Abstract
From January 1, 1990 to December 31, 1994, DBCG conducted a randomised trial in 1 615 postmenopausal women with operable, high-risk, receptor-positive or -unknown breast cancer. The patients were after surgery randomised to Tamoxifen for 1 year (TAM1), Tamoxifen for 2 years (TAM 2) or Tamoxifen for 6 months followed by megestrol acetate for 6 months (TAM/MA). When the preplanned sample size of 1 500 patients was reached it was decided to continue randomisation to TAM1 or TAM2 and the study was finally closed December 31, 1996. With a median follow-up of more than 10 years, there was no difference in disease-free survival (DFS) or overall survival (OS) among the three treatment arms. Similar results were obtained in the original and extended comparisons of Tamoxifen for 1 versus 2 years. A multivariate analysis in the per-protocol treated patients did not show significant differences in hazard ratios for DFS or OS among the three arms. Side-effects were rare but more common in the TAM2 and TAM/MA arms.
The Danish Breast Cancer Cooperative Group (DBCG) 77c trial compared Tamoxifen for 1 year to no further treatment in postmenopausal patients with high-risk operable breast cancer. A significant improvement of disease-free survival and a reduction in mortality in favour of the Tamoxifen group was observed Citation[1]. A similar study performed by NATO showed Tamoxifen for 2 years to be superior to control Citation[2], and the Scottish trial found 5 years of Tamoxifen superior to control Citation[3]. In advanced disease, second-line treatment with gestagens after progression on Tamoxifen was well known to be of benefit Citation[4], and in a randomised study, sequential therapy with Tamoxifen and medroxyprogesterone actetate was superior to Tamoxifen alone Citation[5]. Based on these data, DBCG decided to perform a randomised three-armed study, in which Tamoxifen for 1 year (TAM1) was compared with Tamoxifen for 2 years (TAM2) and Tamoxifen for 6 months followed by megestrol acetate for 6 months (TAM/MA).
Patients and methods
The DBCG prepared the original protocol (DBCG trial 89C). This open-label, randomized, phase III trial involved centres nation-wide. The study was conducted in accordance with the Helsinki declaration and was approved by the regional ethical committees. Informed consent was obtained before randomisation. Patients were randomized and data collected by the DBCG data centre and subsequently accumulated by the DBCG Registry. The DBCG Data Centre undertook central review, queries, and analysis of data.
Patients
The study included women <75 years of age with completely resected, unilateral, invasive carcinoma of the breast. Eligible patients had distant metastases excluded by physical examination, chest radiography, and blood tests. Further imaging was done in case of symptoms or signs, i.e. elevated ALAT/ASAT, elevated alkaline phosphatase, elevated calcium, bone pain etc. Breast-conserving surgery or mastectomy, both in combination with lower axillary clearance (level I and part of level II) was required. The patients had to be postmenopausal and to have a hormone receptor-positive (ER and/or PgR) or -unknown tumour. The patients were required to be high-risk defined as either having axillary lymph node metastases or tumours with a size larger than 5 cm. A patient was classified as postmenopausal if she had undergone an oophorectomi, was amenorrhoic for more than 12 months, had amenorrhea for 2–12 months and FSH in the postmenopausal range, or was more than 50 years of age in the case of hysterectomy.
Pathological procedures
Classification of histological type and grade (ductal carcinomas) was done according to WHO. Examination of tumour margins, invasion of skin or deep fascia, measurement of gross tumour size, the number of lymph nodes identified and the number of metastatic nodes was mandatory. ER and PgR were analyzed using immunohistochemical assays (IHC) or dextran-coated charcoal assays (DCC). Receptor-positive was defined as ≥10% positive tumour cells or ≥10 fmol of receptor per mg of cytosol protein. The laboratories participated in the EORTC quality control program for DCC or the DBCG quality control program for IHC.
Treatment
Patients were assigned to either Tamoxifen 30 mg once daily for 1 year, Tamoxifen 30 mg once daily for 2 years, or Tamoxifen 30 mg once daily for 6 months followed by megestrol acetate 160 mg once daily for 6 months. Loco-regional radiotherapy was administered according to national guidelines to all patients after lumpectomy, to patients with involvement of deep fascia after mastectomy, and to women ≤45 years of age with four or more involved lymph nodes. Radiotherapy was given concomitantly with Tamoxifen. Chemotherapy was not permitted.
Follow-up
Symptoms, side-effects, and findings on clinical examination were recorded every 3 months during the first year, every 6 months during the second through fifth year, and then annually to a total of 10 years. Additional biochemical tests and imaging examinations were done when indicated by symptoms or signs. A complete follow-up on vital status was obtained for all patients through linkage to the Danish civic registry. The last follow-up was July 1, 2007.
Study design
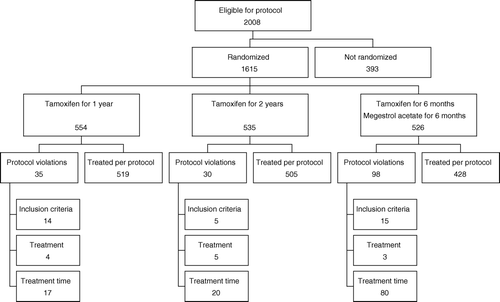
The trial was planned as an open randomised study with equal number of patients in the three treatment arms. The pre-planned sample size was 1 500 patients in 5 years, with an additional five-year follow-up. Assuming a five-year disease-free survival in the control group of 50%, the study was designed to detect a 20% improvement in DFS with a power of 90%. The study was open from January 1, 1990 to December 31, 1994 and recruited 1 615 patients nationwide. An interim analysis at that time showed that TAM/MA could not gain superiority compared to TAM1, and in addition more side effects were reported on megestrol acetate than on Tamoxifen. Patients already on treatment in the sequential arm was continued on or changed to Tamoxifen for a total treatment time of 1 year. This caused a relatively larger number of protocol violations in the TAM/MA arm. The trial profile is shown in . The study was extended and continued to randomise between TAM1 and TAM2 until December 31, 1996 when the results of the Swedish 2 versus 5 years Tamoxifen trial was published Citation[6]. Patients on treatment were advised to continue Tamoxifen for 5 years. This means that the intention to treat analysis of the study extension was highly biased and the primary analysis of efficacy in this part of the study was therefore performed on both an ITT (n=1 795) and a per-protocol population (n=1 304).
Statistical methods
Two endpoints were considered: overall survival (OS) and disease free survival (DFS). OS was defined as the elapsed time from randomization until death from any cause. Death was an event and a patient being alive at the end of follow-up was censored. Because of the linkage to the Danish CPR-register (Danish Civil Registration System), no patients were lost to follow-up in relation to OS.
DFS was defined as the time from randomization to an event or censoring. An event was defined as relapse (local-regional or distant), contralateral breast cancer, second malignancy, or death, whichever came first. A censoring was defined as ‘lost to follow-up. Follow-up time was quantified in terms of a Kaplan-Meier estimate of potential follow-up. Time to event for OS and DFS respectively, was described by Kaplan-Meier curves, and the stratified log-rank test was the primary method to compare the effect of the 3 treatment regimes in all patients randomised until December 31, 1994. Additionally the two regimes, which were continued until December 31, 1996, were compared in all patients randomised to these two regimes and treated according to the protocol.
The multivariate Cox proportional hazards model was used to adjust the observed treatment effect for the influence of various prognostic factors, and to detect the effect of these factors on the outcome. Factors included in the multivariate analyses were treatment (TAM1, TAM2, TAM/MA), age (≤40–59, 60–69, ≥70), tumour size (≤20 mm, 21–50 mm, >50 mm), nodal status (0, 1–3, ≥4), malignancy grade in ductal carcinomas (grade I, grade II, grade III, unknown) and surgery (mastectomy, lumpectomy).The assumptions of proportional hazards were assessed by log(-log) S plots, Schoenfeld residuals, and by including in the model a time-dependent component for each factor at a time. The effects are described by the estimated hazard ratios and tested by a maximum likelihood test (χ2 test).
The distribution of end-point event, the frequency of patients stopping the treatment and the reason for stopping, and the frequency of side effects were compared between treatment groups by using χ2 test or Fisher's exact test. P-values are two-tailed. Statistical analyses were done with the SAS 8.2 program package.
Results
The distribution of the demographic data was well balanced between the treatment groups. Ninety-eight percent of the patients had node-positive disease, more than 50% had tumours larger than 20 mm, and 86% underwent mastectomy (). Steroid receptor analysis was mainly by DCC (60%), but also by IHC in frozen tissue (17%), or in paraffin-embedded tissue (10%). Receptors were not determined in the remainder of the patients. The distribution of methods for determination of receptors was evenly distributed among the groups (data not shown).
Table I. Base-line characteristics of the ITT population (n=1 615)
Study outcome
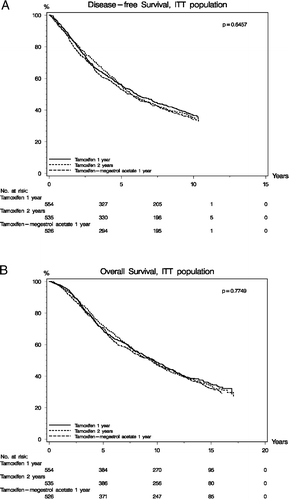
The analysis was conducted 10 years after closure of randomisation with a last follow-up as of July 1, 2007. Median estimated potential follow-up was 10 years for DFS as the patients stopped routine follow-up after 10 years, and 14.7 years for OS as survival data were available for all patients in the Danish civic registry per July 1, 2007. Recurrence, other malignancy including contralateral breast cancer, and any death prior to recurrence was all considered DFS events. Death from any cause was considered an OS event. A total of 972 first events were observed, 324 in the TAM1 group, 327 in the TAM2 group, and 321 in the TAM/MA group (). As the patients went off-study at 10 years, the table shows the distribution of first events within this period. We did not find statistically significant differences among the three treatment arms for any of the main endpoints nor in the death rate in the first 10 years or per July 1, 2007, but the were significantly more other malignancy in the TAM2 and TAM/MA arms than in the control arm (small numbers). panel A shows the disease-free survival in the intention-to-treat population. The absolute 10-year disease-free survival was around 40%, and the difference between the treatment arms was not statistically significant (p = 0.65). panel B shows overall survival with no statistically significant difference between the three arms (p = 0.77). A supportive multivariate analysis evaluated the three treatment arms in the per-protocol treated patients. The prognostic factors included in the Cox model were treatment, age, type of surgery, grade, and tumour size. The analysis was stratified according to lymph-node status, as this factor did not fulfil proportionality assumptions. shows the results. No statistically significant differences were found comparing the three treatments, TAM1, TAM2, or TAM/MA with respect to DFS (p = 0.47) and OS (p = 0.74). The hazard ratio for DFS and OS varied as expected among the known prognostic factors: age, tumour size, and malignancy grade. A corresponding ITT analysis of patients randomised to TAM1 vs. TAM2 in the combined early and extended study period (n = 908 vs. 887) did not reveal differences in DFS (p = 0.10) or OS (p = 0.28), and the corresponding multivariate analysis in the per-protocol treated TAM1 and TAM2 patients (n = 690 vs. 614) found no differences in DFS (p = 0.16) or OS (p = 0.37) among the two groups (data not shown).
Table II. End-point events
Table III. Multivariate analysis of the per-protocol population (n = 1 452)
Toxicity
One hundred and thirty-nine patients stopped their allocated treatment and the reasons for this are shown in . Significantly more patients stopped treatment in TAM2 and TAM/MA compared to TAM1 (p < 0.002) The main reason for stopping was side-effects, and in addition a number of patients stopped treatment at their own request in all three arms. The reasons for stopping treatment was significantly related to treatment (p = 0.04). The higher frequency of side-effects reported in the TAM2 arm compared to the TAM1 arm was probably related to the longer time on treatment. Toxicity grading was not required in the study, and shows the frequency of reported side effects (any grade) versus allocated treatment. The data shows that relatively few side-effects were reported. The most common was vasomotor symptoms, depression, and nausea. There were statistically significant differences among the three treatment arms with vasomotor symptoms, nausea, depression, weight gain, fluid retention, and dyspnoea reported more frequently in the TAM2 and TAM/MA arms than in TAM1.
Table IV. Reasons for stopping treatment
Table V. Side-effects
Discussion
The DBCG 89C trial is an early example of an adjuvant trial exploring the possible benefits of sequential endocrine therapies with dissimilar mechanism of action in patients with early and hormone responsive breast cancer. This approach has later turned out to be beneficial in the IES and ABCSG/ARNO trials with Tamoxifen followed by an aromatase inhibitor Citation[7], Citation[8]. Megestrol acetate was chosen because it is effective in advanced breast cancer after Tamoxifen failure with second-line response rates of 12–16% Citation[9], Citation[10], and a cyclic approach using Tamoxifen and megestrol acetate seems to be superior to Tamoxifen alone in advanced breast cancer Citation[5]. In the adjuvant setting, Fjøsne randomised 489 patients to 2 years of Tamoxifen or 2 years of cyclic Tamoxifen and megestrol acetate, and found similar DFS and OS in the two arms Citation[11]. In our study the sequential arm was not superior to the two Tamoxifen-alone arms. A poor or even detrimental effect of megestrol acetate cannot be ruled out, but the lack of superiority of the sequential arm in the present study is likely to be caused by a too short duration of Tamoxifen treatment though. This is supported by substantial data showing that Tamoxifen for 1 year is superior to control Citation[12], Tamoxifen for 2 years likewise Citation[2], and that Tamoxifen for 5 years is superior to 2 years Citation[6]. The patients in the sequential arm received Tamoxifen for 6 months, and in a similar study, Focan et al. found medroxyprogesterone acetate alone for 9 months to be inferior to Tamoxifen for 5 years Citation[13]. Thus, the evidence seems to suggest that megestrol acetate as given in this study cannot enhance the effect of adjuvant Tamoxifen.
The present study did not demonstrate an advantage of prescribing Tamoxifen for 2 years compared to 1 year. Considering the estimated proportional reductions in trials of Tamoxifen for 1 year, 2 years, and about 5 years of 18%, 25%, and 42% for disease-free recurrence Citation[14], an increment of 7% between TAM1 and TAM2 would not be expected to show up in the present study which was not powered to pick up a difference of this magnitude. The observed 10-year disease-free survival of 40% in the two Tamoxifen arms was superior to the 30% observed in the previous DBCG 77c studyCitation[12], and comparable to the node-positive subset in the 1992 oxford overview Citation[15]. The present study included a number of receptor-unknown patients, of whom 20% can be estimated to be receptor-negative. This may have contributed to dilute the difference between TAM1 and TAM2.
Toxicity was relatively uncommon in this study. The most common side-effect was vasomotor symptoms reported in 1–4% of the patients across the three treatment groups. Fisher et al. reported a 64% frequency of hot flashes in the NSABP five vs. more than five years Tamoxifen trial Citation[16]. In a more recent Tamoxifen trial, Coombes found a 38% hot flashes rate Citation[7]. This indicates that side effects were underreported in the present study. Most side-effects were nevertheless more common in the TAM/MA arm compared to the TAM1 arm, and in view of the efficacy data, the decision only to extend recruitment onto the two Tamoxifen-alone arms was well justified.
In conclusion the present study failed to find a 1-year sequential approach with Tamoxifen followed by megestrol acetate superior to Tamoxifen alone for 1 or 2 years.
Acknowledgements
Preliminary data from this study has in part been presented as a poster at the First European breast cancer conference, Firenze, Italy, 1998.
The following Danish institutions participated in the study: DBCG Registry, Copenhagen: Susanne Møller, M.Sc.; Aalborg Hospital, Århus University Hospital, Aalborg: Marianne Ewertz, MD; Århus University Hospital, Århus: Jørn Andersen, MD; Odense University Hospital, Odense: Søren Cold, MD; Esbjerg County Hospital, Esbjerg: Brita Bjerregaard, MD; Herlev University Hospital, Herlev: Dorte L. Nielsen, MD, Herning Hospital, Herning: Knud Aage Møller, MD; Hillerød Hospital, Hillerød: Lene Adrian, MD; Næstved Hospital, Næstved: Preben Philip, MD; Rigshospitalet, Copenhagen: Bent Ejlertsen, MD; Roskilde Hospital, Roskilde: Peter Grundtvig, MD; Rønne Hospital, Rønne: Ditte Nielsen, MD; Sønderborg Hospital, Sønderborg: Ebbe Lindegaard-Madsen, MD; Vejle Hospital, Vejle: Erik H. Jakobsen, MD; Viborg Hospital, Viborg: Vera Haahr, MD.
References
- Mouridsen HT, Rose C, Overgaard M, Dombernowsky P, Panduro J, Thorpe S, et al. Adjuvant treatment of postmenopausal patients with high risk primary breast cancer. Results from the Danish adjuvant trials DBCG 77 C and DBCG 82 C. Acta Oncol 1988; 27: 699–705
- Anonymous. Controlled trial of Tamoxifen as single adjuvant agent in management of early breast cancer. Analysis at six years by Nolvadex Adjuvant Trial Organisation. Lancet 1985; 1(8433)836–40
- Anonymous. Adjuvant Tamoxifen in the management of operable breast cancer: The Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh. Lancet 1987; 2(8552)171–5
- Rose C, Mouridsen HT. Endocrine therapy of advanced breast cancer. Acta Oncol 1988; 27: 721–8
- Gundersen S, Kvinnsland S, Lundgren S, Klepp O, Lund E, Bormer O, et al. Cyclical use of Tamoxifen and high-dose medroxyprogesterone acetate in advanced estrogen receptor positive breast cancer. Breast Cancer Res Treat 1990; 17: 45–50
- Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant Tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 1996; 88: 1543–9
- Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. Survival and safety of exemestane versus Tamoxifen after 2-3 years' Tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet 2007; 369(9561)559–70
- Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant Tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005; 366(9484)455–62
- Kaufmann M, Bajetta E, Dirix LY, Fein LE, Jones SE, Zilembo N, et al. Exemestane is superior to megestrol acetate after Tamoxifen failure in postmenopausal women with advanced breast cancer: Results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol 2000; 18: 1399–411
- Dombernowsky P, Smith I, Falkson G, Leonard R, Panasci L, Bellmunt J, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: Double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol 1998; 16: 453–61
- Fjosne HE, Jacobsen AB, Lundgren S. Adjuvant cyclic Tamoxifen and Megestrol acetate treatment in postmenopausal breast cancer patients—Long-term follow-up. Eur J Surg Oncol 2007.
- Rose C, Andersen JA, Andersen KW, Axelsson CK, Blichert-Toft M, Dombernowsky P, et al. Adjuvant endocrine treatment of postmenopausal patients with breast cancer with high risk of recurrence. 5. Results from the DBCG (Danish Breast Cancer Cooperative Group) 77C randomized trial. Ugeskr Laeger 1991; 153: 2283–7
- Focan C, Beauduin M, Majois F, Canon JL, Cusumano G, Focan-Henrard D, et al. High-dose oral medroxyprogesterone acetate or Tamoxifen as adjuvant hormone therapy for node-negative early-stage breast cancer: Randomized trial with 7-year update. Clin Breast Cancer 2004; 5: 136–41
- Tamoxifen for early breast cancer. Cochrane Database Syst Rev 2001; 1:CD000486.
- Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 1992; 339(8784)1–15
- Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, et al. Five versus more than five years of Tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 1996; 88: 1529–42