Abstract
Introduction. Danish experience from the first five years with sentinel lymph node biopsy (SLNB) as a routine staging procedure in early breast cancer is reported. Methods. During the period January 1, 2002 to December 31, 2006, 14 923 patients were diagnosed at Danish breast surgical centers certified for the sentinel node method. SLNB was performed in 8 338 patients (55.9%). The fraction increased steadily from 43% in 2002 to 67% in 2006. The median follow-up was 1.7 year (range 0–5.2 years). Results. Patients staged with SLNB were younger, had more often BCS, had smaller tumor size, were more often hormone receptor positive, and had lower grade, than patients staged with lymph node dissection (ALND). Blue dye and radio colloid were used in combination in 82%. Lymphoscintigraphy was performed in 61%, and frozen section was performed in 87%. Originally, peritumoral injection of tracer was most often used, but the recommendations have changed, and in 2006 90% of cases had sub-or periareolar injection of radioactive tracer. In the sentinel nodes 25% had macrometastases, 17% micrometastases only, and 3.2% isolated tumor cells only (ITC). ALND was performed in 2 714 patients, whose lymph node classification by SN was known. In the group of 1 563 patients with macrometastases in SN, 45% had non-sentinel node metastases, and in the group of 942 patients with micrometastases only, 23% had more positive nodes. Regional lymph node metastases were found in 15% with ITC in sentinel nodes. Lymph node recurrence among node negative patients was observed more often after staging by SLNB (0.5%) than after ALND (0.2%, p =0.04). Conclusion. Two thirds of breast cancer patients can be safely staged with the sentinel node technique, half of these will need no further axillary surgery. The loco-regional control in node negative patients classified by SLNB is high, but seems not quite comparable to what is seen after ALND.
The sentinel node technique has been tested and found effective in axillary staging of patients with early breast cancer in a large number of evaluation studies Citation[1], and the method is now considered the gold standard in patients in whom lymph node metastases are not expected Citation[2]. In 2001 the Danish Breast Cancer Cooperative Group (DBCG) defined a protocol for the implementation of the method which included a backup-series of 30 patients at each center (see paper by Friis et al. in this issue), and from 2002 and onward gradually more and more patients were staged by sentinel lymph node biopsy (SLNB) in Denmark. When the SLNB method was introduced in the beginning of 2002 the inclusion in the DBCG program was restricted to clinical node negative patients with unifocal tumors up to 4 cm. The program was revised in 2006, and thereafter tumor size no longer excludes patients, and the centers are free to apply the method in patients with multifocal and multicentric tumors. At the same time DBCG has activated a multicentre study in which patients with multifocal or multicentric tumors are operated with SLNB combined with backup axillary lymph node dissection (ALND).
Currently, it is believed that the improvement in overall survival in early breast cancer is a consequence of improved systemic treatment protocols, and focus of surgical treatment has been brought on minimizing the surgical trauma as for instance the SLNB method. On the other hand, it has been documented that poor local control is associated with decreased survival Citation[3]. It is therefore essential that the loco-regional recurrence rate after SLNB does not exceed what is seen after ALND, and it is mandatory that this is convincingly documented. Otherwise the SLNB as a sole surgical staging procedure would not be acceptable.
This is a report of the experience from the first five years with SLNB as a routine staging procedure. Special focus is brought on the loco-regional control in node negative patients classified by SLNB.
Material and methods
All Danish hospital departments engaged in treatment of breast cancer patients report prospectively to the DBCG database. The information collected includes surgical, pathological and oncologic data and clinical follow-up data. For the present analysis we have included information on age, type of surgery, tumor size, number of foci, histological type, grade of malignancy, hormone receptor status, number of lymph nodes removed, and number of positive lymph nodes. For patients where SLNB was used, the following information was available: use of lymphoscintigraphy, technique and site of injection localization of sentinel lymph nodes (SN), and number of removed nodes, if simultaneous ALND was performed, and for what reason. After 2004 the number of positive sentinel nodes was also reported.
The DBCG follow-up program consists of clinical examination twice a year for 5 years and once a year for additional 5 years to a total of 10 years of follow-up. Date and location of recurrences, if any, or date of last follow-up without recurrence within 10 years after surgery, are reported to the DBCG. Information on date of death is retrieved by record linkage to the Central Population Registry, which keeps electronically updated data on vital status and on emigration on the entire Danish population. Linkage with the registry is possible by using the civil person registration number, a unique personal identification number given to all Danes upon birth. Follow-up ended on April 1, 2007.
The sentinel node biopsy technique
The original DBCG program did not give specific recommendations on type of tracer (color dye, technetium-99-labelled tracer) injection technique (intra- or peritumoral, subareolar or subcutaneous), and the use of lymphoscintigraphy, but the methods used are reported to the database. From 2006 the guidelines were revised and from that time sub-/periareolar injection of radioactive tracer was advised. The injection site of color dye was not included in this recommendation. From the beginning of the study frozen section of removed SN has been recommended and immediate completion ALND to be performed in SLNB positive cases. If metastases or isolated tumor cells (ITC) are found later at the conventional examination on slides from paraffin embedded tissue including immunohistochemical cytokeratin staining, ALND is performed as a separate later operative procedure. Presence of ITC alone is not regarded as expression of metastatic disease, but ALND is recommended in order to exclude metastases in non-sentinel nodes.
Regarding axillary surgery, reports concerning specific information about formal ALND or lymph node extirpation were lacking in a substantial part of the patients. As the number of excised nodes was available in all cases, we have instead defined two groups based on total number of removed nodes. The ALND group if 10 or more nodes were excised and the lymph node extirpation group if fewer than 10 nodes were harvested.The argument for the split up into two groups is also that, according to the DBCG guidelines, at least 10 lymph nodes were demanded for complete ALND.
Histopathology
Histological type was defined according to the WHO classification (WHO, Lyon 2002). Tumor grade was assessed according to the method of Bloom and Richardson modified by Elston and Ellis (Elston and Ellis 1991). Hormone receptor status was determined by immunohistochemistry and tumors were classified as receptor positive by 10% or more positivity for estrogen and/or progesterone receptors.
Lymph node metastases were classified as macrometastases if the tumor deposit exceeded 2 mm and as micrometastases if the diameter was 0.2–2 mm or there were 10–100 tumor cells. ITC were defined as clusters of epithelial cells not exceeding 10 cells and a diameter below 0.2 mm.
Statistical methods
The patient characteristics are compared between patients treated with and without SNLB by Chi square test. The comparison of injection methods was performed by a multivariate logistic regression analyses, including the prognostic parameters in the model. The recurrence free survival (RFS) was defined as time until recurrence, second malignant disease, or death, whichever came first. If no events have occurred, the observations are censored at end of follow-up. RFS were analyzed in patients with negative nodal status comparing the outcome in patients classified by SLNB only versus patients classified by ALND, using a multivariate Cox model with proportional hazard ratios, including the important prognostic factors in the model. The effects are described by the estimated hazard ratios and tested by a maximum likelihood test (chi test). The type of recurrence: regional recurrence, defined as recurrence in the ipsilateral axilla, supra- or infra clavicular, isolated local recurrence, defined as a recurrence in the remaining breast or scar, and distant metastases defined as recurrences at other sites, were given separately and shown as cumulative incidences.
Results
All patients with primary breast cancer diagnosed from January 1, 2002 to December 31, 2006 at breast surgical centers after they were certified for the sentinel node method were included in this investigation (N = 14 923). SLNB was performed in 8 338 patients (55.9%), but the fraction has increased from just above 40% in 2002 to two thirds in 2006 (). Eleven thousand, one hundred and four patients (74.4%) were included in one of DBCG's programs (DBCG A, B, C, or D), and for these patients follow-up data of recurrence are available, whereas information of vital status are available for all patients. The median follow-up was 1.7 year (range 0–5.2 years). Considering survival, there were 18 200 person-years at risk in the SLNB group and 15 227 person-years at risk in the non-SLNB group of patients. Corresponding figures for disease free survival were 15 190 and 10 954 person-years at risk. General characteristics of the patients are given in . The distributions were different depending on the treatment (+/− SLNB). Patients treated with SLNB were younger, had more often BCS, smaller tumor size, were more often hormone receptor positive, and had lower grade, compared to patients not treated by SLNB.
Figure 1. The number of patients at surgery in Danish centers after certification and the frequency of SLNB.
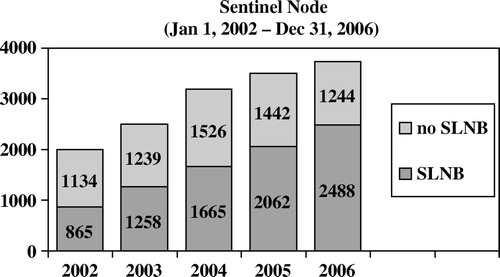
Table I. General characteristics of patients diagnosed at breast surgical centers in Denmark in the period 2002–2006 after certification for the SLNB method (N = 14923). The distributions were for all characteristics significantly different between treatment groups (SLNB/no SLNB) (p < 0.0001).
Information on the technical aspects of sentinel lymph node biopsy was reported in 7 927 of the 8 338 cases where SNLB was performed (). Lymphoscintigraphy was performed in 60.5% of the cases. In the vast majority of cases (82.2%) blue dye and radio colloid were used in combination. In most primary procedures, frozen section was performed (86.9%). Completion ALND was performed in almost one third of the patients. The main reason for this was finding of metastasis in SN on frozen section (78.6%) or intraoperative discovered suspicious lymph nodes (3.9%). In a few patients (N = 37, 1.6%) the explanation was validation of the method. In 168 patients (7.6%) ALND was performed because a SN was not found. In the whole material there were 219 patients in whom SLNB was intended but no SN was found (2.6%). In this group there were only four patients from whom no lymph nodes were harvested at all.
Table II. Technical aspects of the sentinel lymph node biopsy technique. Report forms were available on 7 927 Danish women with breast cancer operated in the period 2002–2006.
The preferred injection technique was from the beginning peritumoral, but from 2006, when the recommendations changed, the sub-/periareolar site has been increasingly popular (90.0% of cases for injection of radioactive tracer in 2006). In 2 191 patients tracer and/or color dye was exclusively injected peritumoral (inj. group A), and in 2 630 patients the injection was given at the sub-or periareolar site without giving any tracer around the tumor (inj. group B) (). The detection rate was significantly different for the two methods, thus, slightly lower in inj. group A, and also the node positive fraction was somewhat higher in inj. group A. Because the patients in the two groups were not similar regarding age at surgery, grade of malignancy, and tumor size, these differences were investigated by a multivariate logistic regression analysis including all the prognostic variables. The difference was significant for non-detection (odds ratio 2.0, p < 0.001) and for node positive detection rate (odds ratio 1.13, p = 0.05) showing that fewer sentinel lymph nodes were detected in inj. group A, but more of these sentinel nodes were positive in that group.
Table III. Comparison of injection performed peritumoral and sub-or periareolar regarding missed detection of sentinel lymph nodes and node positivity in Danish women with breast cancer operated in the period 2002–2006.
Lymph node surgery
Lymph node surgery was performed in 13 279 patients (89.0%). Lymph node status is missing in seven cases, though. The procedures performed in the remaining 13 272 cases are described in . There were no lymph nodes harvested in 1 644 patients (11%). This group included 1 098 cases where the diagnosis was secured by biopsy alone without surgical treatment, and 546 patients who were operated without lymph node surgery (in 27 cases (4.9%) SLNB was intended, but no SN was found). The 1 644 patients were mainly elderly patients, and 69.5% were 70 years of age or older in contrast to 24.2% of patients, from whom lymph nodes were removed (p < 0.0001).
Table IV. Lymph node surgery in all patients operated at breast surgical centers in Denmark after certification for the SNLB method in the period 2002–2006. (n = 13 272) In 1 644 patients no lymph nodes were harvested (among these 27 where SLNB was intended).
The number of sentinel nodes removed was median 2 (range 1 to 13). In case of ALND the number removed was median 16 (range 10 to 55). In the group of patients where lymph node extirpation was performed the median number of lymph nodes was 7 (range 1 to 9).
Lymph node status
The lymph nodes status could be determined for 13 272 patients (). Of these 6 752 were node positive (50.9%), and they were distributed as 5 535 (41.7%) with macro metastatic disease and 1 217 (9.2%) with micrometastases only. From 2003 and thereafter, the number and type of metastases in SN were available and could be related to the final lymph node status (N = 6 879) (). In the sentinel nodes 1 713 (24.9%) had macrometastases, 1 167 (17.0%) micrometastases only, and 183 ITC only (2.7%), however data on ITC were only available from 2004 in 5 707 patients. Thus, the adjusted frequency of finding ITC only was 3.2%. In 3 816 (55.5%) cases the sentinel nodes were negative. The result of the examination of the SN underestimated the final lymph node status in 200 cases, as 141 (12.1%) with micrometastases turned out to have macrometastases, 17 (9.3%) patients with ITC had either micro- or macrometastases, and 42 patients (1.1%) sentinel node negative patients showed to be node positive.
Table V. Status of sentinel lymph nodes and final lymph node status including non-sentinel nodes removed by ALND or by more limited dissection in Danish women with breast cancer operated in the period 2002–2006. SN positive lymph nodes were registered only from 2003, N = 6 879. Axillary lymph node dissection was performed in 2 505 cases.
ALND was performed in 2 505 sentinel node positive patients, in 110 patients with ITC in sentinel nodes, and in 99 patients with SN negative status (). In the group of 1 563 patients with macrometastases in SN 708 (45.3%) had non-sentinel node metastases, and in the group of 942 patients with micrometastases only in SN 213 (22.6%) had more nodes (125 [13.3%] as macrometastases and 88 [9.3%] as micrometastases). Furthermore, 16 of 110 patients (14.5%) with isolated tumor cells in the sentinel nodes turned out to have regional lymph node metastases (5 patients had micrometastases [4.5%] and 11 patients had macrometastases [10.0%]). Among the 99 SN node negative patients, 32 had non-sentinel node metastases (28 as macrometastases and 4 as micrometastases). The indications for performing ALND in these patients are not available.
Loco-regional control
In the node negative group of patients, 5 626 were followed according to the DBCG protocols, and for these the follow-up data of recurrence were available. In 4 061 patients the negative lymph node staging was based exclusively on SLNB whereas ALND was performed in 1 469 patients. Less than 10 nodes were removed without SLNB in 96 patients (). The apparent differences in total number of events (breast cancer recurrence, contra lateral breast cancer, second non-breast malignancy, and death) were not statistical significant in the multivariate model taking in consideration the prognostic factors described in (p = 0.09). Both distant recurrence and local recurrence showed the same pattern as seen for the total number of events. Regional axillary lymph node recurrence had another pattern; it was observed in 0.5, 0.2, and 1.0% of patients in the SLNB group, ALND group, and lymph node extirpation groups, respectively. The difference observed between the SLNB and the ALND group was small but statistically significant (p = 0.04). The cumulative incidence of distant, local, and regional recurrences for each of the two methods of staging is shown in . Until now, no axillary recurrence has been seen in the group of patients with ITC in SN where ALND was not performed (N = 73). Likewise, regional recurrence is not reported for the 225 registered patients where micrometastases in SN did not lead to ALND.
Figure 2. Cumulated incidence of local, regional, and distal recurrence after treatment of node negative Danish breast cancer patients (N = 5 530) staged by sentinel lymph node biopsy (N = 4 061) and axillary lymph node dissection (N = 1 469), respectively, in the period 2002–2006. Median follow-up was 1.7 years.
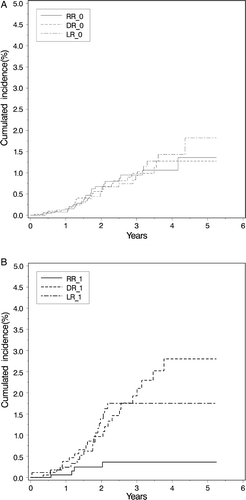
Table VI. Clinical outcome for node negative Danish women with breast cancer operated in the period 2002–2006 followed according to DBCG-recommendations. Total number of events includes breast cancer recurrence, contra lateral breast cancer, second non-breast malignancy, and death.
There was no difference in regional recurrence rate between inj. group A and B ().
Discussion
This validation study showed that the sentinel node technique shortly after its introduction as a standard procedure was used in a substantial proportion of patients, and after 5 years two thirds of Danish breast cancer patients have a SLNB included as part of the standard surgical treatment. Consequently, more and more patients are classified as node negative as result of examination of only a few sentinel lymph nodes. It is documented that the regional recurrence rate is low in these patients, although higher than seen after classification based on ALND.
Our data indicate that the detection rate is 98%. This may well be an overestimate and may reflect that not all cases, where a sentinel node was not found, were reported to the database. Thus, we have no reason to believe that the detection rate in our hands are better or worse than the 90–95% given as the gold standard Citation[2].
In accordance with the international trend sub-/periareolar injection is now recommended. So far the change in strategy has had not lead to marked changes in results. The present data indicate that the detection rate was higher, but the improvement was only marginal. On the other hand, more node positive patients were found after peritumoral injection. As the fraction of small tumors in inj. group A was less than in inj. group B, the slightly increased number of node positive probably to some extent reflects differences in tumor characteristics, but the multivariate analysis indicate that the peritumoral injection technique leads to a more accurate staging. This is a surprising result and to and to our knowledge it is not supported by other reports. We therefore recommend that this should be explored further in prospective, randomized trials.
There is good evidence supporting the therapeutic benefit associated with lymph node clearance in node positive breast cancer leading to better regional control, and thereby patients are spared from significant morbidity and have a better survival Citation[3]. Consequently, most guidelines recommend ALND in SN positive patients. In case of micrometastases, there is some controversy, though. Some papers have suggested a differentiated approach, where patients with small tumor deposits in SN receives no further axillary surgery, as the risk of non-SN metastases is small, typically less than 5%, whereas others have found that the risk of non-SN metastases may be substantial in such cases. In a meta-analysis Cserni et al. Citation[4] found that non-SN involvement was present in 15–20% after ALND in patients with “small metastases” defined as micrometastases and/or ITC and/or metastases detected by IHC. In patients with immunohistochemical positive SNs only the risk of non-SN involvement was about 10%. Based on this review the authors recommend the current strategy of performing ALND in micrometastatic SN-positive patients to be continued. Our finding of 22% occurrence of non-SN metastases in patients with micrometastases in the SNs, of which more than 50% were macrometastases, supports this conclusion and also justifies our policy of offering ALND in these cases. Furthermore, our data also supports the recommendation of ALND in case of finding of ITC in SN, as almost 15% of these patients had non-SN metastases. On the other hand, no axillary recurrences were found in the SN ITC-positive patients where ALND had not been performed. This is in keeping with observations from other investigators Citation[5], Citation[6] and might suggest a more conservative approach in at least sub-groups of patients. Others have looked into this Citation[7] but were not able to identify a subset of patients where the risk of non-SN metastases was less than 5%. At this moment we do not want to put too much emphasis to this finding in our final conclusions. Firstly, the number of observations is low, as these data were only reported within the last 2 years. Secondly, we fear that the classification might not have been precise in all cases, as the definition of isolated tumor cells was not very clear from the beginning. Thirdly, we are not able to tell whether the ALND in ITC was based on finding of palpable suspect lymph nodes intraoperatively, as recommended in the guidelines. We assume this has been the main reason for performing ALND performed in 99 SN negative patients, and, as the finding in these patients indicate, such nodes are often metastatic. Based on these considerations, we are looking into these data, and a reexamination of all sentinel node ITC positive cases is ongoing.
Although it is generally accepted that omission of ALND is safe in node negative patients staged by SLNB Citation[2], Citation[8], there are no published randomized studies comparing the regional recurrence rate. Most of the evidence come from observational studies from single institutions Citation[9–11]. Our study demonstrates a small, but significant, increase in the axillary lymph node recurrence in SLN negative patients without ALDN. The number of observations, however, is small and longer observation is necessary before conclusions can be drawn. In the study by Veronesi et al. Citation[11] the 5 year cumulative incidence of axillary metastases was 0.43%. A comparison with ALND was not reported, but the incidence of axillary metastases was lower than expected based on previous experience from a study with axillary back-up. In that study the occurrence of metastases in non-sentinel nodes was 6% Citation[12]. They point at radiotherapy after breast conserving surgery and adjuvant systemic treatment as possible explanations, but they also consider it plausible that a number of occult metastases never become clinical evident. In this context we have found it reassuring that the axillary lymph node recurrence rate in the present series was only 0.5% after SLNB, but on the other hand there was an even lower regional recurrence rate following ALND. Furthermore, as distant recurrence was seen more often after ALND, we do not think that the observed difference could be explained by more serious tumor characteristics in the SLNB group. Consequently, some concern remains, and emphasizes the need for continued close observation of this group of patients. Hopefully, this question will be properly addressed when the final results from the randomized NSABP B32 is reported.
Conclusion
In a setting where sentinel node biopsy is performed as a routine staging procedure two thirds of breast cancer patients can be safely staged with the technique, half of which will need no further axillary surgery. The loco-regional control in node negative patients classified by SLNB is high after short observation, but may not be quite comparable to results seen after ALND.
References
- Lauridsen MC, Garne JP, Sorensen FB, Melsen F, Lernevall A, Christiansen P. Sentinel lymph node biopsy in breast cancer–experience with the combined use of dye and radioactive tracer at Aarhus University Hospital. Acta Oncol 2004; 43: 20–6
- Lyman, GH, Giuliano, AE, Somerfield, MR, Benson Iii, AB, Bodurka, DC, Burstein, HJ, et al. American Society of Clinical Oncology Guideline Recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005.
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005; 366(9503)2087–106
- Cserni G, Gregori D, Merletti F, Sapino A, Mano MP, Ponti A, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg 2004; 91: 1245–52
- Herbert GS, Sohn VY, Brown TA. The impact of nodal isolated tumor cells on survival of breast cancer patients. Am J Surg 2007; 193: 571–3
- Calhoun KE, Hansen NM, Turner RR, Giuliano AE. Nonsentinel node metastases in breast cancer patients with isolated tumor cells in the sentinel node: Implications for completion axillary node dissection. Am J Surg 2005; 190: 588–91
- Bolster MJ, Peer PG, Bult P, Thunnissen FB, Schapers RF, Meijer JW, et al. Risk factors for non-sentinel lymph node metastases in patients with breast cancer. The outcome of a multi-institutional study. Ann Surg Oncol 2007; 14: 181–9
- Cremonesi M, Ferrari M, Sacco E, Rossi A, De CC, Leonardi L, et al. Radiation protection in radioguided surgery of breast cancer. Nucl Med Commun 1999; 20: 919–24
- Naik AM, Fey J, Gemignani M, Heerdt A, Montgomery L, Petrek J, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: A follow-up study of 4008 procedures. Ann Surg 2004; 240: 462–8
- Takei H, Suemasu K, Kurosumi M, Horii Y, Yoshida T, Ninomiya J, et al. Recurrence after sentinel lymph node biopsy with or without axillary lymph node dissection in patients with breast cancer. Breast Cancer 2007; 14: 16–24
- Veronesi U, Galimberti V, Mariani L, Gatti G, Paganelli G, Viale G, et al. Sentinel node biopsy in breast cancer: Early results in 953 patients with negative sentinel node biopsy and no axillary dissection. Eur J Cancer 2005; 41: 231–7
- Veronesi U, Paganelli G, Viale G, Galimberti V, Luini A, Zurrida S, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: Results in a large series. J Natl Cancer Inst 1999; 91: 368–73