mutations occurring in West Denmark have also been observed in other Scandinavian countries whereas the mutations occurring in East Denmark were more often reported from other European countries and the Baltic countries. The pattern of mutation distributions are comparable with observations from other Scandinavian and European studies and indicate that the Danish BRCA1 and BRCA2 mutations are a mixture of Scandinavian mutations and other European mutations including two of the Ashkenazi mutations.
Even though a tendency towards founder mutations was observed most mutations were only detected once. Based on these observations we recommend that the mutation screening strategy of the BRCA1 and BRCA2 genes in Danish HBOC families comprises full screening of both genes including analysis for large genomic rearrangements.
Familial occurrence of breast and/or ovarian cancer is observed in a minor proportion of patients with breast or ovarian cancer. Hereditary breast and/or ovarian cancer (HBOC) families are identified by genetic risk assessment and genetic diagnostic testing. HBOC families are characterised by the presence of early onset breast cancer and/or ovarian cancer in first degree female relatives or second degree relatives related through a male family member. The family history is the major indication of whether genetic diagnostic testing should be initiated and which genes should be analysed. The BRCA1 and BRCA2 genes are most frequently mutated and other genes are only analysed if the pedigree is suspicious of another cancer syndrome. BRCA1 and BRCA2 mutations have been identified in approximately 28.5% of the families from Southwest Denmark with HBOC or hereditary breast cancer without ovarian cancer Citation[1].
The frequency and distribution of BRCA1 and BRCA2 mutations reflect the genetic composition of the studied population and such issues are important components of a molecular diagnostic strategy. This is the case for populations with a high frequency of Ashkenazi Jews. It is very important to collect national data concerning BRCA1 and BRCA2 mutations in order to get the best estimate of the mutation distribution but it is also important to evaluate if there are regional differences even in a small country as Denmark. The aim of the present study was to develop the optimal diagnostic strategy based on the report of all BRCA1 and BRCA2 mutations identified in Denmark by April 1, 2006.
When a BRCA1 or BRCA2 mutation is identified in a family other family members are offered predictive testing for the mutation. The utilization of predictive genetic testing varies according to many variables such as gender, age, marital status, children and whether there are any cancer preventive possibilities. As part of this study we were interested in evaluating the utilization of predictive genetic testing for BRCA1 and BRCA2 mutations in Denmark.
Material and methods
Mutation screening and testing of the BRCA1 and BRCA2 genes were performed in four laboratories (Odense, Copenhagen, Herlev, and Aalborg) and have also earlier been performed in Lund, Sweden, and to a lesser extent at Medical Genetics Clinic at Copenhagen University. BRCA1 and BRCA2 testing was requested after genetic risk assessment and counselling by the departments of Clinical Genetics.
Mutation screening was performed by slightly different techniques, but most laboratories used DHPLC (Denaturing High Performance Liquid Chromatography) and sequencing. All suspected abnormal results from DHPLC were sequenced. Some laboratories performed PTT (Protein Truncation Test) in exon 11 in BRCA1 and exons 10 and 11 in BRCA2 to screen for truncating mutations. There was no difference in mutation detection frequency between the PTT method compared to the DHPLC method (data not shown). MLPA (Multiplex Ligation dependent Probe Amplification) analysis was used to identify large genomic rearrangements. Analysis of a known mutation in the family was usually performed by sequencing or MLPA.
Pathogenic mutations were defined as in BIC (http://research.nhgri.nih.gov/bic). In brief, all mutations introducing premature stop codons (except the terminal frame shift mutations in exon 27 of BRCA2, see http://research.nhgri.nih.gov/bic), larger genomic rearrangements, and splice site mutations in the positions +1, +2, −1, −2 were classified as pathogenic. If the deletion/insertion only involved one exon the mutation was verified by other methods such as long range PCR or other comparable methods. If a missense mutation was located in the last nucleotide in an exon further analyses were performed to confirm the aberrant splicing Citation[2]. Three missense mutations in BRCA1 (5208T>C, 5332G > A, and 5544G > T) were classified as pathogenic mutations according to functional assays which supported this assumption Citation[3–5]. The nomenclature of mutations followed the guidelines described in BIC.
Results
Three hundred and twenty two BRCA1 and BRCA2 positive families were identified by April 1, 2006, and 206 (64%) had mutations in BRCA1 and 116 (36%) in BRCA2. After the primary identification of a mutation in a family at risk, family members were tested for the familial mutation. Four hundred and four persons were tested positive for the mutation identified in the family, and of these 275 were BRCA1 carriers (207 females and 68 males) and 129 were BRCA2 carriers (106 females and 23 males). In total 726 mutation positive individuals were identified: 402 female BRCA1 carriers, 79 male BRCA1 carriers, 213 female BRCA2 carriers, and 32 male BRCA2 carriers.
The utilization of predictive testing did not differ between BRCA1 and BRCA2 families. Four hundred and four persons tested positive for the familial mutation which gives a ratio of 1.25 (404/322) carrier pr. family. We did not have information about the family members who tested negative for the familial mutation, but if we anticipate that this number is equal to the number of mutation positive individuals this results in an average number of 2.5 persons pr. family who ask for predictive genetic testing. The utilization of predictive testing did not differ significantly between BRCA1 families and BRCA2 families as the ratio of carries pr. family was 1.3 for BRCA1 families and 1.1 for BRCA2 families. Female relatives requested predictive testing more often than male relatives. The ratio of females versus males requesting predictive testing in BRCA1 families was 3.0 and in BRCA2 families the ratio was 4.7. This difference was not statistically significant by Fisher's Two-Tail Exact Test.
Most of the mutations in BRCA1 were only identified once () and occurred in exon 11 (62%). This is not surprising as exon 11 comprises 61% of the coding sequences in the BRCA1 gene. The most common BRCA1 mutations were 2594delC in 32 families (16%), 3438G > T in 19 families (9%), 5382insC in 16 families (8%), and 3829delT in 11 families (5%).
Most of the mutations in BRCA2 occurred in exons 11 and 10 (71%: 56% in exon 10 and 15% in exon 11) (), and like in BRCA1 many BRCA2 mutations were only identified once. The frequency of 71% for mutations in exons 10 and 11 is a little higher than the size of these exons (59%) in the BRCA2 gene, but the observed frequency is identical to the reported mutations in BIC. The three most common BRCA2 mutations were 6601delA in 13 families (11%), 1538del4 in 12 families (10%), and 6714del4 in 10 families (9%).
The majority of BRCA1 and BRCA2 mutations were frame shift mutations (54% and 78%, respectively), nonsense mutations occurred in 24% of the BRCA1 families and 15% of the BRCA2 families, missense mutations were only reported in BRCA1 (17%) families, splice site mutations were observed in 0.5% of the BRCA1 families and 8% of the BRCA2 families, and large genomic rearrangements were only observed in BRCA1 (4% deletions and 0.5% duplication). A deletion of an exon in BRCA2 has later been detected. As pathogenic missense mutations were only identified in BRCA1 we calculated the frequencies of different BRCA1 mutation types excluding the missense mutations. The combined frequencies of frame shift mutations together with the nonsense mutations were very similar for the two genes: 95% in BRCA1 and 93% in BRCA2.
The number of mutation positive families identified by each laboratory was 145 families at Odense University Hospital, 120 families at Rigshospitalet in Copenhagen and Skejby Hospital in Århus in collaboration, 38 families at Lund University Hospital, 11 families at Herlev Hospital, 5 families at Ålborg Hospital, and 4 families at Medical Genetics Clinic at Copenhagen University.
Two laboratories are located in West Denmark (Odense University Hospital and Ålborg Hospital) and two laboratories (Rigshospitalet and Herlev Hospital) are located in Copenhagen in East Denmark. The mutations identified at Lund University Hospital could be allocated to West or East Denmark, respectively, according to the requesting Departments of Clinical Genetics. This resulted in 167 families (102 BRCA1 and 65 BRCA2) from West Denmark and 155 (104 BRCA1 and 51 BRCA2) families from East Denmark. Of the 206 BRCA1 families 50% were from West and 50% from East Denmark and of the 116 BRCA2 families 56% were from West and 44% from East Denmark.
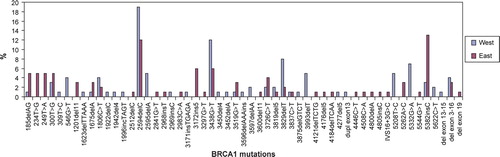
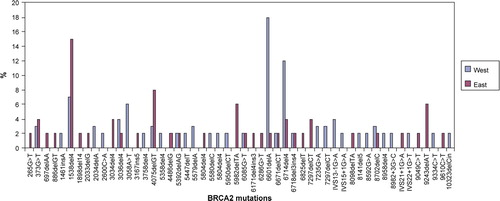
There was a tendency towards a different distribution of the specific BRCA1 mutations and also of the specific BRCA2 mutations in the two regions of Denmark. Seven BRCA1 mutations occurred more frequently in West Denmark: 2594delC, 2595delA, 3438G > T, 3829delT, 3993delT, 5208T > C, and 5332G > A. Five BRCA1 mutations occurred more frequently in East Denmark: 185delAG, 234T > G, 249T > A, 3172ins5, and 5382insC (). In BRCA2 three mutations occurred more frequently in West Denmark: 3058A > T, 6601delA, and 6714del4, and four mutations occurred more frequently in East Denmark: 1538del4, 4075delGT, 5982delTA, and 9243delAG ().
Discussion
The distribution of mutations in a population is characterised by the genetic homogeneity or heterogeneity of that population. Knowledge about mutation distributions is important when a population specific strategy for mutation screening is considered. If mutation screening could be limited to a small number of founder mutations the costs and amount of time for laboratory work could be reduced Citation[6]. Specific mutations in BRCA1 are very common in Norway Citation[7] and this is also observed for people of Ashkenazi Jewish ancestry where two BRCA1 mutations and one BRCA2 mutation are very common Citation[8]. Our study included all the identified Danish BRCA1 and BRCA2 positive HBOC families by April 2006 in a population of approximately 5.5 million inhabitants. A total of 322 BRCA1 and BRCA2 positive families were identified with 64% BRCA1 families and 36% BRCA2 families. This was a slightly higher frequency of BRCA2 mutations compared to Norway and Poland Citation[7], Citation[9] but the results were comparable to Sweden Citation[10].
If a BRCA1 or BRCA2 mutation is identified in a family other family members can be offered predictive testing for the mutation. Our study revealed that on average 1.25 individuals were tested positive pr. family. We did not have information about the family members who tested negative for the familial mutation, but if we anticipate that this number is equal to the number of mutation positive individuals this results in an average number of 2.5 persons pr. family who ask for predictive genetic testing. The utilization of predictive testing did not differ significantly between BRCA1 and BRCA2 families, but female relatives requested predictive testing more often than male relatives. The ratio of females versus males requesting predictive testing was 3.0 in BRCA1 families and 4.7 in BRCA2 families. It is not surprising that women request predictive testing for BRCA1 and BRCA2 mutations more often than men because the increased cancer risks are primarily female related cancers, but this trend might change as we become more aware of an increased risk for prostate cancer in BRCA2 male carries Citation[11]. Furthermore, the utilization of predictive genetic testing is closely correlated to preventive interventions which are not yet part of a routine clinical setting for male carriers concerning prostate cancer. There were relatively more females than males who tested positive for the familial BRCA2 mutation compared to BRCA1. This difference could be explained by a higher number of living female family members in BRCA2 families caused by a better survival among BRCA2 breast cancer cases and a lower risk of ovarian cancer. It could also just be caused by chance due to a limited number of BRCA2 families, and the difference was not statistically significant.
The exonic location and the type of mutations in BRCA1 and BRCA2 did not differ from other studies as most of the mutations were located in the large exon 11 in BRCA1 and exons 10 and 11 in BRCA2. Most of the mutations were either frame shift or nonsense mutations, and large genomic rearrangements were rare Citation[12]. Two missense mutations in BRCA1 were reported several times in Danish HBOC families, namely 5208G > T (C1697R) and 5332G > A (G1738E), and most often these families were from West Denmark. These missense mutations have been shown to be most likely pathogenic by functional assays Citation[3], Citation[4]. However, in general it is difficult to interpret missense mutations concerning pathogenecity because of lack of functional assays.
There was a tendency towards founder BRCA1 and BRCA2 mutations in Danish HBOC families, but most mutations only occurred in one family. The most common BRCA1 mutations were 2594delC, 3438G > T, 5382insC, and 3829delT (). The three most common BRCA2 mutations were 6601delA, 1538del4, and 6714del4 (). The 2594delC BRCA1 mutation has been reported from Sweden, Norway and Western Europe (http://research.nhgri.nih.gov/bic/), and the 3438G > T BRCA1 mutation is also prevalent in Norway. The 5382insC BRCA1 mutation is one of the Ashkenazi Jewish founder mutations with a high frequency in many populations worldwide including Europe. Two of the recurrent BRCA2 mutations (1538del4 and 6714del4) have also been observed in many other populations whereas the 6601delA BRCA2 mutation is less frequent. It has been reported five times in the BIC database, once by our group and four times by Myriad, where no information about patients was available.
There was a slight tendency towards a higher frequency of BRCA2 families in West Denmark compared to East Denmark. Of the 116 BRCA2 families 56% were from West and 44% from East Denmark whereas there was an equal distribution of the 206 BRCA1 families. We do not have the clinical data of the families so it is not possible to evaluate whether different criteria for selection of families for mutation analysis was used.
There was a slightly different distribution of the specific BRCA1 and BRCA2 mutations in the two regions of Denmark (). Four of the BRCA1 mutations with an increased frequency in West Denmark have all been reported in Norway (2594delC, 3438G > T), Sweden (2594delC, 2595delA, 3829delT) and Western Europe (3829delT). The 3993delT mutation is not listed in BIC. The missense mutation 5208G > T (C1697R) has been reported in BIC twice, and both times from Denmark (once by our group), and the second missense mutation 5332G > A (G1738E) has been reported three times by Myriad. One of these reports by Myriad states that the studied family was of Western European ethnicity.
Two of the BRCA1 mutations with an increased frequency in East Denmark are the Ashkenazi mutations 185delAG and 5382insC, which have been reported worldwide especially from Europe and the Baltic countries. The BRCA1 mutation 234T > G is also frequent in East Denmark and this mutation is located in the RING domain in exon 3 of BRCA1 where other mutations have been shown to be pathogenic. These mutations are T > C and T > A (instead of T > G) and both have been reported from many European countries in BIC. The 249T > A mutation has only been reported once in BIC by Myriad and no clinical data was available. The 3172ins5 mutation has been reported from Western Europe.
In BRCA2 three mutations occurred more frequently in West Denmark (). The 3058A > T mutation has been reported from Denmark and Sweden in BIC, but also from Myriad with Western European and also African American ethnicity. The 6601delA mutation has only been reported in BIC by Myriad except once by our group and no information about the tested persons was available from Myriad. The 6714del4 mutation has been reported from United Kingdom, Australia and Italy. Two of the mutations with an increased frequency in East Denmark (1538del4, 4075delGT) have been reported from many countries in Western Europe. The 5982delTA mutation is not listed in BIC, and the 9243delAT mutation has only been reported once in BIC from Denmark Citation[13].
In conclusion, the presence and frequency of the Danish BRCA1 and BRCA2 mutations are comparable with observations from other Scandinavian and European studies and indicate that the Danish BRCA1 and BRCA2 mutations are a combination of Scandinavian (South Swedish and Norwegian) mutations and other European mutations including one of the Ashkenazi mutations. These observations are in agreement with the Gothic-Germany ancestry of the Danes with a close relation to the other Scandinavian countries.
Even though a tendency towards founder mutations was observed most mutations were only detected in one family. Based on these observations we recommend that the mutation screening strategy of the BRCA1 and BRCA2 genes in Danish HBOC families comprises a full screening of both genes including analysis for large genomic rearrangements.
Acknowledgements
All the Departments of Clinical Genetics and Oncology are greatly acknowledged for collaboration. All the families are thanked for contributing to this study.
References
- Gerdes AM, Crüger DG, Thomassen M, Kruse TA. Evaluation of two different models to predict BRCA1 and BRCA2 mutations in a cohort of Danish hereditary breast and/or ovarian cancer families. Clin Genet 2006; 69: 171–8
- Thomassen M, Kruse TA, Jensen PK, Gerdes AM. A missense mutation in exon 13 in BRCA2, c.7235G > A, results in skipping of exon 13. Genet Test 2006; 10: 116–20
- Vallon-Christersson J, Cayanan C, Haraldsson K, Loman N, Bergthorsson JT, Brondum-Nielsen K, et al. Functional analysis of BRCA1 C-terminal missense mutations identified in breast and ovarian cancer families. Hum Mol Genet 2001; 10: 353–60
- Carvalho MA, Marsillac SM, Karchin R, Manoukian S, Grist S, Swaby RF, et al. Determination of cancer risk associated with germ line BRCA1 missense variants by functional analysis. Cancer Res 2007; 67: 1494–501
- Phelan CM, Dapic V, Tice B, Favis R, Kwan E, Barany F, et al. Classification of BRCA1 missense variants of unknown clinical significance. J Med Genet 2005; 42: 138–46
- Simard J, Dumont M, Moisan AM, Gaborieau V, Malouin H, Durocher F, et al. Evaluation of BRCA1 and BRCA2 mutation prevalence, risk prediction models and a multistep testing approach in French-Canadian families with high risk of breast and ovarian cancer. J Med Genet 2007; 44: 107–21
- Borg A, Dorum A, Heimdal K, Maehle L, Hovig E, Moller P. BRCA1 1675delA and 1135insA account for one third of Norwegian familial breast-ovarian cancer and are associated with later disease onset than less frequent mutations. Dis Markers 1999; 15: 79–84
- Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 1999; 91: 1241–7
- Gorski B, Jakubowska A, Huzarski T, Byrski T, Gronwald J, Grzybowska E, et al. A high proportion of founder BRCA1 mutations in Polish breast cancer families. Int J Cancer 2004; 110: 683–6
- Hakansson S, Johannsson O, Johansson U, Sellberg G, Loman N, Gerdes AM, et al. Moderate frequency of BRCA1 and BRCA2 germ-line mutations in Scandinavian familial breast cancer. Am J Hum Genet 1997; 60: 1068–78
- van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J Med Genet 2005; 42: 711–9
- Thomassen M, Gerdes AM, Crüger D, Kruse TA. Low frequency of large genomic rearrangements of BRCA1 and BRCA2 in Western Denmark. Cancer Genet Cytogenet 2006; 168: 168–71
- Bergthorsson J, Ejlertsen B, Olsen JH, Borg A, Nielsen KV, Barkardottir RB, et al. BRCA1 and BRCA2 mutation status and cancer family history of Danish women affected with multifocal or bilateral breast cancer at a young age. J Med Genet 2001; 38: 361–8