Abstract
Objective
This retrospective observational study described baseline characteristics, real-world treatment patterns, and outcomes among patients with metastatic breast cancer treated with abemaciclib in the United States.
Methods
De-identified electronic health record-derived data were used to describe patients who began abemaciclib treatment on or after 30 June 2016 and ≥4 months before data cutoff (31 December 2018). Real-world response (rwR) and real-world progression assessments were abstracted from clinical documentation. Descriptive statistics were used to calculate the real-world best response. The Kaplan–Meier method estimated real-world time to first response (rwTTFR) and real-world progression-free survival (rwPFS).
Results
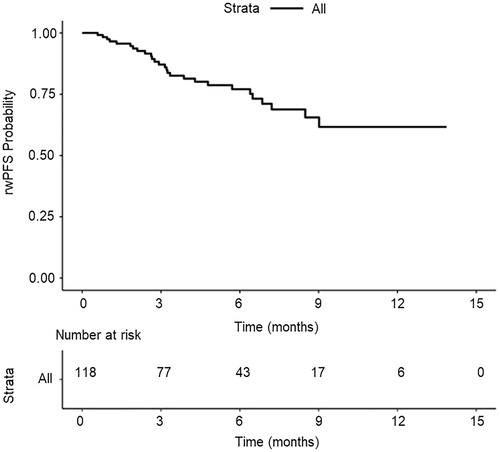
The median age of 118 female patients at abemaciclib initiation was 66.5 years (interquartile range, 57.0, 73.0). The breakdown of patients who received abemaciclib in first, second, third, or later lines was 28.8%, 21.2%, 20.3%, and 29.7%, respectively. Patients received abemaciclib as monotherapy (12.7%) or in combination with endocrine therapy: fulvestrant (59.3%); aromatase inhibitor (22.9%); aromatase inhibitor and fulvestrant (5.1%). There were 68 patients (57.6%) with ≥1 rwR assessment: 41.2% with a real-world complete response or real-world partial response. Median rwTTFR was 3.6 months (95% confidence interval, 3.5, 5.2). Twelve-month rwPFS probability was 61.7%.
Conclusions
This study represents utilization and outcomes associated with abemaciclib approximately 1 year following FDA approval. Treatment patterns demonstrated heterogeneity and, as in clinical trials, patients appeared to benefit from abemaciclib treatment in the real world. More research investigating outcomes associated with abemaciclib treatment is needed, with larger samples and longer follow-up to enable closer evaluation by subgroup, regimen, and line of therapy.
Introduction
Cyclin-dependent kinase 4 and 6 (CDK4 and 6) inhibitors, a relatively new class of approved drugs, are considered a standard-of-care option, in combination with endocrine therapy (ET) and as monotherapy (for abemaciclib), for patients with hormone-receptor-positive (HR+), human epidermal growth factor receptor-2 negative (HER2−) metastatic breast cancer (mBC)Citation1. In February 2015, palbociclib was the first CDK4 and 6 inhibitor in combination with hormonal treatment to receive United States (US) Food and Drug Administration (FDA) approvalCitation2 for the treatment of HR+, HER2− mBC, followed by ribociclib (initial FDA approval: March 2017)Citation3 and abemaciclib (initial FDA approval: September 2017)Citation4.
Abemaciclib clinical trials have demonstrated efficacy as monotherapy and in combination with ET among patients with HR+, HER2− mBC across various treatment linesCitation5–11. However, few studies have examined abemaciclib utilization in routine clinical practice, including patient characteristics, treatment patterns, clinical effectiveness, and safety. Real-world observational data are an important complement to clinical trials, given the more heterogeneous patient populations they representCitation12. This study characterized abemaciclib use during the first year of adoption into routine clinical practice among patients with HR+, HER2− mBC in the US, primarily in the community oncology setting.
Methods
Study design
This retrospective observational study utilized data from the Flatiron Health electronic health record (EHR)-derived de-identified database. Demographically and geographically diverse, the database contains data from over 280 cancer clinics (approximately 800 sites of care), representing more than 2.2 million patients with cancer from the USCitation13. As of November 2018, this database included approximately 18,000 patients with mBC treated at community and academic practices.
Patient-level data were obtained from structured (e.g. vital signs, laboratory analysis) and unstructured (e.g. clinic notes, radiology reports) sources. Data were processed with technology-enabled chart abstraction, reviewed by trained abstractors, and de-identified with provisions to prevent re-identification to protect patient confidentiality. Approval of the study protocol was obtained from the Copernicus Group Institutional Review Board prior to study conduct and included a waiver of informed consent (IRB # RWE-001, “The Flatiron Health Real-World Evidence Parent Protocol”, Tracking # FLI118044).
Study population
Patients were eligible for inclusion if they met the following criteria: (1) breast cancer diagnosis (ICD-9 174.x or 175.x or ICD-10 C50.x); (2) ≥2 visits in the database on or after 1 January 2011; (3) pathology consistent with breast cancer; and (4) confirmed the diagnosis with stage IV or recurrent HR+, HER2− mBC with a metastatic diagnosis date on or after 1 January 2011. Confirmation of HR+, HER2− status was required before or up to 60 days after metastatic diagnosis. HR+ status was defined as any estrogen receptor-positive and/or progesterone receptor-positive test. HER2− was defined by any negative HER2 test result and the absence of a positive test (immunohistochemistry positive [3+], Fluorescence In Situ Hybridization positive/amplified, positive not otherwise specified). The overall study design included patients with abemaciclib, palbociclib, or ribociclib use (Supplemental Figure 1). All patients in this analysis received abemaciclib (monotherapy or combination therapy across all lines, excluding lines with a clinical study drug) as systemic therapy in the metastatic setting on or after 30 June 2016. Because abemaciclib was not approved until 28 September 2017, some patients received ribociclib or palbociclib between 30 June 2016 and 28 September 2018; however, patients who specifically received first line (1 L) palbociclib + aromatase inhibitor (AI), 1 L or second line (2 L) palbociclib + fulvestrant, or 1 L ribociclib + AI prior to abemaciclib were excluded to enable mutually exclusive cohorts (Supplemental Figure 1). Abemaciclib initiation had to be ≥4 months prior to data cutoff (31 December 2018, inclusive), to allow ≥4 months of potential follow-up. Patients with a gap of over 90 days between metastatic diagnosis and first subsequent structured EHR activity were excluded due to possible missing data. Female sex was not an inclusion criterion, but no males were included in these analyses.
Key variables
The index date was defined as the earliest abemaciclib initiation date, and line of therapy (LoT) as the earliest distinct treatment regimen number in the metastatic setting where abemaciclib was given. Dose change was defined as a ≥ 50 mg increase or decrease in daily abemaciclib dose, and schedule change was an alteration in abemaciclib administration frequency. Schedule changes did not consider if the daily dose was also changed. Dose modification encompassed dose reductions, increases, and/or schedule changes.
Patient baseline demographic and clinical characteristics, and prior CDK4 and 6 inhibitor use were recorded at index date unless otherwise specified. Menopausal status was not available in this data set; a proxy for menopausal status was age categorized as ≤60 versus >60 years. The modified Charlson Comorbidity Index (CCI)Citation14,Citation15 was calculated using comorbidities documented by the treating physician at any time prior to the index date (note: cancer was excluded from the CCI calculation).
The number and location of metastatic sites were recorded (specifically, metastases that occurred before or in the same month/year as the index date), and included: liver, lung, bone, brain, other central nervous systems, and/or distant lymph node. Visceral metastases were defined as the presence of lung and/or liver metastases. Cancer stage and tumor grade were collected at initial breast cancer diagnosis dates. The time between initial and metastatic diagnoses was measured among those who were diagnosed at a stage earlier than metastatic. Follow-up time was measured from the index date until the patient’s last known structured EHR activity (most recent visit).
Real-world response (rwR) assessments were defined as the treating clinician’s assessment of change in disease burden following radiographic imaging over the treatment course with a given therapy, using previously described methodsCitation13. RwR assessments during abemaciclib therapy were abstracted based on documentation recorded between 30 days post-index date to the end of abemaciclib therapy, defined as the day before the start of subsequent therapy, the end of EHR activity, or date of death for patients not initiating a subsequent LoT. These assessments were subsequently used to calculate rwR among patients with ≥1 rwR assessment, defined as the best of real-world complete response (rwCR), real-world partial response (rwPR), real-world stable disease (rwSD), or real-world progressive disease (rwPD). Real-world time to first response (rwTTFR) was defined as the time from index date to first rwCR or rwPR, among patients achieving rwCR or rwPR.
Previously described real-world progression eventsCitation16 were abstracted from clinician notes and documented by a direct or indirect description of disease progression, or by clinician acknowledgement of source evidence (e.g. radiology reports) consistent with progression. The date on which progression events were observed was recorded. Real-world progression-free survival (rwPFS) was defined as the time from index date to first real-world progression date >14 days after the index date, or death during abemaciclib therapy (if the patient did not experience progression).
Real-world adverse effects (rwAEs) of interest included diarrhea, venous thromboembolic events (VTE), neutropenia, and liver enzyme elevation. Neutropenia was derived using structured laboratory data and defined as decreased neutrophil or granulocyte counts (Supplemental Table 1). Elevated liver enzymes were derived from structured data and defined as increased aspartate aminotransferase (AST), alanine aminotransferase (ALT), or total bilirubin levels (Supplemental Table 1). Grades for structured laboratory-based rwAEs were derived based on The National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 5Citation17. Grade of diarrhea or VTE was not available as commonly used AE severity scales (e.g. CTCAE) were developed for clinical trial purposes and are not typically used or recorded in the routine clinical practice setting. RwAEs derived from structured data were investigated during index therapy and the baseline period (i.e. 30 days prior to index date).
The first incidence of diarrhea and VTE (including pulmonary embolism and/or deep vein thrombosis), and the earliest onset date during index therapy were abstracted from unstructured data and defined as an unfavorable sign, symptom, or diagnosis that began or worsened (if the rwAE was present in the medical history or prior to index date). The following outcomes due to diarrhea and VTE were captured when available: therapy (any component drug of the index line of therapy) dose or schedule change, hold, or discontinuation; medication for the rwAE (if any pharmacological or non-pharmacological treatment was recommended, prescribed, or administered to treat the rwAE); and hospitalization.
If a dose change was attributed to multiple rwAEs in the index line of therapy, the dose change was listed with each AE.
Statistical analysis
Descriptive statistics summarized baseline characteristics, treatment patterns, rwR, and rwAEs. Time-to-onset for rwAEs was calculated as the time between index date and earliest date of rwAE onset during index therapy (amongst patients who experienced the rwAE during index therapy). Because this analysis was calculated only among patients who experienced the AE of interest, censoring was not needed. The Kaplan–Meier method estimated rwTTFR, rwPFS, time to first dose hold, and time to dose reduction. Because rwTTFR analysis included only patients who experienced a CR or PR, censoring was not needed. Patients without an rwPFS event were censored at their last clinic note date. Patients without a dose hold/reduction were censored at the documented end date of the CDK4 and 6 inhibitor episodes of interest. Due to limited follow-up time for this cohort, the estimated median time to event (e.g. rwPFS, time to first dose reduction, etc.) was not reached if Kaplan–Meier curves did not cross the 50% threshold. All analyses were performed in R version 3.3.1 (R Foundation for Statistical Computing) or SAS Enterprise Guide 7.1.
Results
Baseline characteristics
There were 118 patients who met the eligibility criteria for this study (Supplemental Figure 2). There was no abemaciclib utilization prior to regulatory approval (28 September 2017)Citation4; 75.4% of patients received abemaciclib as their first CDK4 and 6 inhibitor. Patients had a median follow-up of 6.4 months (interquartile range [IQR], 4.1, 9.5). All patients in this cohort were female. The median age at metastatic diagnosis was 65.0 years (IQR, 55.2, 71.0), and at abemaciclib initiation was 66.5 years (IQR, 57.0, 73.0; ). In this cohort, 63.6% of patients were White, 9.3% were Black or African American, 8.5% were classified as other race, and 2.5% were Asian; 16.1% of patients had missing race data (). Most patients (88.9%) had a modified CCI of 0/unknown or 1 at index date (). A majority of patients received treatment in a community setting (98.3%) (). At initial diagnosis, most patients had Stage III (28.8%) or Stage IV (34.7%) disease and Grade 2 tumor (45.8%; ). Among patients who were not metastatic at initial diagnosis, most patients (79.2%) had ≥2 years between initial and metastatic diagnosis (). At abemaciclib initiation, Eastern Cooperative Oncology Group performance status (ECOG PS) scores were: 0 (31.4%), 1 (27.1%), 2 (8.5%), and 3 (3.4%); ECOG PS was unknown for 29.7% of patients (). At abemaciclib initiation, the median number of involved metastatic sites was 2, with 82.2% having metastases to the bone (). Nearly half of all patients (49.2%) had visceral metastases (), including lung (34.7%) and/or liver (22.9%); 7.6% had brain metastases.
Table 1. Baseline patient and clinical characteristics.
Treatment regimens
In this cohort, 28.8% of patients received abemaciclib in 1 L, 21.2% in 2 L, 20.3% in the third line (3 L), and 29.7% in later lines (4 + L; ). Patients received abemaciclib in combination with ET, including fulvestrant (59.3%), AI (22.9%), or fulvestrant plus AI (5.1%). Abemaciclib was given as monotherapy in 12.7% of patients.
Table 2. Abemaciclib treatment regimens in a real-world setting.
Among patients who received abemaciclib in ≥2 L (n = 84), 29 (34.5%) received a prior CDK4 and 6 inhibitor in the metastatic setting, 42 (50.0%) received prior chemotherapy in the metastatic setting, and 13 (15.5%) received a prior CDK4 and 6 inhibitor and prior chemotherapy, both in the metastatic setting.
Abemaciclib dosing
Among patients taking abemaciclib in combination with ET (n = 103), 87.4% (n = 90) initiated abemaciclib at 150 mg twice daily. Among patients taking abemaciclib monotherapy (n = 15), 40.0% (n = 6) initiated at 200 mg twice daily ().
Table 3. Abemaciclib treatment attributes in a real-world setting.
Among patients in this cohort, 37.3% had a dose hold (i.e. withheld a dose of treatment, irrespective of subsequent dose modifications), 6.8% had a dose reduction, and 21.2% had a schedule change (). Among patients who experienced a dose hold, the median time to first hold was 10.4 months. Median time to first dose reduction was not reached (). Among patients with a schedule change, 72.0% changed from twice to once daily.
Real-world time to response
At least one rwR assessment was observed among 68 patients in the response assessment cohort, of whom 4 (5.9%) had an rwCR, and 24 (35.3%) had an rwPR. Among those 28 patients who experienced rwCR or rwPR, the median rwTTFR across all index abemaciclib-containing regimens was 3.6 months (95% CI, 3.5, 5.2). RwSD was observed for 33.8% and rwPD for 25.0% (). There was a trend toward higher rates of rwPR in 1 L (60.0%) compared to 2 L (31.2%), 3 L (28.6%), and 4 + L (16.7%).
Table 4. Real-world best response.
Real-world progression-free survival
Median rwPFS was not reached in this cohort (95% CI, 9.0, not reached) (). The overall 12-month rwPFS probability across all regimens and lines of therapies was 61.7%.
Real-world adverse effects
Diarrhea occurred during index therapy for 79 (67.0%) patients, of whom 5 (6.3%) had diarrhea at baseline. The median time from index date to diarrhea onset was 0.9 months (IQR, 0.5, 1.7). Among 118 abemaciclib patients, 6.8% were hospitalized due to diarrhea, 46.6% were prescribed anti-diarrheal medication, 11.9% experienced treatment discontinuation (any treatment component), 14.4% had a dose hold, and 12.7% had a treatment dose or schedule change due to diarrhea ().
Table 5. Real-world adverse effects of interest during index therapy (new or worsening event) and medical history of these real-world adverse events.
Forty-three (36.4%) patients experienced any-grade neutropenia during index therapy, of whom 4 (9.3%) had a medical history of neutropenia at baseline. During index therapy, 8 (6.8%) patients experienced grade 3, and 1 (0.8%) experienced grade 4 neutropenia. The median time from index date to earliest neutropenia onset was 1.0 month (IQR, 0.7, 1.6), regardless of neutropenia history.
Filgrastim/pegfilgrastim use during index therapy on or after initial neutropenia onset was observed in 2 (1.7%) patients ().
During index therapy, any elevated liver enzyme occurred in 9 (7.6%) patients, 5 (55.6%) of whom had a medical history of any elevated liver enzyme. During index therapy, grade 3 elevated liver enzymes occurred in 2 (1.7%) patients, and no patients experienced grade 4. For all patients with elevated liver enzymes during index therapy, the median time from index date to onset was 1.8 months (IQR, 1.2, 2.4). For patients with no elevated liver enzymes at baseline but who developed this rwAE during index therapy, the median time from index date to onset was 3.0 months (IQR, 2.1, 6.9). Three (2.5%) patients had any-grade AST elevation, 3 (2.5%) had any-grade ALT elevation, and 4 (3.4%) had an elevated bilirubin levels during index therapy (). One patient (0.8%) had grade 3 AST elevation and 2 (1.7%) had grade 3 ALT elevation; no patients had grade 4 AST or ALT elevation, or grade ≥3 bilirubin.
VTE occurred in 4 (3.4%) patients during index therapy, 1 (25.0%) of whom had a medical history of VTE. Median time from index date to earliest onset was 1.3 months (IQR, 0.9, 1.8). During index therapy, 1 (0.8) patient was hospitalized due to VTE, 4 (3.4%) received medication for VTE; no patient had a therapy hold due to VTE.
In this study, no patients had an AE outcome of death.
Discussion
This study evaluated real-world abemaciclib outcomes and utilization among patients with HR+, HER2− mBC, within the first year following initial FDA approval. These results complement clinical trials characterizing abemaciclib effectivenessCitation5–11 and support clinical benefit in a predominantly community oncology patient population. In this cohort, >80% of patients were treated with abemaciclib in combination with fulvestrant or AI, which is likely a reflection of the timing of regulatory approval and the study period and is consistent with treatment in MONARCH 2 and 3Citation6,Citation11. Just over a third of patients experienced a rwPR or rwCR, and an additional third experienced rwSD. These results are generally consistent with overall response rates of 35.2% in MONARCH 2Citation11 and 59% in MONARCH 3Citation6.
Differences in a clinical trial versus real-world tumor assessments should be noted. Clinical trials often utilize Response Evaluation Criteria in Solid Tumors (RECIST)Citation18; however, in real-world studies such as this one, tumor response was based on the treating clinician’s assessment of disease burden change as documented in the patient chart, which may be influenced by the heterogeneous interpretation of radiological reports, and/or missing data. These differences may bias tumor response estimates in the real world, with the potential for the bias to be in either directionCitation16,Citation19. In spite of these differences, the 12-month rwPFS probability of 61.7% directionally aligns with RECIST determined 12-month PFS rates in MONARCH 2 (61.1%) and MONARCH 3 (73.0%; unpublished data).
RwAE diarrhea incidence was lower than clinical trials (∼85%)Citation6,Citation7,Citation10,Citation11; however, abemaciclib discontinuation due to diarrhea was higher in the real-world, occurring in 11.9% of this cohort compared to <3.0% in MONARCH 2 and 3Citation20. Anti-diarrheal medication use among all patients with diarrhea was consistent with clinical trial results (∼70%)Citation21. Median time-to-onset of diarrhea symptoms was longer in the real-world (27 days versus 6–8 days in clinical trials)Citation20. Differences in abemaciclib discontinuation due to diarrhea and median time-to-onset of diarrhea symptoms may be due to heterogeneity in side effect management; clinical trial physicians follow a strict protocol and monitor patients in pre-specified ascertainment windows, which is not done in the real-world setting. Since grading is not typically used in a clinical practice setting, grade was not available for the rwAE of diarrhea or VTE, unlike CTCAE grade information available in clinical trials. RwAE incidence of any grade and grade ≥3 neutropenia was lower (36% and 8%, respectively) than in MONARCH 2 (46% and 27%) and 3 (44% and 24%)Citation21; this may be due to laboratory measurement occurring at a different frequency in clinical trials versus the real-world setting, or due to applied derivation rules. Elevated liver enzymes occurred at lower rates in the real world than in clinical trials. Incidence of any-grade VTEs was consistent with clinical trials.
A majority of patients did not require a dose reduction (93.2%) or schedule change (78.8%), suggesting abemaciclib was tolerable. Tolerability was further supported by median time to dose reduction not being reached, and a median time to first dose hold of 10.4 months. Interestingly, in MONARCH trials, the median time to dose reduction occurred within the first 3 treatment cycles, and the median time to dose hold was not reachedCitation5,Citation11. Differences are possibly explained by a standard protocol for physicians to follow in a clinical trial compared to the real world where each physician follows the best judgment considering the label, personal experience, and other patient factors.
These results were consistent with real-world utilization studies of new drugs showing the early post-approval period is often characterized by heterogeneity in useCitation12,Citation22, as demonstrated by varying abemaciclib use across lines of therapy and regimens. In this period, there is also often a channeling of therapy to patients with more severe disease and a poorer prognosisCitation12; patients receiving abemaciclib in this cohort tended to have characteristics indicating a less favorable prognosisCitation23. For example, approximately 50% of patients had visceral metastases, >70% received abemaciclib in the 2 L or later, 20.8% had <2 years between initial and metastatic diagnosis, and 12% had ECOG PS of 2 at baseline (∼30% missing data for ECOG PS). We did not investigate the association between patient characteristics and line of therapy. This is likely due to patients who have been in the metastatic setting for a longer time or who have had disease progression on other therapies/therapy combinations being selected for treatment with abemaciclib as soon as it received FDA approval. Furthermore, the frontline indication for abemaciclib was not approved until February of 2018, thus there was a very limited window of time in which patients receiving front-line abemaciclib could have been included in this study.
Among 118 patients in this cohort, 25% received a prior CDK4 and 6 inhibitor. Moreover, there were 34 patients excluded from the abemaciclib cohort, due to the overall broader study design, who received prior 1 L or 2 L palbociclib or ribociclib. The reason for discontinuation (e.g. disease progression, toxicity) of the CDK4 and 6 inhibitor used prior to abemaciclib was not evaluated in this study. It is important to note that currently there is no strong evidence in the literature to support the use of a CDK4 and 6 inhibitor following progression on another CDK4 and 6 inhibitor; specific to abemaciclib, there is no regulatory-approved indication supporting the use of abemaciclib following either palbociclib or ribociclib treatment for patients with mBC.
Some potential limitations should be considered when interpreting these results. Of note is the relatively small sample of patients receiving abemaciclib, which limited the extent of analyses and potential generalizability of these results. A small sample size was a result of the timing of this report, where data were examined approximately 1-year post-initial FDA approval. Future real-world abemaciclib studies with longer follow-up are planned. Response assessments were not available for 50 patients, which prevented stratification by subgroups that may be more or less likely to receive abemaciclib benefitCitation10,Citation23. This may be due to lack of eligible scans during the relevant response window and/or responses for these patients that were indeterminate (e.g. “clinician notes indeterminate response”, or “clinician interpretation cannot be mapped to assessment category”), or not documented (e.g. “patient had a recent scan, but no mention of results”). Time-to-event analyses were also limited by sample size, therefore rwPFS investigation by subgroups of interest was not possible. Future studies should investigate real-world abemaciclib-associated outcomes with larger sample sizes. Common to an observational, retrospective study design with an EHR-derived database, data are subject to missing data and miscoding errors. For example, there may be limited or no information about patients’ treatment outside the specific clinical oncology practice, and the extent to which historical data are entered into the EHR varies widely across practices. Missing data may include information such as race (16% missing here), ECOG PS (30% missing here), and dose/schedule changes (dose/schedule changes here do not indicate whether the daily dose was affected). RwAE data may be limited by incomplete documentation in the patient record or in structured data used to derive rwAEs. Miscoding or misclassifications may occur when deriving variables based on what is entered into the EHR (e.g. LoT, menopausal status, historical data artifacts). For example, some patients received abemaciclib simultaneously in combination with AI and fulvestrant which could reflect treatment misclassification or clinician decisionCitation24. Neutrophil and granulocyte count were used interchangeably when deriving neutropenia; granulocyte count includes cell types other than neutrophils (e.g. basophils, eosinophils), therefore neutropenia may be overestimated in those cases, albeit by a very small amount. Median follow-up time was limited to 6.4 months, and time-dependent clinical outcomes, such as PFS, are not mature given the short follow-up. Due to cohort exclusions in study design and a relatively higher proportion of community practice use, generalizability to other populations may be limited. Finally, real-world data reflect how abemaciclib is being used in a clinical practice setting, therefore some treatment and utilization patterns may not be fully aligned with the label (e.g. abemaciclib + AI + fulvestrant).
Conclusion
These results, representing abemaciclib utilization in the first year following initial FDA approval, were consistent with real-world utilization of new drugsCitation12,Citation22, demonstrating significant heterogeneity in treatment patterns and abemaciclib administration to patients with characteristics typically indicative of a less favorable prognosis. Despite this, abemaciclib-based therapy demonstrated responses, PFS rates, and a safety profile that generally complement prior MONARCH studiesCitation5–7,Citation10,Citation11. These results support clinical benefit in the real-world setting. Future research should monitor evolving treatment patterns and outcomes for patients in a real-world setting, with a longer post-FDA approval period. This includes future exploration of practice patterns and patient outcomes of CDK4 and 6 inhibitor sequencing observed in the clinical practice setting.
Transparency
Declaration of funding
This study was funded by Eli Lilly and Company. Authors on this manuscript who are employees of Eli Lilly and Company participated in the design and conduct of this study, and in the writing of this manuscript.
Declaration of financial/other relationships
GCC, KMS, Y-JH, YEZ, LB, ENS, CMG, and SR are all employees and shareholders at Eli Lilly and Company. AG, RM, ABC, and SB are all employees of Flatiron Health, Inc., which is an independent subsidiary of the Roche group, and owns stock in Roche; AG, ABC, and SB own stock in Flatiron Health. ER is a former employee of Flatiron Health, Inc. ADS reports receiving personal fees from Eli Lilly and Company, Novartis, and Pfizer, and institutional research funding from Novartis. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
GCC, KMS, AG, RM, ABC, ER, SB, and SR contributed to study conception and design. Material preparation, data collection, data analysis, and interpretation of data were performed by GCC, KMS, AG, ENS, RM, ABC, ER, SB and AS. The manuscript was drafted and edited by all authors, who provided detailed comments on all versions of the manuscript. All authors read and approved the final manuscript.
Supplemental Figure 2
Download TIFF Image (79.1 KB)Supplemental Figure 1
Download TIFF Image (401.9 KB)Supplemental Material
Download MS Word (24.4 KB)Acknowledgements
This work was funded by Eli Lilly and Company. Eli Lilly and Company contracted with Syneos Health for writing and editorial support from Andrea Metti, Ph.D., MPH, and Antonia Baldo.
Data availability statement
The data that support the findings of this study have been originated by Flatiron Health, Inc. These de-identified data may be made available upon request, and are subject to a license agreement with Flatiron Health; interested researchers should contact <[email protected]> to determine licensing terms.
References
- Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634–1657.
- Dhillon S. Palbociclib: first global approval. Drugs. 2015;75:541–543.
- Syed YY. Ribociclib: first global approval. Drugs. 2017;77:799–807.
- Kim ES. Abemaciclib: first global approval. Drugs. 2017;77:2063–2070.
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224.
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646.
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5.
- Neven P, Johnston SRD, Toi M, et al. MONARCH 2: subgroup analysis of patients receiving abemaciclib + fulvestrant as first- and second-line therapy for HR+, HER2- advanced breast cancer [abstract]. J Clin Oncol. 2020;38(15):1061–1061.
- Rugo HS, Tolaney SM, Cortes J, et al. MONARCH 1: final overall survival analysis of a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. Cancer Res. 2017;77(13):CT044.
- Sledge GW, Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy - MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124.
- Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884.
- Schneeweiss S, Gagne JJ, Glynn RJ, et al. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. 2011;90:777–790.
- Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR [Internet]. New York (NY): Cold Spring Harbor Laboratory; 2020 [cited 2020 Dec 9]. Available from: https://www.medrxiv.org/content/10.1101/2020.03.16.20037143v2
- Charlson M, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Quan H, Li B, Couris C, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682.
- Griffith SD, Tucker M, Bowser B, et al. Generating real-world tumor burden endpoints from electronic health record data: comparison of RECIST, radiology-anchored, and clinician-anchored approaches for abstracting real-world progression in non-small cell lung cancer. Adv Ther. 2019;36:2122–2136.
- National Cancer Institute [Internet]. Cancer therapy evaluation program. Bethesda (MD): Common Terminology Criteria for Adverse Events (CTCAE), version 5. 2020 [cited 2020 July 8]. Available from: https://ctep.Cancer.Gov/protocoldevelopment/electronic_applications/ctc.Htm#ctc_50
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Feinberg BA, Bharmal M, Klink AJ, et al. Using response evaluation criteria in solid tumors in real-world evidence cancer research. Future Oncol. 2018;14:2841–2848.
- Rugo HS, Sledge GW, Johnston SRD, et al. The association of early toxicity and outcomes for patients treated with abemaciclib [abstract. J Clin Oncol. 2018;36(15):1053–1053.
- Rugo HS, Tolaney SM, Huober J, et al. Management of abemaciclib associated adverse events in patients with hormone receptor positive (HR+), HER2- advanced breast cancer: analysis of the MONARCH trials. Ann Oncol. 2018;29(8):viii90–viii121.
- Rassen JA, Schneeweiss S. Newly marketed medications present unique challenges for nonrandomized comparative effectiveness analyses. J Comp Eff Res. 2012;1:109–111.
- Di Leo A, O’Shaughnessy J, Sledge GW Jr, et al. Prognostic characteristics in hormone receptor-positive advanced breast cancer and characterization of abemaciclib efficacy. NPJ Breast Cancer. 2018;4(1):41.
- Mehta RS, Barlow WE, Albain KS, et al. Overall survival with fulvestrant plus anastrozole in metastatic breast cancer. N Engl J Med. 2019;380:1226–1234.