Abstract
Improvement of the management of breast cancer patients has high priority. In this regard, prognostic stratification needs to be improved in order to ensure proper medical treatment of all patients and furthermore predictors of response to chemotherapy are urgently needed. As new treatment opportunities emerge in the future this need will continue to grow. Thus, the search for molecular markers of prognosis and prediction is ongoing. Tissue Inhibitor of Metalloproteinases-1 (TIMP-1) has been suggested as a marker of both prognosis and response to treatment. Several studies have demonstrated the association between TIMP-1 and prognosis in breast cancer and new studies within this area have focused on the possibility of using blood samples or paraffin embedded tissue instead of tumor tissue extracts for measurements of TIMP-1. Interestingly, recent studies have investigated the association between TIMP-1 and response to treatment showing that TIMP-1 may also carry predictive information on response to treatment. In this regard, results from studies of the molecular functions of TIMP-1 point to a role of TIMP-1 in the inhibition of tumor cell apoptosis as an explanation for the clinical findings. This review gives an update on the ongoing investigation of the potential role of TIMP-1 as a tumor marker in breast cancer. Furthermore, we link the clinical findings with studies of the molecular actions of the TIMP-1 protein, raising hypotheses that may explain why TIMP-1 could play an important role in future management of breast cancer patients.
Being the most common type of cancer among women in the western part of the world, breast cancer is the subject of much research and debate. With the aim of improving management of breast cancer patients, areas of particular interest to many clinicians and researchers are prognostic stratification and prediction of response to therapy.
Today, prognosis is determined by a number of clinical and biological parameters currently including lymph node status, tumor size, grade of malignancy, age, absence or presence of peritumoral vascular invasion and HER2/neu gene expression Citation[1]. Using these parameters, patients are allocated to one of three risk groups (low-, intermediate- and high risk, respectively) and adjuvant therapy is planned according to this. However, it is becoming increasingly clear that the currently available prognostic parameters are relatively inadequate to precisely define the prognosis of individual patients and therefore a substantial proportion of breast cancer patients are offered adjuvant therapy although they are cured by surgery alone Citation[2]. Also, some patients do not receive adjuvant therapy although their tumors later prove to recur. Thus, additional prognostic markers need to be identified.
In addition to estimating risk of relapse, there is a need to develop biomarkers that can be used to predict which tumors are responsive to specific systemic treatments. Today, the only predictive markers available to guide breast cancer treatment are the presence of estrogen- and progesterone receptors (ER and PR) in the tumors, which are used to select patients to receive endocrine therapy, and expression of HER-2/neu for selection of patients to receive trastuzumab (Herceptin®) Citation[1], Citation[3]. At present, there are no markers routinely to be used to predict response to chemotherapy in its broad sense or to specific types of chemotherapy. In addition, a large proportion of patients with ER/PR positive and HER-2/neu positive tumors do not benefit from therapy with endocrine agents or trastuzumab, respectively, which further supports the need to identify more precise predictive markers Citation[4].
One molecule that has been extensively studied as a possible new biomarker in breast cancer is Tissue Inhibitor of Metalloproteinases-1 (TIMP-1). TIMP-1 is one of four naturally occurring inhibitors of the matrix metalloproteinases (MMPs), the latter being enzymes playing important roles in both normal physiological processes as well as pathological conditions of tissue remodelling Citation[5], Citation[6]. In recent years, novel functions of TIMP-1 have been demonstrated. These functions include several cancer promoting effects such as stimulation of growth Citation[7], Citation[8] and inhibition of apoptosis Citation[9–14]. In accordance with the recent findings of a tumor promoting function of TIMP-1, several studies have shown that high tumor tissue extract levels of TIMP-1 are associated with a poor prognosis of breast cancer patients Citation[15–18] and based on these studies TIMP-1 has been proposed as a new prognostic marker in this disease. However, since the use of tumor tissue extracts as a specimen in biomarker studies has been highly criticized because of the heterogeneity of this material and because of the increasing difficulty in acquiring freshly frozen tumor material, more recent studies have aimed at investigating the prognostic value of TIMP-1 in blood, i.e. plasma and serum. The first results from these studies have now emerged and seem promising Citation[19–22]. An alternative to blood would be to investigate whether scoring of TIMP-1 in paraffin embedded tissue carries prognostic information as this specimen type is more readily available from individual patients. This is also an area which researchers currently focus on and preliminary data on the subject will be presented in this review Citation[20], Citation[23], Citation[24]. Interestingly, high tumor tissue and plasma or serum levels of TIMP-1 have recently also been associated with a poor response to chemotherapy and endocrine treatment indicating that besides being associated with prognosis TIMP-1 may also carry predictive information Citation[25–27]. A close association between TIMP-1 levels and tumor cell sensitivity to treatment is supported by recent in vitro cell system studies Citation[28], Citation[29].
Taken together, there is now a considerable amount of data pointing to a potential role for TIMP-1 in the management of breast cancer patients. TIMP-1 appears to be a potential prognostic as well as predictive marker and this review will systematically summarize published data in these areas and link them with biological aspects.
TIMP-1 as a prognostic marker
As mentioned in the introduction of this review, several studies have investigated the association between TIMP-1 and prognosis in breast cancer. summarizes the published articles on this subject; these were identified by searching the PubMed Database using the words “TIMP-1 and prognosis in breast cancer”. Only articles, which were clearly outside the area of interest, were excluded. Besides aspects of study design, main conclusions of the studies are listed. Although many investigators perform survival analyses not all do and therefore hazard ratios are not listed in the table. We thus refer to original references for details.
Table I. Overview of published articles studying the association between TIMP-1 and prognosis. All articles were identified via PubMed using the search criteria described in the text.
In general, high levels of TIMP-1 are associated with a poor prognosis both at the mRNA and at the protein level (). There are few exceptions to this general finding as two studies show the opposite association, namely that high tumor levels of TIMP-1 are associated with a favourable prognosis Citation[24], Citation[30]. One of these is the study by Sieuwerts et al., which is one of very few studies investigating mRNA levels of TIMP-1 and the authors of this article point to the fact that several regulatory mechanisms determinative of protein amount and function are present at the posttranscriptional level Citation[30]. Thus, a substantial amount of data is present pointing to a potential role of TIMP-1 as a prognostic marker, however, probably only when studied at the protein level.
Looking at of this review it is clear that the studies of the association between TIMP-1 and prognosis are very different in size and design; TIMP-1 mRNA as well as protein has been studied, patient cohorts of different sizes have been included, different assays have been applied and the obtained data have been analyzed in various ways. It is a positive thing that studies being that different almost all point to the same association, namely that high levels of TIMP-1 are associated with a poor prognosis. This proves the robustness of the marker itself. However, for the potential clinical use of TIMP-1 we need more uniform studies. Recently, uniform recommendations for the design and reporting of prognostic tumor marker studies were published Citation[31] and future studies should adhere to these in order to facilitate evaluation and improve quality.
Several published studies, including the largest one, have been performed using extracts of fresh tumor tissue Citation[15–18], Citation[30]. The use of tumor tissue extracts in tumor marker studies has nevertheless been criticized because of the heterogeneity of this material. In addition, because of more sensitive diagnostic methods and thereby earlier detection of the disease, tumors of still smaller size are obtained from the patients making it increasingly difficult to obtain freshly frozen tumor samples. In this regard, blood-based markers would be of great importance because of the accessibility of this specimen type and therefore, newer clinical studies have been aimed at investigating the prognostic value of TIMP-1 measurements in blood, i.e. plasma and serum Citation[19–22]. In the first of these studies, performed by Talvensaari-Mattila et al., comprising 71 breast carcinoma patients, high serum TIMP-1 levels (>196 ng/ml) were significantly correlated with a poor relapse-free survival in univariate survival analysis of lymph node-negative patients. In multivariate analysis TIMP-1 was still significantly correlated with survival when including other prognostic parameters in the analysis Citation[19]. These results were later reproduced in another laboratory analyzing the same serum samples but using another ELISA platform. In the validation study the same association between high TIMP-1 serum levels and poor prognosis was demonstrated thereby further supporting the prognostic value of TIMP-1 in serum Citation[22]. A large independent validation study has recently been performed in our laboratory Citation[21]. This study comprised prospectively collected preoperative plasma and corresponding serum samples from 519 patients with primary breast cancer and it was shown that patients with high plasma TIMP-1 levels had a significantly shorter disease free survival compared with patients with lower plasma levels of TIMP-1. The same tendency applied to serum however the association was not statistically significant. In sub-group analysis of node-negative patients, TIMP-1 predicted prognosis when measured both in plasma and in serum. Interestingly, when analysing the subgroup of node-negative patients defined as low-risk patients, who currently are offered no adjuvant systemic therapy, both plasma and serum TIMP-1 predicted prognosis as it was shown that low-risk patients with high levels of TIMP-1 had a significantly worse disease-free survival when compared to patients with lower levels. Thus, based on this study it seems that plasma or serum TIMP-1 can further stratify this patient group and thereby be used to identify a group of patients within the low-risk group that may benefit from treatment in spite of their expected favourable prognosis. A recent study performed by Wu et al. also showed that high levels of serum TIMP-1 were associated with poor recurrence-free and overall survival Citation[20]. Importantly, this study also showed that serum levels of TIMP-1 correlated with tissue levels, determined by immunohistochemistry, and that immunohistochemical scoring of TIMP-1 was associated with prognosis, i.e. high expression levels of TIMP-1 were associated with significantly poorer overall survival. Similar findings have been obtained in our laboratory as we have shown that low-risk patients have significantly lower immunohistochemical TIMP-1 scores compared with high-risk patients (Bartels et al., unpublished observations). Although preliminary, these findings are of high importance as they suggest that immunohistochemical assessment of TIMP-1, using paraffin embedded tissue, may be useful in prognostic evaluation. As opposed to freshly frozen tumor tissue, which is used to make tumor tissue extracts, paraffin embedded tissue is readily available from all patients as this is used for the assessment of traditional prognostic and predictive markers
Regardless of the sample type chosen (fresh or paraffin-embedded tissue, plasma or serum), a number of technical issues, i.e. pre-analytical, analytical and post-analytical aspects, need to be addressed. In this regard, a number of pre-analytical issues concerning the use of blood, especially serum, in tumor marker studies have been discussed. In general, levels of TIMP-1 measured in serum are higher than the levels in corresponding plasma samples. This is believed to be caused by the fact that during the preparation of serum, platelets, which store TIMP-1 in their alpha-granules Citation[32], will be activated and disintegrated and may thus release TIMP-1 into the sample resulting in an increase in the level of the inhibitor. This was recently supported by a study by Lomholt et al. showing that contamination of plasma with platelets lead to increased concentrations of TIMP-1 as well as beta-thromboglobulin as a result of platelet degranulation Citation[33]. Thus, plasma has been suggested to be the best choice for TIMP-1 measurements Citation[34–36]. As described above, some studies have investigated TIMP-1 levels in serum and it should be thoroughly investigated whether associations found in these studies are real or if they are artefacts influenced by the coagulation process. Furthermore, if blood is going to be used in tumor marker measurements other important pre-analytical conditions, e.g. the impact on TIMP-1 concentrations of blood sampling procedures, diurnal variations and menstrual cycle need to be elucidated. Moreover, analytical issues, i.e. aspects related to measuring TIMP-1 in the samples, have been investigated for some ELISAs but should be validated for all TIMP-1 assays to be used in clinical material. Finally, post-analytical issues, e.g. reference intervals and cut-off values, need to be addressed.
In conclusion, the prognostic value of TIMP-1 in primary breast cancer when measured in tumor tissue extracts is well established. With the aim of bringing TIMP-1 closer to the clinical setting, research is currently focused on the identification of other more easily accessible specimens for TIMP-1 measurements. In this regard, blood and paraffin embedded tumor tissue seem to be promising candidates and future studies should be aimed at validating these two materials.
TIMP-1 as a predictive marker
Chemotherapy and endocrine therapy is widely used in the treatment of breast cancer. However, a large proportion of the patients does not benefit from the treatment but still suffer from the substantial side-effects caused by the drugs. The only currently available markers to be used as predictors of benefit from a specific treatment is the presence of ER and PR receptors for predicting endocrine responsiveness (i.e. treatment with tamoxifen and/or aromatase inhibitors) and HER-2/neu expression for predicting the benefit from trastuzumab Citation[1], Citation[3]. Thus, there is an urgent need to identify new predictive markers in breast cancer especially seen in the light of the continuous introduction of new drugs in the clinical setting. Furthermore, in the case of ER/PR and HER-2/neu, a substantial part of ER/PR- and HER-2/neu positive tumors remain resistant to endocrine therapy and trastuzumab, respectively. This indicates that there are limitations in predicting the efficacy of these treatments based on the currently available markers Citation[4].
TIMP-1 has for some years been known to play a role in inhibition of apoptosis. Since chemotherapy works by inducing apoptosis in cancer cells through damaging of DNA and activation of p53, a hypothesis could be raised that tumors having high levels of TIMP-1 are less sensitive to chemotherapeutic drugs as compared with tumors with no or low levels of TIMP-1. It could therefore be speculated that TIMP-1 may be useful as a predictive marker in breast cancer. Articles within this area are still few but those published support the hypothesis described above.
Articles describing the association of TIMP-1 with response to therapy were identified by searching the PubMed Database using the words “TIMP-1 and breast cancer” combined with “prediction” or “therapy response”. One article, which was not related to breast cancer, was excluded.
TIMP-1 and prediction of response to chemotherapy
To our knowledge, the association between the level of TIMP-1 and response to chemotherapy in breast cancer patients has been investigated in one clinical study Citation[25]. In a study performed in our laboratory, tumor tissue levels of TIMP-1 were measured in 173 samples from patients with metastatic breast cancer. This study showed that increasing TIMP-1 levels were significantly associated with lack of response to cyclophosphamide/methotrexate/5-fluorouracil and anthracycline-based chemotherapy (cyclophosphamide/epirubicin/5-fluorouracil, cyclophosphamide/adriamycin/5-fluorouracil, or single agent adriamycin). In multivariate analysis TIMP-1 remained significantly associated with response to therapy when including lymph node status, hormone receptor status, menopausal status, dominant metastases site, type of chemotherapy and disease-free interval. Similar results were recently obtained in colorectal cancer patients Citation[37].
These clinical findings are in agreement with results from recent in vitro studies, which have demonstrated an association between the TIMP-1 level and cell survival following treatment with chemotherapeutic drugs, and thus these studies may contribute to the molecular explanation of the clinical findings. Li et al. investigated the response of TIMP-1 transfected MCF10A cells to adriamycin and showed that cell survival was significantly enhanced in the cell lines over-expressing TIMP-1 following treatment with the drug Citation[29]. Likewise, in a study comprising TIMP-1 gene deficient fibrosarcoma cells from mouse lung tissue and their genetically identical wild type controls, our laboratory has demonstrated that TIMP-1 deficiency considerably increased tumor cell sensitivity to three types of chemotherapeutic drugs, i.e. etoposide, cytosar and vincristine Citation[28]. The increased sensitivity was shown to be the result of excessive apoptosis induced in the TIMP-1 gene deficient cells. In summary, these in vitro studies support the hypothesis that by preventing apoptosis TIMP-1 protects the cancer cells against chemotherapy-induced cell death.
The ability of TIMP-1 to inhibit apoptosis has been demonstrated in a number of different cell lines e.g. B cells, Burkitt's lymphoma cells, hepatic stellate cells, human breast epithelial cells and human breast carcinoma cells Citation[9–14], in mouse fibrosarcoma cells Citation[28], and in transgenic mice Citation[38]. Controversy still exists as to how TIMP-1 regulates apoptosis and both MMP-dependent Citation[14] and MMP-independent mechanisms Citation[11–13], Citation[29] have been proposed (reviewed in details elsewhere Citation[39], Citation[40]). Results from a number of recent studies indicate that TIMP-1 activates the focal adhesion kinase (FAK)/phosphatidylinositol-3 kinase (PI-3 kinase)/Akt/Bad/Bcl-XL/Bcl-2 survival signalling pathway thereby inhibiting apoptosis Citation[11], Citation[13], Citation[29]. For example, in the MCF10A breast epithelial cell line, over-expression of TIMP-1 was shown to induce constitutive activation of FAK through tyrosine phosphorylation Citation[11], Citation[29]. FAK has previously been shown to be upstream regulator of the PI-3 kinase leading to regulation of the Bcl-2 family members, a well-characterised signalling pathway leading to cell survival. Phosphorylated FAK associates with and thereby activates the PI-3 kinase, which in turn activates the Akt-kinase. Akt phosphorylates the protein Bad, which as a result is sequestered in the cytoplasm by the capture protein 14–3–3 and Bad can therefore no longer interact with and inhibit Bcl-2 and Bcl-XL. Bcl-2 and Bcl-XL are proteins situated in the mitochondrial membrane and when activated these anti-apoptotic proteins inhibit Bax thereby preventing the release of cytochrome c from the mitochondria. This in turn prevents activation of the caspases and accordingly prevents apoptosis. Thus, TIMP-1 may inhibit apoptosis by acting as a trophic factor initiating the survival pathway including FAK, PI-3 kinase, Akt and Bcl-2 family members, resulting in inhibition of caspase activation. In support of this model, several studies have demonstrated phosphorylation and activation by TIMP-1 of the PI-3 kinase, Akt and Bad as well as TIMP-1 induced up-regulation of expression of Bcl-XL and Bcl-2.
The existence of a signalling pathway possibly regulated by TIMP-1 indicates that a cell surface binding protein with affinity for TIMP-1 exists, which mediates the anti-apoptotic signal into the cell. Until quite recently such a receptor had not been identified but in 2006 Jung et al. identified, using yeast two-hybrid screening, CD63, a member of the tetraspanin family, as a TIMP-1 binding protein on the surface of the human breast epithelial cell line MCF10A Citation[41]. This interaction on the cell surface was validated by confocal microscopic analysis and co-localization studies by immunofluorescence staining, which also showed that CD63 and TIMP-1 form a complex with integrin β1. Down regulation of CD63 using small hairpin RNA resulted in lesser binding of TIMP-1 to the cell surface as well as abrogated the co-localization of TIMP-1 with integrin β1 significantly reducing the anti-apoptotic effect of TIMP-1 on the cells.
In summary, it has been shown that TIMP-1 inhibits apoptosis in a number of different cell types. Based on the results from several studies a model is proposed in which TIMP-1 inhibits apoptosis by binding to CD63 at the cell surface leading to activation of the CD63/integrin β1 complex and thereby initiation of the cell survival signalling pathway including FAK, PI-3 kinase, Akt, Bad and bcl-proteins, which in turn prevents the execution of apoptosis Citation[39], Citation[41]. This interaction with cell signalling could be responsible for the decreased sensitivity to apoptosis-inducing drugs observed in cells and tumors expressing high levels of TIMP-1 (). Future studies should be aimed at further confirming this relationship between TIMP-1 and apoptosis and then at exploiting this association therapeutically, e.g. by inactivating TIMP-1 before chemotherapeutical treatment.
Figure 1. Chemotherapy works by initiating a stress signal in the cell by damaging the DNA. This stress signal activates p53, which in turn induces apoptosis. Since TIMP-1 has been shown to inhibit apoptosis, probably by activating the cell survival signalling pathway involving FAK, PI-3 kinase, Akt and Bcl-proteins, a model is proposed in which TIMP-1 neutralizes the chemotherapeutic stress stimuli by inhibition of apoptosis. See the text for further details. Modified from Citation[47].
![Figure 1. Chemotherapy works by initiating a stress signal in the cell by damaging the DNA. This stress signal activates p53, which in turn induces apoptosis. Since TIMP-1 has been shown to inhibit apoptosis, probably by activating the cell survival signalling pathway involving FAK, PI-3 kinase, Akt and Bcl-proteins, a model is proposed in which TIMP-1 neutralizes the chemotherapeutic stress stimuli by inhibition of apoptosis. See the text for further details. Modified from Citation[47].](/cms/asset/48cc8e11-7c99-4f33-a308-d3635c775800/ionc_a_302465_f0001_b.gif)
TIMP-1 and prediction of response to endocrine therapy
The association between TIMP-1 and response to endocrine therapy has still not been investigated in detail. However, two clinical studies have revealed a correlation between elevated plasma or serum TIMP-1 levels and a decreased response to hormone therapy in metastatic breast cancer. In a study by Lipton et al., TIMP-1 levels were determined in pre-treatment EDTA plasma samples from 251 metastatic breast cancer patients. In these patients, increasing TIMP-1 levels predicted decreased response to 2nd-line hormone therapy with an aromatase inhibitor or with megestrol acetate Citation[27]. In another study performed by the same group, TIMP-1 was measured in pre-treatment serum from 522 patients and as in the previous study, these results showed that patients with elevated TIMP-1 levels had significantly reduced response to letrozole and tamoxifen Citation[26]. Thus, these clinical data suggest that TIMP-1 may be associated with response to endocrine therapy.
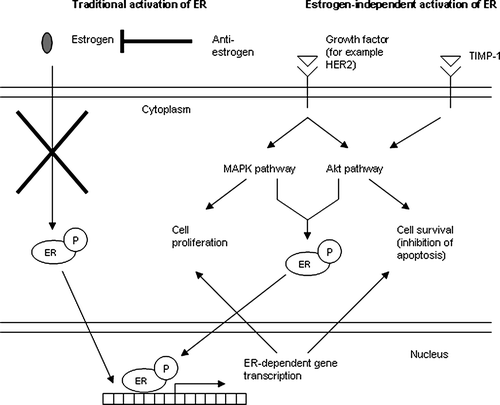
The molecular explanation for this apparent interference with response to endocrine therapy by TIMP-1 has not yet been elucidated. Future studies should both validate the clinical studies mentioned above as well as study the molecular basis for the clinical observations. However, it could be speculated that TIMP-1 interferes with ligand-independent signalling from the ER as described below.
Ligand-independent phosphorylation of ER at key positions has been shown to be elicited by members of the MAP-kinase pathway and by Akt, which is involved in the survival pathway described above. Ligand-independent phosphorylation of ER activates the receptor in the absence of estrogen and promotes ER dependent gene transcription resulting in stimulation of proliferation/cell survival Citation[42]. In support of an Akt-mediated activation of the ER signalling pathway, breast cancer cell lines with constitutively activated Akt have been demonstrated to be resistant to endocrine therapy with tamoxifen Citation[43]. Moreover, in clinical studies ER/PR-positive breast cancer patients with activated Akt had a significantly poorer response to tamoxifen compared with patients with no activated Akt Citation[44], Citation[45]. Blockage of Akt signalling by inhibition of mammalian target of rapamycin (mTOR), a downstream target of Akt, restores the sensitivity of MCF-7 breast cancer cells expressing a constitutively active Akt to tamoxifen Citation[46].
In several studies, TIMP-1 has been shown to activate the Akt survival pathway and it is thus reasonable to believe that high levels of TIMP-1 would be associated with a poor response to endocrine therapy because of an estrogen-independent activation of the ER. Because of the TIMP-1 mediated activation of the Akt pathway and thereby estrogen-independent activation of the ER signalling pathway, ER/PR-positive tumors would then become resistant to endocrine therapy. Therefore, it could be speculated that TIMP-1 and/or other molecules involved in the Akt-survival pathway used together with ER/PR would probably be more precise predictive markers compared with ER and PR used alone (). As mentioned earlier, resistance to endocrine therapy in patients with ER/PR expressing tumors is an actual clinical problem seen in approximately half of these patients, thus illustrating the need for improving prediction of response to endocrine treatment. Future studies should be aimed at testing other signal transduction inhibitors in combination with endocrine therapy.
Figure 2. Endocrine therapy, i.e. anti-estrogen treatment, prevents estrogen from binding ER and inhibits thereby estrogen/ER dependent transcription of ER-responsive genes such as genes coding for proteins stimulating cell proliferation/survival. By activating ER in a ligand-independent manner through Akt, TIMP-1 stimulates cell survival in spite of the anti-hormone treatment and thereby protects the cancer cell against endocrine therapy.
Conclusive remarks and future perspectives
As is evident from this review there has over the last couple of years been an extensive development in the knowledge concerning TIMP-1 and its use as a tumor marker in breast cancer.
First, the association between tumor tissue extract levels of TIMP-1 and prognosis is well established. It is noteworthy that an additive prognostic effect seems to exist between tumor tissue TIMP-1 levels and levels of Plasminogen Activator Inhibitor-1 (PAI-1), another proteinase inhibitor with highly validated prognostic value Citation[15]. Importantly, recent studies have suggested that the prognostic value of TIMP-1 also applies to TIMP-1 measurements in blood, i.e. plasma and serum. When using blood, sample collection and processing is much easier and the problem of tissue heterogeneity is circumvented and therefore identification of blood as a suitable specimen for tumor marker measurements brings the use of TIMP-1 as a prognostic marker closer to implementation into the clinical setting although further validation is still needed. It is important to emphasize that if blood is going to be used for TIMP-1 measurements several pre-analytical issues have to be addressed. In addition, immunohistochemical scoring of TIMP-1 appears to carry prognostic information and as paraffin embedded tissue is readily available from the patients, as opposed to freshly frozen tumor tissue, this is a highly important finding. Thus, the search for more favourable specimens for TIMP-1 tumor marker measurements is successfully progressing.
Second, since TIMP-1 has been shown to protect tumor cells from chemotherapy-induced apoptosis both in vitro and in clinical studies, TIMP-1 is a potential marker for prediction of response to chemotherapy in breast cancer. Preliminary data suggest that this is also the case concerning endocrine treatment. Future studies should be aimed at validating the clinical uses of TIMP-1 in larger and independent patient populations and these studies are currently ongoing in our laboratory (Klintman et al., unpublished observations). It would be of high clinical value if TIMP-1 could predict the effect of specific chemotherapeutic drugs thereby aiding in individualizing treatment. In addition, the fact that TIMP-1 plays a central role in protecting cancer cells against chemotherapy and, possibly, anti-hormone treatment suggests that inhibition of TIMP-1 represents a novel approach as a sensitising treatment before conventional therapy. Besides direct inhibition of TIMP-1 using antibodies, a possibility could be to inhibit CD63, the recently discovered TIMP-1 binding protein, thereby indirectly preventing the anti-apoptotic signal from entering the cells.
Acknowledgements
We thank the Danish Cancer Society, The Foundation of Clinical Experimental Cancer Research Especially Concerning Breast Cancer, Kai Lange and Gunhild Kai Lange's Foundation and Danish Centre For Translational Breast Cancer Research, for financial support.
References
- Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007; 18: 1133–44
- Thomssen C, Janicke F. Do we need better prognostic factors in node-negative breast cancer?. Pro Eur J Cancer 2000; 36: 293–8
- Goldhirsch A, Coates AS, Gelber RD, Glick JH, Thurlimann B, Senn HJ. First-select the target: Better choice of adjuvant treatments for breast cancer patients. Ann Oncol 2006; 17: 1772–6
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365((9472)):1687–717.
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 161–74
- Toi M, Ishigaki S, Tominaga T. Metalloproteinases and tissue inhibitors of metalloproteinases. Breast Cancer Res Treat 1998; 52: 113–24
- Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett 1992; 298: 29–32
- Luparello C, Avanzato G, Carella C, Pucci-Minafra I. Tissue inhibitor of metalloprotease (TIMP)-1 and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res Treat 1999; 54: 235–44
- Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, et al. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest 1998; 102: 2002–10
- Guedez L, Courtemanch L, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood 1998; 92: 1342–9
- Liu XW, Bernardo MM, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem 2003; 278: 40364–72
- Liu XW, Taube ME, Jung KK, Dong Z, Lee YJ, Roshy S, et al. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: A potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res 2005; 65: 898–906
- Lee SJ, Yoo HJ, Bae YS, Kim HJ, Lee ST. TIMP-1 inhibits apoptosis in breast carcinoma cells via a pathway involving pertussis toxin-sensitive G protein and c-Src. Biochem Biophys Res Commun 2003; 312: 1196–201
- Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: Implications for reversibility of liver fibrosis. J Biol Chem 2002; 277: 11069–76
- Schrohl AS, Christensen IJ, Pedersen AN, Jensen V, Mouridsen H, Murphy G, et al. Tumor tissue concentrations of the proteinase inhibitors tissue inhibitor of metalloproteinases-1 (TIMP-1) and Plasminogen Activator Inhibitor Type 1 (PAI-1) are complementary in determining prognosis in primary breast cancer. Mol Cell Proteomics 2003; 2: 164–72
- McCarthy K, Maguire T, McGreal G, McDermott E, O'Higgins N, Duffy MJ. High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int J Cancer 1999; 84: 44–8
- Schrohl AS, Holten-Andersen MN, Peters HA, Look MP, Meijer-van Gelder ME, Klijn JG, et al. Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res 2004; 10: 2289–98
- Ree, AH, Florenes, VA, Berg, JP, Maelandsmo, GM, Nesland, JM, Fodstad, O. High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res 1997;3:1623–8.
- Talvensaari-Mattila A, Turpeenniemi-Hujanen T. High preoperative serum TIMP-1 is a prognostic indicator for survival in breast carcinoma. Breast Cancer Res Treat 2005; 89: 29–34
- Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer 2008; 122: 2050–6
- Wurtz SO, Moller S, Mouridsen H, Hertel PB, Friis E, Brunner N. Plasma and serum levels of tissue inhibitor of Metalloproteinases-1 are associated with prognosis in node-negative breast cancer: A prospective study. Mol Cell Proteomics 2008; 7: 424–30
- Kuvaja P, Wurtz SO, Talvensaari-Mattila A, Brunner N, Paakko P, Turpeenniemi-Hujanen T. High serum TIMP-1 correlates with poor prognosis in breast carcinoma – A validation study. Cancer Biomark. 2007; 3: 293–300
- Nakopoulou L, Giannopoulou I, Stefanaki K, Panayotopoulou E, Tsirmpa I, Alexandrou P, et al. Enhanced mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in breast carcinomas is correlated with adverse prognosis. J Pathol 2002; 197: 307–13
- Nakopoulou L, Giannopoulou I, Lazaris AC, Alexandrou P, Tsirmpa I, Markaki S, et al. The favorable prognostic impact of tissue inhibitor of matrix metalloproteinases-1 protein overexpression in breast cancer cells. APMIS 2003; 111: 1027–36
- Schrohl AS, Meijer-van Gelder ME, Holten-Andersen MN, Christensen IJ, Look MP, Mouridsen HT, et al. Primary tumor levels of tissue inhibitor of metalloproteinases-1 are predictive of resistance to chemotherapy in patients with metastatic breast cancer. Clin Cancer Res 2006; 12: 7054–8
- Lipton, A, Leitzel, K, Chaudri-Ross, HA, Evans, DB, Ali, SM, Demers, L, et al. Elevated serum TIMP-1/HER-2 predicts decreased response and survival in metastatic breast cancer. Breast Cancer Research and Treatment 2007;106(S1):S84.
- Lipton A, Ali SM, Leitzel K, Demers L, Evans DB, Hamer P, et al. Elevated plasma tissue inhibitor of metalloproteinase-1 level predicts decreased response and survival in metastatic breast cancer. Cancer 2007; 109: 1933–9
- Davidsen ML, Wurtz SO, Romer MU, Sorensen NM., Johansen SK, Christensen IJ, et al. TIMP-1 gene deficiency increases tumour cell sensitivity to chemotherapy-induced apoptosis. Br J Cancer 2006; 95: 1114–20
- Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res 1999; 59: 6267–75
- Sieuwerts AM, Usher PA, Meijer-van Gelder ME, Timmermans M, Martens JWM, Brunner N, et al. Concentrations of TIMPI mRNA splice variants and TIMP-I protein are differentially associated with prognosis in primary breast cancer. Clin Chem 2007; 53: 1280–8
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97: 1180–4
- Cooper TW, Eisen AZ, Stricklin GP, Welgus HG. Platelet-derived collagenase inhibitor: Characterization and subcellular localization. Proc Natl Acad Sci USA 1985; 82: 2779–83
- Lomholt, AF, Frederiksen, CB, Christensen, IJ, Brunner, N, Nielsen, HJ. Plasma tissue inhibitor of metalloproteinases-1 as a biological marker? Pre-analytical considerations. Clin Chim Acta 2007.
- Jung K, Meisser A, Bischof P. Blood sampling as critical preanalytical determinant to use circulating MMP and TIMP as surrogate markers for pathological processes. Int J Cancer 2005; 116: 1000–1
- Jung K, Nowak L, Lein M, Henke W, Schnorr D, Loening SA. Role of specimen collection in preanalytical variation of metalloproteinases and their inhibitors in blood. Clin Chem 1996; 42: 2043–5
- Holten-Andersen MN, Schrohl AS, Brunner N, Nielsen HJ, Hogdall CK, Hogdall EV. Evaluation of sample handling in relation to levels of tissue inhibitor of metalloproteinases-1 measured in blood by immunoassay. Int J Biol Markers 2003; 18: 170–6
- Sorensen NM, Bystrom P, Christensen IJ, Berglund A, Nielsen HJ, Brunner N, et al. TIMP-1 is significantly associated with objective response and survival in metastatic colorectal cancer patients receiving combination of irinotecan, 5-fluorouracil, and folinic acid. Clin Cancer Res 2007; 13: 4117–22
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol 1996; 135 (6 Pt 1): 1669–77
- Wurtz SO, Schrohl AS, Sorensen NM, Lademann U, Christensen IJ, Mouridsen H, et al. Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr Relat Cancer 2005; 12: 215–27
- Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 2006; 25: 99–113
- Jung KK, Liu XW, Chirco R, Fridman R, Kim HR. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J 2006; 25: 3934–42
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: A new model for anti-estrogen resistance. J Biol Chem 2001; 276: 9817–24
- Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther 2002; 1: 707–17
- Tokunaga E, Kataoka A, Kimura Y, Oki E, Mashino K, Nishida K, et al. The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. Eur J Cancer 2006; 42: 629–35
- Tokunaga E, Kimura Y, Mashino K, Oki E, Kataoka A, Ohno S, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer 2006; 13: 137–44
- Degraffenried LA, Friedrichs WE, Russell DH, Donzis EJ, Middleton AK, Silva JM, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res 2004; 10: 8059–67
- Pettmann B, Henderson CE. Neuronal cell death. Neuron 1998; 20: 633–47
- Kronqvist P, Kuopio T, Collan Y. Morphometric grading of invasive ductal breast cancer. I. Thresholds for nuclear grade. Br J Cancer 1998; 78: 800–5
- Zhang YG, Du J, Tian XX, Zhong YF, Fang WG. Expression of E-cadherin, beta-catenin, cathepsin D, gelatinases and their inhibitors in invasive ductal breast carcinomas. Chin Med J (Engl) 2007; 120: 1597–605
- Jinga D, Stefanescu M, Blidaru A, Condrea I, Pistol G, Matache C. Serum levels of matrix metalloproteinases MMP-2 and MMP-9 and their tissue natural inhibitors in breast tumors. Roum Arch Microbiol Immunol 2004; 63: 141–58
- Jinga DC, Blidaru A, Condrea I, Ardeleanu C, Dragomir C, Szegli G, et al. MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: Correlations with prognostic factors. J Cell Mol Med 2006; 10: 499–510