ABSTRACT
Very early precursors of disrupted social behaviours are significant to understanding the possibility of mitigating or changing behaviours through interventions. Spontaneous play situations between infant and parent in two groups of infants aged 8.5–9 months were observed. First, a large number of videos were analysed to develop an observational schedule of play behaviour. Second, 135 videos were used in the comparison of atypical (AT; n = 23) and typically developed (TD; n = 22) infants. Frequency and duration of infants’ orientation towards the parent’s eye zone was significantly higher among TD infants than AT, indicating a stronger social gaze behaviour. Both groups were as competent at screening the surroundings, grabbing, and looking at toys. Finding may indicate an equal competence at reacting to reflexive stimuli. The quality of gaze behaviour provides important information for child healthcare professionals and parents when deciding whether to start supportive intervention or diagnostic investigation.
Introduction
The main reason for studies of early precursors of disrupted social orientation is to make it possible to start an early intervention while the plasticity of the brain is high (Shonkoff & Phillips, Citation2000). The rapid development of knowledge about early screening and intervention in the past five years has shifted the debate towards favouring an early start of both (Dawson et al., Citation2010; Rogers et al., Citation2014; Ryberg, Citation2015; Vivanti, Trembath, & Dissanayake, Citation2014). When exploring early atypical (AT) behaviours in infants, it is essential to focus on behaviours that might be of interest to the early social learning process. Vivanti and Rogers (Citation2014) discuss the role of the mirror neuron system and autism in the perspective of bodily and affective atypicalities in dyadic infant–parent interactions. Synchrony is vital during infant–parent interactions in activities such as imitation, reciprocal vocalization, and sharing of affect.
The social gaze behaviour of infants and toddlers has been hypothesized as one of the important behaviours for the early identification of autism spectrum condition (ASC) also used for autism spectrum disorder (ASD) (Barbaro & Dissanayake, Citation2012; Chawarska et al., Citation2014; Chawarska, Macari, & Shic, Citation2013; Saint-Georges et al., Citation2010; Thorup, Nystrom, Gredeback, Bolte, & Falck-Ytter, Citation2016). Jones et al. (Citation2016) hypothesized that a reduction in attention engagement to faces is associated with later autism. The conclusion of their study was that there might be fundamental early disruptions to attention engagement with consequences for future development. Chawarska, Ye, Shic, and Chen (Citation2016) recently reported that gaze behaviour among infants/toddlers aged 11–26 months with ASD is characterized by limited tuning into the social environment, combined with AT targets for processing. The study concluded that toddlers with ASD were generally less adept at tuning in to social scenes and at choosing to process the most context-relevant social targets. Chawarska et al. (Citation2013) previously found that six-month-old infants later diagnosed with ASD were less spontaneously attentive to the social scene, in particular, the face zone. However, the results of two studies of infants previously identified as showing lower rates of eye contact at 6 months that used an eye-tracking technique during a live mother–infant interaction involving a still-face procedure did not show diagnostic data for autism upon follow-up at 18 months (Merin, Young, Ozonoff, & Rogers, Citation2007; Young, Merin, Rogers, & Ozonoff, Citation2009). Ozonoff et al. (Citation2014) compared 294 high-risk infants with 116 low-risk infants for AT development at 6, 12, 18, 24, and 36 months of age. The results showed that the high-risk group could not be distinguished from the low-risk group at 6 months, but could be distinguished on multiple measures at 12 months.
There has also been a growing interest in the role of biological and behavioural rhythms in the typical and atypical early development of social communication such as gaze rhythmicity (Srinivasan, Eigsti, Gifford, & Bhat, Citation2016; Tordjman et al., Citation2015). The role of neuropeptide hormones like melatonin has been highlighted in relation to rhythmicity and synchrony in child development. In a prospective study, Jones and Klin (Citation2013) demonstrated that attention to eyes is present but in decline in two- to six-month-old infants later diagnosed with autism. These observations are the earliest known indicators of social disability in infancy. The authors state that eye looking appears to begin at normative levels prior to decline. However, the decline is gradual and they suggest that the gaze can be used for early identification of initial orientation towards the eyes.
Recent research has shed new light on gaze behaviour (Greene et al., Citation2011; Jones et al., Citation2016; Kirchgessner, Chuang, Patel, & Sereno, Citation2015; Tordjman et al., Citation2015). Kirchgessner et al. (Citation2015) refer to the observation that laboratory experiments testing results of social orientation in individuals with ASC are inconclusive and they propose that the experimental design is not entirely successful at isolating a reflexive (stimulus-driven) gaze from those that are voluntary. They claim that children with ASC are entirely competent at stimulus-driven reflexive gaze but have severe problems with voluntary socially driven gaze. This may be the reason why individuals with ASC often avoid social gaze as they cannot read faces and do not see the point of it. Greene et al. (Citation2011) found no difference in behavioural performance and social gaze behaviour between infants/toddlers, but a clear difference (MRI) can be observed in brain activity. They suggest that the normative (typical) behavioural performance in the ASD group in a laboratory setting may reflect compensatory mechanisms rather than intact social attention. Despite the mixed results yielded from a large amount of studies of social gaze, our impression is that there is growing preliminary evidence to support there being a difference in social gaze behaviour in infants/toddlers with ASD and those developing typically (Chawarska et al., Citation2016). The laboratory research setting with eye tracking of gaze has contributed fruitfully to the research field, but our intention in this study is to contribute to the social gaze research using an alternative methodology to study gaze behaviour because new findings suggest this might make a difference to the results. Our choice to investigate infants’ interest in the surroundings emanates from the observation that infants aged 8–9 months show an attentiveness to environmental stimuli such as sounds, movements, and light flashes.
The present study aims to make detailed observations of infants’ social gaze in an interactive and spontaneous play session between infant and parent by looking at voluntarily eye contact with the parent. Videos from two groups of infants aged between 8.5 and 9 months are compared; one group having previously demonstrated AT behaviour during several clinical occasions and the other not having done so (typically developed, TD).
Hypotheses
The number of times infants’ voluntary gaze towards their parent’s eyes during spontaneous play sessions and the duration of these gazes differ between infants with signs of AT development and TD infants.
The number of times infants screen the play surroundings during spontaneous play sessions and the duration of this screening differ between infants with signs of AT development and TD infants.
The number of times infants look at parent’s body during spontaneous play sessions and the duration of these looks differ between infants with signs of AT development and TD infants.
The number of times infants look at toys during spontaneous play sessions and the duration of these looks differ between infants with signs of AT development and TD infants.
Method
Instruments
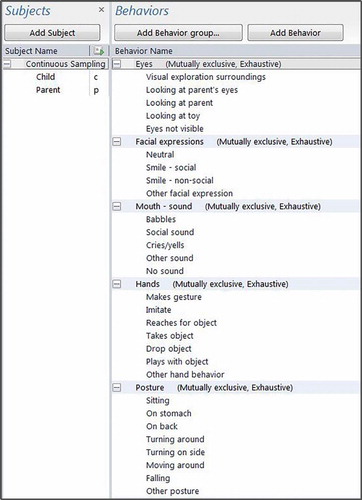
Infants were recruited from a screening study using an observational tool named SEEK (Sivberg, Lundqvist, Johanson, Nordström, & Persson, Citation2015). The first version of SEEK was developed in 2004–2006 (Persson, Nordstrom, Petersson, Mansson, & Sivberg, Citation2006). The development started with a systematic literature review of existing early screening instruments for ASD and was further developed by studying CHAT (Baron-Cohen, Allen, & Gillberg, Citation1992) and the Modified Checklist for Autism in Toddlers (Chlebowski, Robins, Barton, & Fein, Citation2013; Robins, Citation2008; Robins et al., Citation2014). Publications by Barbaro, Ridgway, and Dissanayake (Citation2011) and Barbaro and Dissanayake (Citation2013) were also scrutinized for information about early screening. SEEK (Sivberg et al., Citation2015; Appendices 1 and 2 for extended information about SEEK) consists of two parts; one direct observational part containing seven dimensions: social interaction (seven items), communication (nine items), coordination/stability (seven items), physical body contact (one item), behaviour patterns (three items), cognition (two items), and additional criteria (two items); and one short parental interview part. The observational scoring options are: 0 points for typical development, 1 for slightly AT development, and 2 for clearly AT development (Persson et al., Citation2006; Sivberg et al., Citation2015). One example of an item in the dimension social interaction is attention, with the scoring alternatives: easy to get the infant’s attention through kind verbal invitation, mimicry/gestures, and eye contact (0 points), takes longer than expected to get the infant’s attention (1 point), it is impossible to get the infant’s attention (2 points). All infants in the study took part in the Swedish national child health programme and child healthcare nurses and paediatricians saw all of the infants at regular check-ups on five or more occasions.
Participants
Child healthcare nurses in Region Kronoberg, Sweden screened 4027 infants aged 8 months from June 2011 until the end of 2013 at child health centres (CHC). Infants with any chromosomal anomaly or other serious health problems were excluded. None of the infants in the present study had received any form of intervention before their inclusion in the study. Infants with 4 or more SEEK-positive points (n = 302, of which 162 were boys) were screened once more by two psychologists within two weeks if they had at least one item with 2 SEEK points. The infants were also examined using the Functional Emotional Assessment Scale’s (FEAS) 7–9 months’ scale (Greenspan, DeGangi, & Wieder, Citation2001). FEAS has been developed by Stanley Greenspan, together with Greenspan, DeGangi, & Wieder (Citation2001), for measuring self-regulation, different levels of relating or attachment, emotional cueing and signalling, expressed affect, co-regulated social problem solving, symbolizing or representing wishes and emotions, expressing wishes and feelings, elaborating themes in pretend play, reasoning about feelings, and reflecting on one’s own feelings and empathizing with the feelings of others (Greenspan et al., Citation2001). There is one clinical version and one research version of FEAS for each age level. We used the research version, the FEAS 7–9 months measure, of both the infant and the caregiver (parent) formulas in our observations. The infant version contains two main dimensions: self-regulation and interest in the world (nine items) and forming relationships, attachment, and engagement (five items). An example of the former is ‘is interested and attentive to play with toys’ and the latter ‘shows emotional interest and connection with caregiver by vocalizing and smiling at her/him. Infants (n = 23, 16 boys) with 8 or more SEEK points (range: 8–25 points, out of which at least two items had 2 SEEK points) (Sivberg et al., Citation2015) and being clearly AT or in the at-risk zone according to the FEAS observational scale, as judged by two independent researchers, were included in the AT group. TD infants (n = 22, 16 boys) were recruited from the same region at ages of between 8 months and two weeks and 9 months. Two of these infants had 2 SEEK points (1 + 1) while the remaining infants had 0 SEEK points. The infants in the two groups were matched concerning sex and age.
Video recordings
The researchers made 3 five-minute video recordings of each of the infants in the two groups. A total of 66 five-minute videos were made with the TD infants and 69 with the infants who showed signs of AT development. The video recordings took place at the CHC in a special room also used by the nurses for the first SEEK observation/examination at eight months. One the camera was used, and it was moved during the play session in order to have an optimal view for catching the infant–parent interaction. The coders were blinded as to whether the infants were in the TD or AT group.
During the video sessions, mother or father and infant played using three boxes containing different types of toys. Box 1 contained very tangible toys such as a doll with an inviting, smiling face, and a ‘bell toy’ (symbolic play materials), Box 2 contained soft textiles such as a blanket, a soft animal (snake), and a textile ball (tactile play materials), and Box 3 contained toys that make noises such as a pop-up machine with wheels, a car with interesting wheels, and a little box to open with toy animals (movement play materials). Parents were asked to sit on a carpet in front of their infant during the play session and were told to play with their infant as if they were at home. The researchers remained silent during the video sessions and did not intervene in the play or draw the infant’s attention away from either the parent or the toy. The temperature was sufficiently high (21–23°C) for the infants to feel comfortable playing.
Schedule of analysis
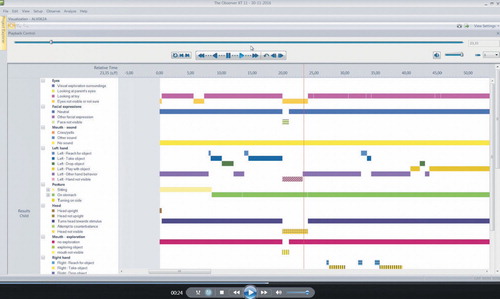
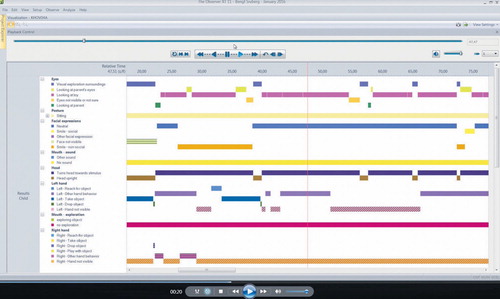
The observational schedule (, Coding Schedule Videos.docx) is based on a large number of videos. All videos included (n = 135) were scrutinized by two of the authors and all the infants’ behaviours were evaluated in order to further simplify the coding process. A code was assigned to each type of behaviour and they were sampled using software designed for the collection and analysis of observational data (The Observer 11.5, Noldus Information Technology; Noldus International 2014). The observational schedule used when coding behaviours was constructed on the basis of the infant’s body and bodily activities: head/face (visual exploration, type of smile/not smiling), type of eye contact (no contact, type of vocalization/no vocalization), body position (tonus), right arm/left arm reaching for toys, movements (turning, moving around, head turning), and the interaction between infant and parent. The videos were coded within The Observer and scored for each second with the opportunity to go back and forth during the coding. If the coders could not decide sufficiently clearly on the coding for a behaviour, the sequence was scored as ‘not visible’, fairly equal in each group.
All items were monitored separately and the videos (, Printscreen Video AT.pdf _Video AT.mp4; , Print screen Video TD.pdf _Video TD.mp4) were subsequently monitored for each body part in order to obtain reliable observations of the behaviours. The behaviours were coded ‘each other excluded’ which means that a behaviour was scored only for one type of behaviour when present in the video sequence. The content validity of the schedule was tested by two members of the research group (BS and PL) and two unbiased external raters who were specialists in scoring using The Observer (The Observer 11.5, Noldus Information Technology; Noldus International 2014). Several changes were implemented during collaborative sessions until we reached full agreement on the content validity of the final schedule. After that, each of the two external coders, who were blinded as to the group of infants, separately scored nine videos (5 minutes each) and reached an interrater reliability (Cohen’s kappa) for the frequency of a behaviour in the range of 0.75–0.83, and for duration of behaviour between 0.82 and 0.93.
Statistical analyses
Descriptive statistics concerning means (M) and standard deviation (SD) were produced. Parametric statistics (Student’s t-test) were used to test the hypotheses, together with Levine’s test of variances and CI. The alpha level of significance was set to .05. The three videos of each infant were handled separately in the analysis since the toys in the three boxes represented different play materials as per the FEAS manual (Greenspan et al., Citation2001, pp. 193–200). The software used to produce the statistics were SPSS version 23 and The Observer version 11.5.
Results
In this study, parents of AT infants talked more frequently than those of TD infants did (M = 10.696 and 6.803, respectively), but the number of times toy objects were presented did not differ significantly between the two groups of parents. The number of playing activities were equal between the two groups of parents. The mean length of time parents talked with their infants was longer among parents of AT infants than parents of TD infants (40.820 and 23.589 seconds, respectively), but the duration of the presentation of toy objects and play activities did not differ between the groups.
Test of hypotheses
The analysis of the videos verified first hypothesis (H1) in terms of both the number of times eye contact was made between infant and parent and the duration of eye contact measured in seconds ( and ). The number of times eye contact were made was significantly higher among the TD infants (p < .001). The duration was also significantly longer among the TD infants (p < .001; M = 4.6, SD = 4.8). The mean number of times eye contact was made was 4.6 times for the TD infants and 1.8 times (M = 1.8, SD = 3.3) for the AT infants. The mean difference was 2.81. However, 11 of the 23 AT infants did not look at their parent’s eyes at all. The range of the CI was 1.40–4.22.
Table 1. Comparisons of the AT group (n = 23) and the TD group (n = 22) regarding the number of occasions/behaviours.
Table 2. Comparisons of the AT group (n = 23) and the TD group (n = 22) regarding the duration of occasions/behaviours.
The second hypothesis (H2), concerning the infants’ screening of the play surroundings, was not supported by the results ( and ). No significant difference between the two groups of videos was demonstrated in terms of either number (TD: M = 11.1, SD = 5.8 and AT M = 10.5, SD = 7.2; p = .607) or duration (TD M = 37.5, SD = 26.0 and, AT M = 43.2, SD = 44.1; p = .364) ().
The third hypothesis (H3), concerning the analysis of the infants’ looking at their parent’s body (not face or eyes), was tested and verified in terms of number of looks and their duration ( and ). There was a significant difference in both the number of looks (p = .016) and in the duration of looking (p < .001). In the videos, the TD infants looked both fewer times and for shorter periods than those in the AT group. The mean number was 4.6 (SD = 4.7) for the TD group and 7.0 (SD = 6.7) for the AT group. The mean difference was −2.42. The range of the CI for number was −4.40 to −0.45. The mean duration was 7.5 (SD = 10.4) seconds for the TD group and 15.7 (SD = 17.2) seconds for the AT group. The mean difference was −8.19. The range of the CI for duration was −13.01 to −3.36.
The fourth hypothesis (H4) about the play behaviour with toys measured as number of looks at toys and the duration of looks was verified in terms of the number (p < .001). In the videos, the TD infants looked at the toys more frequently than the AT infants. The duration was not significantly different (p = .220). The mean number was 53.4 (SD = 16.6) for the TD group and 38.7 (SD = 15.1) for the AT group. The mean difference was 14.8. The range of the CI was 9.39–20.18. The mean duration was 227.5 (SD = 55.8) seconds for the TD group and 241.2 (SD = 71.8) seconds for the AT group. The mean difference was −13.69. The range of the CI was −35.65–8.27. The large CI means that the difference in terms of duration is not significant.
Discussion
Our results show that there is a significant difference between TD and AT infants in terms of how often (number) they orient towards their parent’s eyes and for how long (duration in seconds) their gaze was towards their parent’s eyes. However, the small size of our sample means this finding must be considered with care. Nevertheless, our sample was a prospective community sample from a full population of infants in a Swedish region (county). Our finding is supported by several earlier studies conducted in a laboratory setting, most recently by Chawarska et al. (Citation2016) and Chawarska, Macari, and Shic (Citation2012). They found a difference between infants at 6 and 12 months of age later diagnosed with ASD in terms of both the frequency (number) and length of time typically developing infants spontaneously oriented to eye contact with their parent. Our study contributes by supporting these earlier findings with results obtained using a different methodology and research design. The Observer analysis controls for possible disturbing stimuli from the play surroundings. In spite of the fact that Young et al. (Citation2009) and Vivanti and Rogers (Citation2014) found no difference in gaze behaviour in infants at 6 months later diagnosed with ASD, and that Greene et al. (Citation2011) reported the same results, Greene et al. questioned their own results in the light of the discovery of a difference in brain activity between ASD infants (13 months) and typically developing infants. The TD group showed an increased activity in frontoparietal attention networks, visual processing regions, and the striatum. The ASD group only showed increased activity in superior parietal lobule. They interpret their result concerning gaze behaviour as a possible compensatory mechanism that infants with ASD employ in a laboratory setting.
Kirchgessner et al. (Citation2015) reported that children with ASC are not able to use social cues in a voluntary, goal-directed way. They also concluded that children with ASC were able to respond to stimuli-driven cues as well as TD children. As far as we are aware, the latter finding of Kirchgessner et al. (Citation2015) is not supported by other published studies, and in spite of the fact that our findings support their findings, caution is advised. In summary, further studies with larger prospectively chosen community samples are needed in order to provide the evidence required for the eye deficits of ASD infants to be used as tools for the diagnosis. Nonetheless, the results currently available from several studies may indicate the pertinence of starting early supportive interventions.
Tordjman et al. (Citation2015) and Srinivasan et al. (Citation2016) highlighted the role of biological and behaviour rhythms such as gaze rhythmicity in typical and atypical development. Our result showed a significant difference in the gaze frequency in the videos of the two groups of infants. Typically developing infants had a more rhythmic gaze behaviour with respect to their parent’s eyes ( and ). This rhythmicity need to be explored further in the data in order to present time intervals in seconds between the gaze occasions.
One reason for using The Observer analysis technique for our analysis was to counter the critique from both Greene et al. (Citation2011) and Kirchgessner et al. (Citation2015) concerning the shortcomings of the laboratory setting, and our intention was to reduce setting errors in the coding. In spite of the fact that The Observer analytical technique is very time consuming, it can be performed with great rigor and reach a very god interrater reliability.
Our study has some methodological limitations. The use of only one camera to record the play sessions was influenced by our ambition to balance rigor in the coding with the potential dropout of infants/parents from the study. Perhaps it would have been better to use two cameras. The background to the screening study (Sivberg et al., Citation2015) was that the screening covered all infants at eight months in a region where there are long distances between CHC. When an infant scored positively on SEEK on the first occasion with the nurse, two members of the research team visited the CHC within two weeks in order to meet the family with the infant and perform SEEK a second time. If the infant again scored positively on SEEK and FEAS, the play session was recorded. It was not possible to ask the family to travel 100–150 kilometres for a short play session in a fully equipped studio without the risk of families dropping out. However, the analysis of the videos was performed with great rigor in The Observer system, in second by second sequences, and if the eyes or faces were not visible, that sequence was coded as ‘not visible’. The coders were blinded to the status of the infants included in the two groups in order to avoid bias. There was no communication between the coders and researchers during or after the coding procedures.
Although the infants with AT development in our study did not have a formal diagnosis of ASC, partly due to their low age and maybe lack of relevant knowledge about ASC in primary healthcare, some of the infants were immediately referred to diagnostic investigation for ASC. It is perhaps possible to speculate about which characteristics of gaze behaviour are most predictive of a subsequent diagnosis of ASC, our findings support the hypothesis that the severity of AT behaviours is crucial. Eleven of the infants in the AT group did not look at their parent’s eye zone at all over the course of 15 minutes. Several of these infants are now being considered for a diagnosis. Many of the infants in the AT group had a gaze that steered towards the parent’s face, but not their eyes in particular. The infants gave the impression they were having difficulties processing facial social cues. Greene et al. (Citation2011) indicate that social cues are not given the same privileged status in the autistic brain as they are in the brains of typically developing infants. This may delay or even disrupt the processing of social cues. Despite intensive ongoing research directed at neurophysiological cues, at present, there are no biomarkers covering the full spectrum of ASD. One major problem is that there is a growing consensus that there are many entries into the autistic spectrum (Leblond et al., Citation2014) and as a consequence of that it would probably be difficult to find one single biomarker of autism (El-Ansary, Bjorklund, Chirumbolo, & Alnakhli, Citation2017; Freedman & Foxe, Citation2017; Hu, Ehli, & Boomsma, Citation2017). Freedman and Foxe (Citation2017) have presented potential biomarkers, having shown consistent deficits of saccades in autism. Onore, Yang, Van de Water, and Ashwood (Citation2017) concluded that there is no known cause for the majority of ASD cases, and that there are no physiological diagnostic biomarkers to support behavioural diagnosis. However, their finding that the activity of several signalling molecules in the Akt/mammalian target of rapamycin (Akt/mTOR) pathway is elevated in young children with ASD is promising in terms of identifying general behavioural, and social behavioural, deficiencies, compared to typically developing children. The findings of Onore et al. (Citation2017) are new and may provide opportunities to focus therapeutic tools on at least a subset of individuals within the ASD spectrum. Further research combining biomarkers and subsets of children with ASD will probably shed light on useful biomarkers in identifying individuals in specific subgroups of ASD children.
Shic, Macari, and Chawarska (Citation2014) also found a reduced attention to facial features in six-month-old infants, and Chlebowski et al. (Citation2013) found a reduced spontaneous attention to social activities in infants later diagnosed with ASC. Jones et al. (Citation2016) concluded that their results are consistent with Chawarska, Volkmar, and Klin’s (Citation2010) findings that infants who are later diagnosed with ASC have a reduced depth in their social attention that affects their capacity to learn. Jones et al. (Citation2016) also stress that it is important to differentiate between processes of attention orientation (number of orientations) and attention maintenance (duration of attention), as we did in our design. The importance of gaze duration, in particular, the duration of direct gaze, can contribute to modulating our perception of the social meaning of gaze cues (Georgescu et al., Citation2013). Our results concerning the difference in duration of direct social gaze may contribute to our understanding of a delayed learning process in children with ASD. Furthermore, our findings that AT infants more frequently looked at their parent’s body and that the total duration of these looks was longer, support the interpretation that AT infants may have longer non-social attention orientation and longer non-social attention maintenance. These findings may indicate that there is a delay in the social learning process. Again, our sample is small and the results must be evaluated in relation to that.
One alternative interpretation is that the AT infants had a greater need to reassure themselves of their parent’s presence. Another more plausible interpretation is that the AT infants had a lower capacity to process perceptual information through sight and to understand the meaning of the parent’s activity (Colombo & Cheatham, Citation2006). An additional interpretation of the results of our study is that the AT infants were more stimulated by details of their parent’s clothing or movements, for example, when they offer a toy, than by a direct mutual eye contact. AT infants were as competent as TD infants in terms of screening the play surroundings and reacting to reflexive stimuli. AT infants looked as frequently at the surrounding as did TD infants, and there was no significant difference in the duration of the looks, which indicates a capacity to react on non-social stimuli. This finding is consistent with Kirchgessner et al. (Citation2015). Jones et al. (Citation2016) concluded that the finding of very early disruption of attention may have cascading negative consequences for later social functioning. Our finding of an impaired social gaze at 8.5–9 months, in terms of both number and duration, supports the finding of Jones et al. (Citation2016) that the disruption of social attention at an age of 6 to 10/11 months is a precursor of later problems in social functioning.
Conclusion
The main clinical implication of the results is that it is vital that paediatric healthcare staff spend enough time observing the frequency and duration of parent–infant gaze behaviour. This is essential in order to differentiate between a stimuli-driven gaze and a voluntary social gaze orientation to the eye zone. This observation must be repeated on several occasions because of the infant’s young age and daily health conditions. If the infant’s gaze behaviour indicates any atypicalities, it is important to investigate their development further using a screening instrument and decide if it is relevant to begin intervention. Further research is needed about the very early signs of atypicalities that may be an indication to start supportive activities. Interventions are probably more successful if they start before the infant has established AT behaviours.
The Observer observational technique is probably a fruitful methodology for studying spontaneous infant–parent play, but it is important to plan the setting carefully in order to ensure the reliability of the results of analysis.
Video_TD.avi
Download Microsoft Video (AVI) (5.2 MB)Video_AT.avi
Download Microsoft Video (AVI) (4.3 MB)Acknowledgements
The authors are grateful to the families who took part in the study and the child healthcare centres Cityläkargruppen and Teleborg in Region Kronoberg, Sweden, without whose help the study would not have been possible. We would also like to thank the psychologists Dr. Berit Nordström and Dr. Bengt Persson for their participation in the data collection process together with BS and PL.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Bengt Sivberg has been active in autism research studies for 20 years and is a Professor in Health Sciences directed on Mental Disabilities at the Faculty of Medicine, Lund University, Sweden.
Ulf Jakobsson is a Professor at Centre for Primary Health Care Research, Faculty of Medicine, Lund University. He has a background District nurse and has a Master’s degree in statistics.
Pia Lundqvist is a paediatric and neonatal nurse with long experience in clinical work. Her experience in paediatric research has been an associate professor at the Faculty of Medicine, Lund University, Sweden.
Additional information
Funding
References
- Barbaro, J., & Dissanayake, C. (2012). Developmental profiles of infants and toddlers with autism spectrum disorders identified prospectively in a community-based setting. Journal of Autism and Developmental Disorders, 42(9), 1939–1948. doi: 10.1007/s10803-012-1441-z
- Barbaro, J., & Dissanayake, C. (2013). Early markers of autism spectrum disorders in infants and toddlers prospectively identified in the social attention and communication study. Autism, 17(1), 64–86. doi: 10.1177/1362361312442597
- Barbaro, J., Ridgway, L., & Dissanayake, C. (2011). Developmental surveillance of infants and toddlers by maternal and child health nurses in an Australian community-based setting: Promoting the early identification of autism spectrum disorders. Journal of Pediatric Nursing, 26(4), 334–347. doi: 10.1016/j.pedn.2010.04.007
- Baron-Cohen, S., Allen, J., & Gillberg, C. (1992). Can autism be detected at 18 months? The needle, the haystack, and the CHAT. The British Journal of Psychiatry, 161, 839–843.
- Chawarska, K., Macari, S., & Shic, F. (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, 53(8), 903–913. doi: 10.1111/j.1469-7610.2012.02538.x
- Chawarska, K., Macari, S., & Shic, F. (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry, 74(3), 195–203. doi: 10.1016/j.biopsych.2012.11.022
- Chawarska, K., Shic, F., Macari, S., Campbell, D. J., Brian, J., Landa, R., … Bryson, S. (2014). 18-month predictors of later outcomes in younger siblings of children with autism spectrum disorder: A baby siblings research consortium study. Journal of the American Academy of Child and Adolescent Psychiatry, 53(12), 1317–1327.e1311. doi: 10.1016/j.jaac.2014.09.015
- Chawarska, K., Volkmar, F., & Klin, A. (2010). Limited attentional bias for faces in toddlers with autism spectrum disorders. Archives of General Psychiatry, 67(2), 178–185. doi: 10.1001/archgenpsychiatry.2009.194
- Chawarska, K., Ye, S., Shic, F., & Chen, L. (2016). Multilevel differences in spontaneous social attention in toddlers with autism spectrum disorder. Child Development, 87(2), 543–557. doi: 10.1111/cdev.12473
- Chlebowski, C., Robins, D. L., Barton, M. L., & Fein, D. (2013). Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics, 131(4), e1121–e1127. doi: 10.1542/peds.2012-1525
- Colombo, J., & Cheatham, C. L. (2006). The emergence and basis of endogenous attention in infancy and early childhood. Advances in Child Development and Behavior, 34, 283–322.
- Dawson, G., Rogers, S., Munson, J., Smith, M., Winter, J., Greenson, J., … Varley, J. (2010). Randomized, controlled trial of an intervention for toddlers with autism: The early start Denver model. Pediatrics, 125(1), e17–e23. doi: 10.1542/peds.2009-0958
- El-Ansary, A., Bjorklund, G., Chirumbolo, S., & Alnakhli, O. M. (2017). Predictive value of selected biomarkers related to metabolism and oxidative stress in children with autism spectrum disorder. Metabolic Brain Disease, 32(4), 1209–1221. doi: 10.1007/s11011-017-0029-x
- Freedman, E. G., & Foxe, J. J. (2017). Eye-movements, sensori-motor adaptation and cerebellar-dependent learning in autism: Towards potential biomarkers and sub-phenotypes. The European Journal of Neuroscience. doi: 10.1111/ejn.13625
- Georgescu, A. L., Kuzmanovic, B., Schilbach, L., Tepest, R., Kulbida, R., Bente, G., & Vogeley, K. (2013). Neural correlates of “social gaze” processing in high-functioning autism under systematic variation of gaze duration. NeuroImage: Clinical, 3, 340–351. doi: 10.1016/j.nicl.2013.08.014
- Greene, D. J., Colich, N., Iacoboni, M., Zaidel, E., Bookheimer, S. Y., & Dapretto, M. (2011). Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage, 56(1), 354–362. doi: 10.1016/j.neuroimage.2011.02.031
- Greenspan, S. I., DeGangi, G., & Wieder, S. ( Eds.). (2001). Functional emotional assessment scale (FEAS) for infancy and early childhood. Clinical and research applications. Washington, DC: Interdiciplinary Council in Developmental and Learning Disorders.
- Hu, Y., Ehli, E. A., & Boomsma, D. I. (2017). MicroRNAs as biomarkers for psychiatric disorders with a focus on autism spectrum disorder: Current progress in genetic association studies, expression profiling, and translational research. Autism Research, 10, 1184–1203. doi: 10.1002/aur.1789
- Jones, W., & Klin, A. (2013). Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature, 504(7480), 427–431. doi: 10.1038/nature12715
- Jones, E. J., Venema, K., Earl, R., Lowy, R., Barnes, K., Estes, A., … Webb, S. J. (2016). Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: A longitudinal prospective study of infants at high familial risk. Journal of Neurodevelopmental Disorders, 8, 694. doi: 10.1186/s11689-016-9139-8
- Kirchgessner, M. A., Chuang, A. Z., Patel, S. S., & Sereno, A. B. (2015). Intact reflexive but deficient voluntary social orienting in autism spectrum disorder. Frontiers in Neuroscience, 9, 1804. doi: 10.3389/fnins.2015.00453
- Leblond, C. S., Nava, C., Polge, A., Gauthier, J., Huguet, G., Lumbroso, S., … Bourgeron, T. (2014). Meta-analysis of SHANK mutations in autism spectrum disorders: A gradient of severity in cognitive impairments. PLoS Genetics, 10(9), e1004580. doi: 10.1371/journal.pgen.1004580
- Merin, N., Young, G. S., Ozonoff, S., & Rogers, S. J. (2007). Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders, 37(1), 108–121. doi: 10.1007/s10803-006-0342-4
- National Research Council, & Institute of Medicine Committee on Integrating the Science of Early Childhood, D., Shonkoff, J. P. & Phillips, D. A. (Eds.) (2000). From neurons to neighborhoods: The science of early childhood development. Washington, DC: National Academies Press (US).
- Onore, C., Yang, H., Van de Water, J., & Ashwood, P. (2017). Dynamic Akt/mTOR signaling in children with autism spectrum disorder. Frontiers in Pediatrics, 5, 43. doi: 10.3389/fped.2017.00043
- Ozonoff, S., Young, G. S., Belding, A., Hill, M., Hill, A., Hutman, T., … Iosif, A. M. (2014). The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child and Adolescent Psychiatry, 53(4), 398–407.e392. doi: 10.1016/j.jaac.2013.12.020
- Persson, B., Nordstrom, B., Petersson, K., Mansson, M. E., & Sivberg, B. (2006). Screening for infants with developmental deficits and/or autism: A Swedish pilot study. Journal of Pediatric Nursing, 21(4), 313–324. doi: 10.1016/j.pedn.2005.07.004
- Robins, D. L. (2008). Screening for autism spectrum disorders in primary care settings. Autism, 12(5), 537–556. doi: 10.1177/1362361308094502
- Robins, D. L., Casagrande, K., Barton, M., Chen, C. M., Dumont-Mathieu, T., & Fein, D. (2014). Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics, 133(1), 37–45. doi: 10.1542/peds.2013-1813
- Rogers, S. J., Vismara, L., Wagner, A. L., McCormick, C., Young, G., & Ozonoff, S. (2014). Autism treatment in the first year of life: A pilot study of infant start, a parent-implemented intervention for symptomatic infants. Journal of Autism and Developmental Disorders, 44(12), 2981–2995. doi: 10.1007/s10803-014-2202-y
- Ryberg, K. H. (2015). Evidence for the implementation of the early start Denver model for young children with autism spectrum disorder. Journal of the American Psychiatric Nurses Association, 21(5), 327–337. doi: 10.1177/1078390315608165
- Saint-Georges, C., Cassel, R., Cohen, D., Chetouani, M., Laznik, M.-C., Maestro, S., & Muratori, F. (2010). What studies of family home movies can teach us about autistic infants: A literature review. Research in Autism Spectrum Disorders, 4, 355–366.
- Shic, F., Macari, S., & Chawarska, K. (2014). Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biological Psychiatry, 75(3), 231–237. doi: 10.1016/j.biopsych.2013.07.009
- Sivberg, B., Lundqvist, B., Johanson, I., Nordström, B., & Persson, B. (2015). Screening of infants at eight months for atypical development in primary health care in southern Sweden. Early Child Development and Care, 186, 1–20. doi: 10.1080/03004430.2015.1029469
- Srinivasan, S. M., Eigsti, I. M., Gifford, T., & Bhat, A. N. (2016). The effects of embodied rhythm and robotic interventions on the spontaneous and responsive verbal communication skills of children with autism spectrum disorder (ASD): A further outcome of a pilot randomized controlled trial. Research in Autism Spectrum Disorders, 27, 73–87. doi: 10.1016/j.rasd.2016.04.001
- Thorup, E., Nystrom, P., Gredeback, G., Bolte, S., & Falck-Ytter, T. (2016). Altered gaze following during live interaction in infants at risk for autism: An eye tracking study. Molecular Autism, 7, 657. doi: 10.1186/s13229-016-0069-9
- Tordjman, S., Davlantis, K. S., Georgieff, N., Geoffray, M. M., Speranza, M., Anderson, G. M., … Dawson, G. (2015). Autism as a disorder of biological and behavioral rhythms: Toward new therapeutic perspectives. Frontiers in Pediatrics, 3, 170. doi: 10.3389/fped.2015.00001
- Vivanti, G., & Rogers, S. J. (2014). Autism and the mirror neuron system: Insights from learning and teaching. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1644), 20130184. doi: 10.1098/rstb.2013.0184
- Vivanti, G., Trembath, D., & Dissanayake, C. (2014). Atypical monitoring and responsiveness to goal-directed gaze in autism spectrum disorder. Experimental Brain Research, 232(2), 695–701. doi: 10.1007/s00221-013-3777-9
- Young, G. S., Merin, N., Rogers, S. J., & Ozonoff, S. (2009). Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science, 12(5), 798–814. doi: 10.1111/j.1467-7687.2009.00833.x