ABSTRACT
Kauri is an ecologically important and culturally treasured tree species in Aotearoa New Zealand. It is under threat from the pathogenic oomycete Phytophthora agathidicida, which causes kauri dieback disease. We hypothesised that mātauranga Māori (Māori knowledge) of kauri forest health could be used to identify native plants that produce anti-Phytophthora compounds. We tested this hypothesis by using knowledge descended from Te Whare Wananga o Ngāpuhi to select and screen four native plants for activity against P. agathidicida and also P. cinnamomi (a broad host-range pathogen). Extracts of kānuka (Kunzea robusta) were active against various life cycle stages. Bioassay-directed isolation led to three flavanones, previously unreported from New Zealand Kunzea, as the main bioactives. These compounds have not previously been reported as having anti-Phytophthora activities. They inhibited P. agathidicida zoospore germination with IC50 values of 1.4–6.5 µg/mL, making them the most potent inhibitors reported against this stage of the life cycle. The three flavanones also inhibited zoospore motility at 2.5–5.0 µg/mL, and showed some inhibition of mycelial growth at 100 µg/mL. They were generally less active against P. cinnamomi. Overall, the results from this study emphasise the value of using mātauranga Māori in the response to kauri dieback.
Introduction
Kauri (Agathis australis) is an important endemic species in New Zealand. It is a foundation species that has a profound influence on the surrounding soil, canopy, and biodiversity (Waipara et al. Citation2013; Wyse et al. Citation2013; Wyse et al. Citation2014). Kauri is also one of the longest-lived species known, with individual trees reported to be more than 1500 years old (Steward and Beveridge Citation2010). Massive trees with heights of up to 60 m and trunk diameters exceeding 7 m have been recorded (Ecroyd Citation1982; Steward and Beveridge Citation2010). However, land clearance and a century of unregulated logging (circa 1830–1930) has dramatically reduced the area of virgin primary kauri forest in New Zealand to less than 1% of that at the time of European settlement (Steward and Beveridge Citation2010). Kauri trees are now also threatened by the pathogen Phytophthora agathidicida, which causes kauri dieback disease (Beever et al. Citation2009; Weir et al. Citation2015).
P. agathidicida is a member of the oomycete genus Phytophthora, other members of which cause diseases in thousands of economically and ecologically important plants worldwide. Often referred to as ‘fungus-like’, Phytophthora are actually more closely related to diatoms and brown algae (Baldauf et al. Citation2000). Practically speaking, this means Phytophthora are unaffected by most agrichemical fungicides. Phytophthora lack many of the common fungicide targets, such as the ergosterol biosynthesis pathway and chitin-based cell walls (Judelson and Blanco Citation2005; Oliver and Hewitt Citation2014). Phytophthora also have several life cycle stages that are not found in most true fungi. In addition to a mycelial growth phase (similar to fungi), P. agathidicida produces two key types of spores: oospores and zoospores (Weir et al. Citation2015). Oospores are non-motile ‘survival’ spores, which are generally known to persist in plant tissues or the surrounding soil for years after the host plant dies (Collins et al. Citation2012; Crone et al. Citation2013). Zoospores are motile ‘dispersal’ spores. They are key to the epidemic spread of disease, facilitating host-to-host transmission (Carlile Citation1985; Tyler Citation2002). Once a zoospore locates a host plant, it encysts and initiates infection (Judelson and Blanco Citation2005).
When a kauri tree is infected by P. agathidicida, several symptoms typically occur including root and collar rot, trunk lesions, canopy thinning, and ultimately tree death (Beever et al. Citation2009; Bellgard et al. Citation2016). Trees of all ages are susceptible. Therefore this disease poses a significant threat to the long-term survival of kauri (Beever et al. Citation2009; Waipara et al. Citation2013).
Globally, there are few anti-oomycete formulations available for controlling Phytophthora diseases. Phosphite is the only treatment currently being used to control P. agathidicida (Horner Citation2013). It is primarily applied via injections into the trunks of trees that are already showing visible signs of infection. Furthermore, the use of existing agrichemicals, including phosphite, is jeopardised by increasing anti-microbial resistance around the world (Parra and Ristaino Citation2001; Dobrowolski et al. Citation2008; Gisi and Sierotzki Citation2008; Miao et al. Citation2016). There is an urgent need to discover and develop novel compounds that target the growth, survival and dispersal of Phytophthora in general, and of P. agathidicida in the particular case of controlling kauri dieback.
Plants are rich sources of known and potential anti-microbial compounds (Bennett and Wallsgrove Citation1994; Cowan Citation1999; Abreu et al. Citation2012; Pusztahelyi et al. Citation2015). In this study, we have begun to explore the anti-Phytophthora potential of New Zealand native plants. There are over 2,300 vascular plants native to New Zealand (de Lange et al. Citation2018) and ∼80% of these are endemic. Many are already known to be useful in rongoā (indigenous plant-based medicine practices). Therefore, we hypothesised that mātauranga Māori (Māori knowledge) could be used to select native plants that were most likely to produce anti-Phytophthora compounds.
Here we report the screening of four plants that were selected based on mātauranga Māori for potential activity against P. agathidicida. In order to assess whether activity was specific to P. agathidicida, or generalisable to other Phytophthora, a second species (P. cinnamomi) was also tested. P. cinnamomi is a broad host-range pathogen capable of infecting thousands of different plant species worldwide (Kamoun et al. Citation2015; Hardham and Blackman Citation2018). In New Zealand, P. cinnamomi is found across native ecosystems, exotic forests, nurseries and both agricultural and horticultural settings (Scott and Williams Citation2014). P. cinnamomi has also been linked to ill-thrift of kauri (Waipara et al. Citation2013). However its overall impact in New Zealand remains poorly understood.
The four plants screened for anti-Phytophthora activities were harvested from Waima, New Zealand. Root and leaf extracts of each plant were screened in vitro for inhibition of three key steps of the Phytophthora lifecycle process: zoospore motility, zoospore germination, and mycelial growth. The results of this screening, as well as identification and further characterisation of three compounds purified from kānuka (Kunzea robusta), are reported.
Materials and methods
Selection of plants for screening
Mātauranga Māori was used as the basis for selection of four endemic plants for anti-Phytophthora screening. The knowledge used to select these plants descends from Te Whare Wananga o Ngāpuhi (the sacred house of learning of Ngāpuhi). More information on the history of this mātauranga can be found in the Supplemental Information.
Intact plant samples of kānuka (K. robusta de Lange et Toelken, family Myrtaceae, voucher code 180508_04), karamū (Coprosma robusta Raoul, Rubiaceae, 180508_05), kawakawa (Piper excelsum Forst. f., Piperaceae, 180508_01) and nīkau (Rhopalostylis sapida Wendl. et Drude, Arecaceae, 180508_02) were collected from the riparian margin of a tributary of the Waima River, Waima, New Zealand in May 2018. We note that Kunzea robusta is often referred to as kānuka; however, native speakers of Ngāpuhi dialect refer to this plant as mānuka – as its flowers and timber are white or mā. In order to avoid confusion, the name kānuka is used throughout this manuscript to describe Kunzea robusta. Other Māori/common names of plants species referred to in this paper are consistent with Ngāpuhi dialect.
One sample of each plant species was collected at the first harvest. Three additional kānuka plants were collected from the same location in July 2018 (180720_03, 04, 06). Botanical voucher samples were prepared for each plant and are available from the Plant & Food Research, University of Otago herbarium collection (see codes above). The identification of the kānuka as K. robusta was made by P. de Lange.
Preparation of crude extracts
Crude root and leaf extracts were prepared as follows: finely ground dried plant material (2 g) was shaken overnight with 95% ethanol (20 mL), then filtered to give extracts. Sub samples were taken and dried overnight in a SpeedVac (Thermo Scientific, Waltham, MA) at ambient temperature. Crude extracts were prepared by resuspending the dried samples in ethanol at approximately 10–20 mg/mL (i.e. the highest concentrations possible, as determined by solubilities of the dried extracts). The crude extract concentrations used in biological assays were 100-fold dilutions of these solutions. The final ethanol concentration in the biological assays was therefore 1% (v/v); this concentration of ethanol was shown to be non-inhibitory in the assays used.
Phytophthora isolates and culture conditions
P. agathidicida isolate NZFS 3770 and P. cinnamomi isolate NZFS 3910 (both provided by Scion, Rotorua, New Zealand) were routinely cultured at 22°C in darkness on clarified 20% V8 juice agar (cV8-agar). Detailed instructions for the preparation of this and other media used are provided in the Supplemental Information.
For zoospore production, 10 agar plugs (6 mm diameter) were removed from the edge of an actively growing mycelial mat and then transferred to a petri dish containing 15 mL of a 1:10 dilution of cV8 broth (P. cinnamomi) or carrot broth (P. agathidicida). These were grown for ∼30 h at 25°C. Broth was then removed and replaced with 15 mL of Chen-Zentmyer salt solution (for P. cinnamomi) or 5% (w/v) sterile soil extract (for P. agathidicida). Dishes were incubated at room temperature for 45 min, then the solutions removed and replaced with fresh salt solution/soil extract. This was repeated after another 45 min and the dishes were then incubated overnight at room temperature under light. The following morning, zoospore release was induced by removing the liquid and washing each dish three times with sterile Milli-Q water that had been cooled to 4°C. Each wash was for 20 min, with the first two at room temperature and the final wash at 4°C. Wash volumes were 15 mL per dish for the first two washes, and 10 mL for the final wash. Following the final wash, dishes were returned to room temperature for 30–90 min until sufficient numbers of zoospores had been released. Zoospore densities were in the range of 103–104 per mL.
Zoospore motility assays
Zoospore motility assays were conducted essentially as described previously (Lawrence et al. Citation2017). Briefly, motility assays were conducted in 24-well plates. Each well contained 1 mL of a zoospore suspension, and crude extracts were added to a final concentration of ∼100–200 µg/mL. Negative control wells contained 1% (v/v) ethanol. Wells were observed at 40 × magnification using an inverted microscope every 5 min for the first 30 min, then at 60 min and every 1 h thereafter. The observation time point at which no zoospores were moving in the well was recorded. The kānuka root and leaf extracts were retested at a range of concentrations (0–100 μg/mL for root extract; 0–200 μg/mL for leaf extract) under the same conditions as above. All assays were performed in duplicate.
Zoospore germination assays
Zoospore suspensions (50 μL) were added to 1 mL cornmeal agar wells amended with either crude extracts (∼100–200 μg/mL) or pure compounds. Negative control wells contained 1% (v/v) ethanol. The plates were incubated at 25°C overnight and 25 zoospores were counted per well. Zoospores with germ tubes greater than twice the spore diameter in length were considered germinated. Germination inhibition rates are reported as the percentage of inhibition relative to the number of germinated spores in the negative (ethanol only) control. All assays were performed in duplicate.
Mycelial growth inhibition assays
Assays were performed in 24-well plates. A 2 mm diameter plug was taken from the edge of an actively growing mycelial mat and placed in the centre of each well. Wells contained cornmeal agar amended with extracts at 100–200 μg/mL. Negative control wells contained 1% ethanol. Plates were incubated at 25°C for ∼24 h (depending on growth rate), and mat diameters were measured (two perpendicular measurements were averaged for each well). Growth inhibition was calculated by dividing the treatment mat diameter by the negative control mat diameter. Following this initial screen, extracts that showed mycelial growth inhibition (kānuka root and leaf) were tested again at a range of concentrations (0–100 μg/mL for root extract; 0–200 μg/mL for leaf extract), in duplicate, under the same conditions as above.
Extract fractionation and active compound preparation
Kānuka dried leaf sample was finely ground and extracted overnight by shaking with 95% ethanol, 5% H2O (1:10 leaf mass to solvent volume) then filtered to give an extract, which was stored at – 20°C. Extract (930 mg) was coated onto 1 g of Reversed-Phase (RP) C18 (Aldrich octadecyl-functionalised silica gel) by rotary evaporating at 30°C; then applied to a 5 g C18 Isolute SPE cartridge preconditioned with ethanol (EtOH), then 1:1 EtOH:H2O, then H2O (10 mL of each). Elution with 2 × 10 mL each of H2O, then 1:4 EtOH: H2O, 1:1 EtOH: H2O, 4:1 EtOH: H2O, EtOH, and ethyl acetate (EtOAc) gave twelve 10 mL fractions. Subsamples (200 μL) were vacuum-dried, resuspended in 96% ethanol, and tested against P. cinnamomi and P. agathidicida in zoospore germination and mycelial growth assays, as described above, to identify the active fractions.
A subsample (28 mg) of active RP fraction 7, eluted from the first RP column with 4:1 EtOH: H2O, was subjected to preparative RP-liquid chromatography (LC) using an Agilent HP1260, controlled with Agilent OpenLab, at 30°C on a C18 column (Phenomenex Luna ODS(3) 5 μm 100 Å 250 × 10 mm) with a 25 × 4 mm C18 guard column. Peaks were detected at 206 nm. The mobile phase was 60% acetonitrile (MeCN) and 40% H2O, both with 0.1% formic acid, with a flow rate of 5 mL/min. Six fractions were collected and compounds in fraction 4 (compound 1, about 8 min, 5 mg) and fraction 5 (compound 2, about 11 min, 5 mg) were identified by comparing nuclear magnetic resonance (NMR) spectra with published data. A later peak at about 15 min was collected from preparative RP-LC of active fraction 8, identified as compound 3 (<1 mg).
Compound characterisation and bio-activity assays
Analytical RP-LC
Analytical reversed phase RP-LC was carried out using an Agilent HP1260 controlled with Agilent OpenLab, at 30°C on a C18 column (Phenomenex Luna ODS(3) 5 μm 100 Å 150 × 3 mm) with a 2 × 4 mm C18 guard column. Peaks were detected at 206 and 295 nm. The mobile phase was MeCN in H2O, both with 0.1% formic acid, using a linear gradient starting at 10% MeCN:H2O to 100% MeCN over 40 min, returning to 10% MeCN:H2O over 5 min with a 5 min equilibration time, with a flow rate of 0.5 mL/min.
Gas chromatography–mass spectrometry (GC–MS)
Pure compounds were dissolved in chloroform to 0.4 mg/mL and analysed by GC–MS. Analyses were performed on an Agilent 7890A gas chromatograph with a CTC Analytics PAL system autosampler and an Agilent 5975C inert XL MSD with triple axis detector (under the control of Enhanced MassHunter software). The injector (260°C) was split 1:5. Injections (1 μL) were made into a 30-m Agilent HP5-ms column with a 0.25 mm internal diameter and 0.25 µm film. The carrier gas used was hydrogen with a flow of 1.5 mL/min. The flow was split using deactivated silica columns between the MS detector (MSD, split arm 0.5 mL × 0.1 mm ID) and the flame ionisation detector (FID) (2 mL × 0.18 mm ID). The oven was heated from 50°C to 175°C at 5°C/min then to 300°C at 20°C/min and held for 8.75 min. Detection was by total ion current mass spectrometry (the MS transfer line was held at 270°C, the MS source was held at 230°C and the MS quad held at 150°C) over the mass range 35–600 Da and FID. A series of n-alkanes was injected separately and used to calculate the retention indices (RIs) of the pure compounds.
NMR spectroscopy
NMR spectra were recorded for D6-acetone solutions at 25°C on a Varian instrument: 1H at 500 MHz, 13C at 125 MHz. 1H NMR spectra of crude extracts were recorded for CDCl3 solutions at 25°C on a Varian instrument at 400 MHz.
5,7-Dihydroxy-6-methylflavanone (strobopinin) 1
CAS Registry Number (RN) 11023-71-5; analytical RP-LC retention 26.67 min; GC Retention Index (RI) 2576; +ve EI-MS m/z (relative intensity %): 270 (M, 100), 193 (75), 166 (100), 138 (95); −ve ESI-MS m/z: 269.0820 (M−H, calc for C16H13O4 269.0819; 1H and 13C NMR) data matching (Mayer Citation1990) allowing for different solvent; [α]D (CHCl3, 0.02 mg/mL) −13.5°.
5,7-Dihydroxy-6,8-dimethylflavanone 2
RN 56297-79-1; RP-LC 28.67 min; GC RI 2592; +ve EI-MS m/z (%): 284 (M, 95), 207 (40), 180 (80), 152 (100); -ve ESI-MS m/z: 283.0981 (M−H, calc for C17H15O4 283.0976; 1H and 13C NMR) data matching (Mustafa et al. Citation2005); [α]D (CHCl3, 0.02 mg/mL) −12.0°.
5-Hydroxy-7-methoxy-6-methylflavanone 3
RN 55743-20-9; RP-LC 32.5 min; GC RI 2532; +ve EI-MS m/z (%): 284 (M, 100), 207 (90), 180 (100), 152 (80); 1H NMR data matching reference sample (Plant & Food Research unpublished).
Compound bio-activity assays
Zoospore germination, chemotaxis and motility, and mycelial growth assays were carried out in duplicate as previously described. No motility assays were done for 5-hydroxy-7-methoxy-6-methylflavanone (3) due to lack of material.
Intraspecific variation in compound abundance
Variation in compound abundance among individual kānuka plants was assessed using leaf and root extracts prepared from three plants collected in Waima, New Zealand, in August 2018. Extraction was done as described above. The concentrations of the three active compounds in each extract were determined by RP-LC.
Results
Selection of plants for screening
From the perspective of Ngāpuhi mātauranga, regenerating native forest of the type found in the northern kauri rainforest, broadly speaking, comes in three waves. The first wave are plants that help to secure, cleanse and prepare the soil for the next generations to follow. The second wave are plants which typically become the second story in the canopy. These plants, typically fruiting plants, bring the fertility and the conditions for high biodiversity. The third wave includes plants that are long lived, such as kauri. Third-wave plants bring stability and relative permanency. Kauri stand above all other plants as the great protector.
Based on this knowledge, four known first wave plants were selected for study: kānuka, karamū, kawakawa and nīkau. While these are all established rongoā (medicine) plants, they are also known (according to the mātauranga of Ngāpuhi) as essential parts of a natural process to establish and maintain the health of kauri forests. Therefore, this knowledge comes from and belongs to mana whenua (the iwi or hapū which hold customary rights and authority over land and taonga in an area).
Plants offered by mana whenua for testing were selected based on this traditional knowledge. While additional first wave plants are known, these are outside the scope of the present study.
Screening crude extracts for potential anti-Phytophthora activities
Crude extracts of kānuka, karamū, kawakawa and nīkau leaves and roots were tested for their ability to inhibit zoospore motility, zoospore germination, and mycelial growth of P. agathidicida and P. cinnamomi.
To assess the ability of the crude extracts to abolish zoospore motility, extracts were added to suspensions of swimming zoospores, and the motility of the spores was monitored over time (). The negative controls (1% ethanol) remained motile for >6 h. As shown in , most of the crude extracts tested had no impact on zoospore motility, with the zoospores remaining motile over the full length of the experiment (6 h). However, karamū root extract caused immediate motility loss for P. agathidicida zoospores and a modest reduction in motility time for P. cinnamomi zoospores. Most notably, the kānuka leaf and root extracts caused immediate loss of zoospore motility for both species of Phytophthora ().
Table 1. Effect of crude extracts on zoospore motility. The length of time zoospores remained motile after the addition of crude extract is shown .
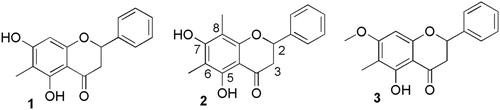
The crude leaf and root extracts of kānuka also completely eliminated germination of P. agathidicida zoospores (A); in these assays, zoospore lysis was observed (data not shown). In contrast, no lysis or appreciable inhibition of P. agathidicida zoospore germination was observed with either the root or leaf extracts of karamū, kawakawa or nīkau (A). For P. cinnamomi (B), kānuka leaf, but not kānuka root, substantially reduced zoospore germination. The other leaf and root extracts had only a moderate impact on P. cinnamomi germination rates (i.e. <50% inhibition) (B).
Figure 1. Effects of crude root and leaf extracts on zoospore germination. A, P. agathidicida zoospore germination. B, P. cinnamomi zoospore germination. Overall, kānuka leaf extract was the most effective at inhibiting zoospore germination of both species tested. Results from root extracts are shown as orange circles, leaf extracts are shown as blue squares, and the negative control (1% (v/v) ethanol) as grey diamonds. Vertical lines indicate the range, and horizontal lines indicate the mean from two independent assays.
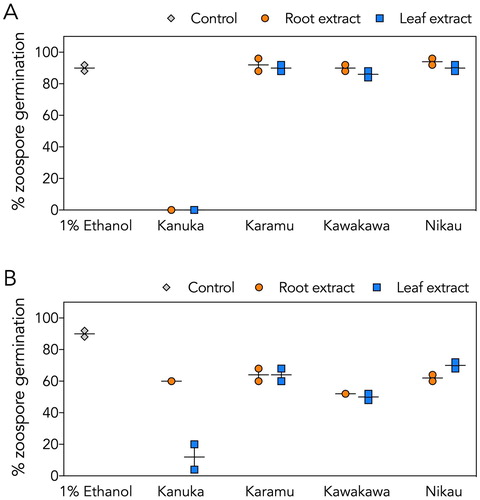
In mycelial growth assays, no inhibition was observed with the karamū, kawakawa or nīkau extracts (data not shown). The crude leaf and root extracts of kānuka showed slight inhibition of mycelial growth for both species (). The greatest inhibition observed was with kānuka leaf extract, which resulted in ∼40% inhibition of P. agathidicida mycelial growth (A).
Figure 2. Effect of crude kānuka root and leaf extracts on mycelial growth. A, P. agathidicida mycelial growth. B, P. cinnamomi mycelial growth. Growth is reported as a percentage relative to the growth of the negative controls (1% (v/v) ethanol). Results from root extracts are shown as orange circles; leaf extracts are shown as blue squares. Vertical lines indicate the range, and horizontal lines indicate the mean from two independent assays.
Overall, the crude kānuka extracts showed the highest activity in all assays performed and kānuka leaf extract was generally more effective than kānuka root. Based on these results, we selected the kānuka leaf extract for further characterisation.
Isolation and identification of kānuka anti-Phytophthora compounds
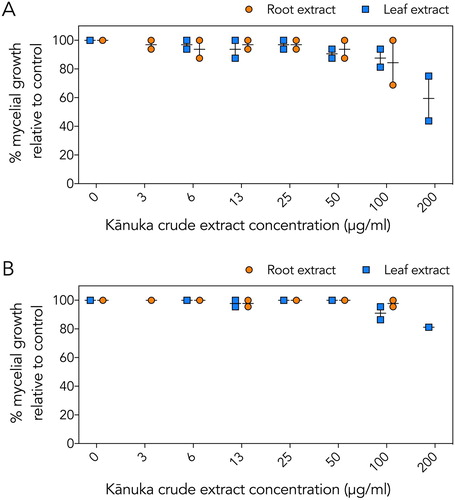
Bioactivity-directed fractionation was used to isolate the active compounds from the kānuka leaf extract. The extract was subjected to a rapid RP fractionation, which separates the molecules based on polarity (Blunt et al. Citation1987). Twelve fractions were generated, and these fractions were tested for bioactivity (). To simplify the screening workflow, we focused on the inhibition of P. agathidicida mycelial growth and zoospore germination as our primary bioactivity screens.
Figure 3. Bioactivity of kānuka leaf extract fractions. Fractions were tested for inhibition of P. agathidicida A mycelial growth and B zoospore germination. 1% (v/v) ethanol was used as the negative control (grey diamonds). Vertical lines indicate the range, and horizontal lines indicate the mean from two independent assays.
In mycelial growth assays, three of the medium polarity fractions (fractions 7, 8, and 9) inhibited growth (A). Fraction 7 displayed by far the highest level of activity, inhibiting mycelial growth by ∼75%. Fractions 7, 8 and 9 also strongly inhibited zoospore germination (B).
The main bioactive compounds in these fractions were purified by RP LC, and identified by high-resolution electrospray ionisation mass spectrometry and NMR spectroscopy. The three active compounds purified from the kānuka leaf extract were identified as flavanones by characteristic H2-H23 spin system in their 1H NMR spectra, which also showed unsubstituted B-rings (). NMR spectra showed that 1 was 5,7-dihydroxy-6-methylflavanone, 2 was 5,7-dihydroxy-6,8-dimethylflavanone, and 3 was 5-hydroxy-7-methoxy-6-methylflavanone. We could not find any previous reports of anti-Phytophthora activity for these flavanones.
Quantification of the anti-Phytophthora activities of isolated kānuka flavanones
The three isolated compounds were tested for their ability to inhibit mycelial growth and zoospore germination of both Phytophthora species. Zoospore motility assays were also conducted with compounds 1 and 2, but not with the small amount of 3 isolated.
Consistent with our observations for the crude extracts (), the purified flavanone compounds did not inhibit mycelial growth of P. cinnamomi, and were only weakly inhibitory to mycelial growth of P. agathidicida (Supplementary Figure 2). Of the compounds tested, 1 showed the strongest effect on P. agathidicida mycelial growth, reducing growth by approximately 50% at the highest concentration tested (100 μg/mL).
All three compounds were strongly inhibitory to zoospore germination, especially for P. agathidicida. The half maximal inhibitory concentration (IC50) for each compound was calculated using dose–response curves (Supplementary Figure 1). The calculated IC50 values for all compounds are presented in . Compound 1 was particularly effective at inhibiting P. agathidicida zoospore germination, with an IC50 value of 1.4 μg/mL. The IC50 values for compounds 1 and 3 were approximately twice as high in P. cinnamomi, while compound 2 was relatively ineffective against this species, with an IC50 around 90 μg/mL.
Table 2. IC50 values for inhibition of zoospore germination by compounds 1–3.a
Compounds 1 and 2 also inhibited zoospore motility in both Phytophthora species. The minimum concentration causing immediate loss of motility was ≤5 μg/mL for both compounds ().
Table 3. Minimum concentration of the compounds 1–3 that resulted in immediate loss of zoospore motility.
Preliminary assessment of plant-to-plant variation in abundance of compounds 1–3
Quantitative RP-LC analysis revealed that the active compounds were much more concentrated in leaf extracts than in root extracts (where they were barely detectable).
The yield of the bioactive compounds (1–3) varied within an order of magnitude between individual kānuka plants that were harvested from the same area but at different times of the year ().
Table 4. Concentrations of flavanones 1–3 in leaf extracts of four individual kānuka plants.
Discussion
Mātauranga-guided biodiscovery
The hit rate in this study, with one out of four rongoā plants showing strong anti-Phytophthora activity, supports the use of mātauranga Māori to focus screening efforts. For comparison, this hit rate is much higher than our previous study, in which we screened over 100 commercially-available antimicrobial compounds and identified only eight that showed activity against both P. agathidicida and P. cinnamomi (Lawrence et al. Citation2017). These results are also consistent with the other larger international studies that demonstrate the predictive power of traditional knowledge systems in bioprospecting efforts (Saslis-Lagoudakis et al. Citation2012; Buenz et al. Citation2018).
The most active extracts obtained by the preparation methods used in this study were from kānuka (Kunzea robusta). We note that other preparation methods (in particular, methods based on rongoā knowledge) may yield different results. Overall, the kānuka leaves contained higher levels of active compounds than roots, therefore we predict that the flavanones are localised within ‘oil glands’, as they are in mānuka (Killeen et al. Citation2015). The main bioactives in kānuka leaves (flavanones 1 and 2) exerted strong inhibitory effects on zoospore motility in both P. agathidicida and P. cinnamomi (). Compounds 1–3 all inhibited the germination of P. agathidicida zoospores with IC50 values in the range 1.4–6.5 µg/mL (). The most efficacious chemical in our previous screen was CuCl2, which showed an IC50 of ∼5 µg/mL (Lawrence et al. Citation2017). Thus, the kānuka-derived compound 1 (IC50 = 1.4 µg/mL) is the most potent inhibitor of P. agathidicida zoospore germination reported to date.
Conversely, compounds 1–3 were all relatively ineffective at inhibiting mycelial growth when tested individually. At the highest concentration tested (100 µg/mL), compound 1 inhibited the growth of P. agathidicida mycelia by ∼50% (Supplementary Figure 2). In contrast, CuCl2 was ∼15-fold more effective (IC50 = 6.8 µg/mL) (Lawrence et al. Citation2017) while the commercially available agrichemical phosphite is 25-fold more effective (IC50 = 4.0 µg/mL) (Horner Citation2013). The biochemical basis of this specificity towards zoospore germination and motility, but not mycelial growth, remains to be elucidated for compounds 1–3.
Kānuka chemistry
Kānuka is mostly used as a name for trees and shrubs of the genus Kunzea, but there are many similarities in form and habitat between Kunzea and Leptospermum scoparium, (most commonly called mānuka). Furthermore, ten Kunzea species have been described in Aotearoa New Zealand (de Lange Citation2014). The Waima kānuka selected for this study have been botanically identified as K. robusta.
Kānuka is commonly found in kauri forests, and it is an effective nurse tree for young kauri (Lloyd Citation1960). Kānuka is also well-known to rongoā practitioners for its medicinal properties. For example, the bark of kānuka was traditionally used for treating diarrhoea and dysentery (Best Citation1905). This has led to a number of previous studies to isolate bioactive compounds from the species. Compounds reported previously from kānuka (i.e. Kunzea from Aotearoa New Zealand) include phloroglucinols (Bloor Citation1992) and terpenes (Perry et al. Citation1997; Porter and Wilkins Citation1999). Kānuka essential oils and/or purified compounds have activity against bacteria (Lis-Balchin et al. Citation2000; Chen et al. Citation2016; Prosser et al. Citation2016), viruses (Bloor Citation1992) and fungi (Chen et al. Citation2016). However, we are the first to report anti-Phytophthora activity of kānuka, and this also seems to be the first report of flavanones from a New Zealand species of Kunzea.
Known sources and activities of identified anti-Phytophthora flavanones 1–3
The three active compounds identified in the present study are flavanones, a sub-group of the flavonoid natural products. Flavonoids are ubiquitous in plants and fulfil numerous important roles, including pigmentation, signalling and defence against pathogenic microbes (Havsteen Citation2002; Samanta et al. Citation2011). Some members of another flavonoid sub-group, the isoflavones, are known to be chemoattractive to Phytopthora spp. (Morris and Ward Citation1992; Tyler et al. Citation1996; Hua et al. Citation2008) and some have been shown to be inhibitory (Rivera-Vargas et al. Citation1993; Subramanian et al. Citation2005).
The three flavanones identified in this study have also been identified from other (non-kānuka) plant species. However, none of these compounds were previously known to have activity against Phytophthora, and this is the first time these compounds have been identified in K. robusta.
The most potent anti-Phytophthora compound we identified was 5,7-dihydroxy-6-methylflavanone (1, also known as strobopinin). It has previously been isolated from a number of other plants including Pinus strobus (Matsuura Citation1957) and L. scoparium (mānuka) (Mayer Citation1990). Strobopinin inhibits the growth of Staphylococcus aureus (a Gram-positive bacterium) (Massaro et al. Citation2014)and also has been reported to have antimalarial activity (Boonphong et al. Citation2007).
5,7-Dihydroxy-6,8-dimethylflavanone (2) has been found previously in a range of plants, including pōhutukawa (Metrosideros excelsa, Myrtaceae) (Mustafa et al. Citation2005). It has been reported to have anti-bacterial (Massaro et al. Citation2014), antimalarial (Boonphong et al. Citation2007; Joseph et al. Citation2007) and antiviral (Dao et al. Citation2010) activities.
5-Hydroxy-7-methoxy-6-methylflavanone (3) has been found in other members of the family Myrtaceae, including Heteropyxis natalensis from South Africa (Maroyi Citation2019), Syzygium samarangense from Thailand (Srivarangkul et al. Citation2018), and L. scoparium from New Zealand (Mayer Citation1990; Häberlein and Tschiersch Citation1998). Antimicrobial properties of this compound are currently unknown, but it has been shown to inhibit dengue virus infectivity (Srivarangkul et al. Citation2018).
Future research and potential applications
In the present study, active flavanone concentrations varied ∼10-fold among foliage extracts from four individual kānuka plants from Waima (). Geographic and seasonal variations in chemical composition and antimicrobial activity have been observed in some Myrtaceae (Häberlein and Tschiersch Citation1998; Demuner et al. Citation2011; Sá et al. Citation2012), but not in others (Demuner et al. Citation2011; Cascaes et al. Citation2015). Overall, more samples are needed to determine potential geographic and/or temporal differences in the production of compounds 1–3.
Further research is also needed to determine the mechanisms of action of these compounds against Phytophthora. Flavonoid bactericidal activity generally occurs through one of three mechanisms: (i) perforation of the plasma membrane; (ii) inhibition of energy metabolism; or (iii) inhibition of nucleic acid synthesis (Ahmad et al. Citation2015). Known antifungal mechanisms of action include membrane disruption, cell wall damage and inhibition of efflux pumps (Seleem et al. Citation2017). Their action in Phytophthora remains unknown, but may involve some of the same processes seen in fungi and bacteria.
In this study, the kānuka extracts and purified compounds were most effective against zoospore motility and germination, but less effective against the mycelial growth that occurs within infected trees. These results suggest that, while not useful for treating existing plant infections, kānuka could have potential applications in limiting zoospore-mediated spread of disease. Possible application methods include companion planting kānuka with host plants, and/or the development of sprays or soil drenches based on the purified compounds. However, any potential applications will require significantly more research. This includes studies of the plant-to-plant variation in flavanone production, the potential of the compounds to leach from roots or leaf matter into surrounding soils, and/or considerations around compound stability and formulation. Furthermore, we caution that while investigation into these (and myriad other) research questions continues, it remains absolutely critical to prevent spread of the disease into uncontaminated kauri forest.
Treaty of Waitangi obligations and Wai 262 obligations
A legal framework for the protection of indigenous knowledge remains elusive, since most provisions for intellectual property law have evolved out of a western view of knowledge as a commodity owned by individuals, not by communities. Under current New Zealand law, researchers can use traditional Māori knowledge without consent or acknowledgement. Similarly, scientific research of taonga plant species is legally allowed to take place without input or consent from mana whenua. The question remains as to how mana whenua can protect their indigenous intellectual property rights and gain economically from the development and implementation of such intellectual knowledge.
We believe that ethical collaboration between mātauranga Māori practitioners and scientists is key. We have specifically sought to frame our research within the principles of the Treaty of Waitangi (i.e. partnership, participation and protection). We support the recommendations from Ko Aotearoa Tēnei: Report on the Wai 262 Claim (Waitangi Tribunal Citation2011). We recognise that although indigenous plants may be valued for their practical benefits, they are not simply resources to be exploited. These plants are taonga to Māori, and therefore the right of mana whenua to practice kaitiakitanga (stewardship) should be acknowledged and respected.
Supplementary information
Download MS Word (274.9 KB)Acknowledgements
SAL, EB, AB, WMP, NBP and MLG gratefully acknowledge the bringing and the sharing of mātauranga Māori and indigenous taonga flora that has underpinned this research. CP and IM are thankful that a pathway was created for rongoā to be tested with integrity, that an approach was made at a cultural and spiritual level, and that an invitation to participate was offered and accepted according to Māori protocol. We also thank the following people: P. de Lange for botanical identification; C. Sansom for GC-MS; I. Fuller, and J. van Klink for advice on Kunzea and Leptospermum chemistry; and J. Pentelow and I. Stewart for NMR and MS support.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Wayne M. Patrick http://orcid.org/0000-0002-2718-8053
Nigel B. Perry http://orcid.org/0000-0003-3196-3945
Monica L. Gerth http://orcid.org/0000-0002-7959-7852
Additional information
Funding
References
- Abreu AC, McBain AJ, Simoes M. 2012. Plants as sources of new antimicrobials and resistance-modifying agents. Natural Product Reports. 29:1007–1021. doi: 10.1039/c2np20035j
- Ahmad A, Kaleem M, Ahmed Z, Shafiq H. 2015. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—a review. Food Research International. 77:221–235. doi: 10.1016/j.foodres.2015.06.021
- Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF. 2000. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 290:972–977. doi: 10.1126/science.290.5493.972
- Beever JE, Coffey MD, Ramsfield TD, Dick MA, Horner IJ. 2009. Kauri (Agathis australis) under threat from Phytophthora? In: Goheen EMF, S.J., editor. Phytophthoras in Forests and Natural Ecosystems Proceedings of the Fourth Meeting of IUFRO Working Party S070209, General Technical report OSW-GTR-221. Albany, CA: USDA Forest Service. p. 74–85.
- Bellgard SE, Padamsee M, Probst CM, Lebel T, Williams SE. 2016. Visualizing the early infection of Agathis australis by Phytophthora agathidicida, using microscopy and fluorescent in situ hybridization. Forest Pathology. 46:622–631. doi: 10.1111/efp.12280
- Bennett RN, Wallsgrove RM. 1994. Secondary metabolites in plant defense-mechanisms. New Phytologist. 127:617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x
- Best E. 1905. Māori medical lore. Notes on sickness and disease among the māori people of New Zealand, and their treatment of the sick; together with some account of various beliefs, superstitions and rites pertaining to sickness, and the treatment thereof, as collected from the tūhoe Tribe. The Journal of the Polynesian Society. 14:1–23.
- Bloor SJ. 1992. Antiviral phloroglucinols from New Zealand Kunzea species. Journal of Natural Products. 55:43–47. doi: 10.1021/np50079a006
- Blunt JW, Calder VL, Fenwick GD, Lake RJ, McCombs JD, Munro MHG, Perry NB. 1987. Reverse phase flash chromatography: a method for the rapid partitioning of natural product extracts. Journal of Natural Products. 50:290–292. doi: 10.1021/np50050a039
- Boonphong S, Puangsombat P, Baramee A, Mahidol C, Ruchirawat S, Kittakoop P. 2007. Bioactive compounds from Bauhinia purpurea possessing antimalarial, antimycobacterial, antifungal, anti-inflammatory, and cytotoxic activities. Journal of Natural Products. 70:795–801. doi: 10.1021/np070010e
- Buenz EJ, Verpoorte R, Bauer BA. 2018. The ethnopharmacologic contribution to bioprospecting natural products. Annual Review of Pharmacology and Toxicology. 58:509–530. doi: 10.1146/annurev-pharmtox-010617-052703
- Carlile MJ. 1985. The zoospore and its problems. In: Ayres PGB L, editor. Water, fungi and plants. Cambridge: University Press, Cambridge; p. 105–118.
- Cascaes MM, Guilhon GMSP, de Aguiar Andrade EH, das Graças Bichara Zoghbi M, da Silva Santos L. 2015. Constituents and pharmacological activities of Myrcia (Myrtaceae): a review of an aromatic and medicinal group of plants. International Journal of Molecular Sciences. 16:23881–23904. doi: 10.3390/ijms161023881
- Chen C-C, Yan S-H, Yen M-Y, Wu P-F, Liao W-T, Huang T-S, Wen Z-H, David Wang H-M. 2016. Investigations of kanuka and manuka essential oils for in vitro treatment of disease and cellular inflammation caused by infectious microorganisms. Journal of Microbiology, Immunology and Infection. 49:104–111. doi: 10.1016/j.jmii.2013.12.009
- Collins S, McComb JA, Howard K, Shearer BL, Colquhoun IJ, Hardy GESJ. 2012. The long-term survival of Phytophthora cinnamomi in mature Banksia grandis killed by the pathogen. Forest Pathology. 41:28–36. doi: 10.1111/j.1439-0329.2011.00718.x
- Cowan MM. 1999. Plant products as antimicrobial agents. Clinical Microbiolgy Reviews. 12(4):564–582. doi: 10.1128/CMR.12.4.564
- Crone M, McComb JA, O'Brien PA, Hardy GE. 2013. Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biology. 117(2):112–123. doi: 10.1016/j.funbio.2012.12.004
- Dao T-T, Tung B-T, Nguyen P-H, Thuong P-T, Yoo S-S, Kim E-H, Kim S-K, Oh W-K. 2010. C-Methylated flavonoids from Cleistocalyx operculatus and their inhibitory effects on novel influenza A (H1N1) neuraminidase. Journal of Natural Products. 73:1636–1642. doi: 10.1021/np1002753
- de Lange PJ. 2014. A revision of the New Zealand Kunzea ericoides (Myrtaceae) complex. PhytoKeys. 40:1–185. doi: 10.3897/phytokeys.40.7973
- de Lange PJ, Rolfe JR, Champion PD, Courtney SP, Heenan PB, Barkla JW, Cameron EK, Norton DA, Hitchmough RA. 2018. Conservation status of New Zealand indigenous vascular plants, 2017. Wellington: Department of Conservation.
- Demuner AJ, Barbosa LCA, Magalhaes CG, da Silva CJ, Maltha CRA, Pinheiro AL. 2011. Seasonal variation in the chemical composition and antimicrobial activity of volatile oils of three species of Leptospermum (Myrtaceae) grown in Brazil. Molecules. 16:1181–1191. doi: 10.3390/molecules16021181
- Dobrowolski MP, Shearer BL, Colquhoun IJ, O’Brien PA, Hardy GES. 2008. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathology. 57(5):928–936. doi: 10.1111/j.1365-3059.2008.01883.x
- Ecroyd C. 1982. Biological flora of New Zealand 8. Agathis australis (D. Don) Lindl.(Araucariaceae) Kauri. New Zealand Journal of Botany. 20:17–36. doi: 10.1080/0028825X.1982.10426402
- Gisi U, Sierotzki H. 2008. Fungicide modes of action and resistance in downy mildews. European Journal of Plant Pathology. 122:157–167. doi: 10.1007/s10658-008-9290-5
- Häberlein H, Tschiersch K-P. 1998. On the occurrence of methylated and methoxylated flavonoids in Leptospermum scoparium. Biochemical Systematics and Ecology. 26:97–103. doi: 10.1016/S0305-1978(97)00084-7
- Hardham AR, Blackman LM. 2018. Phytophthora cinnamomi. Molecular Plant Pathology. 19(2):260–285. doi: 10.1111/mpp.12568
- Havsteen BH. 2002. The biochemistry and medical significance of the flavonoids. Pharmacology & Therapeutics. 96:67–202. doi: 10.1016/S0163-7258(02)00298-X
- Horner IJH EG. 2013. Phosphorous acid for controlling Phytophthora taxon Agathis in kauri: glasshouse trials. New Zealand Plant Protection. 66:242–248. doi: 10.30843/nzpp.2013.66.5673
- Hua C, Wang Y, Zheng X, Dou D, Zhang Z, Govers F, Wang Y. 2008. A Phytophthora sojae G-protein alpha subunit is involved in chemotaxis to soybean isoflavones. Eukaryotic Cell. 7:2133–2140. doi: 10.1128/EC.00286-08
- Joseph CC, Magadula JJ, Nkunya MHH. 2007. A novel antiplasmodial 3′,5′-diformylchalcone and other constituents of Friesodielsia obovata. Natural Product Research. 21:1009–1015. doi: 10.1080/14786410701194310
- Judelson HS, Blanco FA. 2005. The spores of Phytophthora: weapons of the plant destroyer. Nature Reviews Microbiology. 3:47–58. doi: 10.1038/nrmicro1064
- Kamoun S, Furzer O, Jones JD, Judelson HS, Ali GS, Dalio RJ, Roy SG, Schena L, Zambounis A, Panabieres F, et al. 2015. The top 10 oomycete pathogens in molecular plant pathology. Molecular Plant Pathology. 16:413–434. doi: 10.1111/mpp.12190
- Killeen DP, van Klink JW, Smallfield BM, Gordon KC, Perry NB. 2015. Herbicidal β-triketones are compartmentalized in leaves of Leptospermum species: localization by Raman microscopy and rapid screening. New Phytologist. 205:339–349. doi: 10.1111/nph.12970
- Lawrence SA, Armstrong CB, Patrick WM, Gerth ML. 2017. High-throughput chemical screening identifies compounds that inhibit different stages of the Phytophthora agathidicida and Phytophthora cinnamomi life cycles. Frontiers in Microbiology. doi.org/10.3389/fmicb.2017.01340.
- Lis-Balchin M, Hart SL, Deans SG. 2000. Pharmacological and antimicrobial studies on different tea-tree oils (Melaleuca alternifolia, Leptospermum scoparium or Manuka and Kunzea ericoides or Kanuka), originating in Australia and New Zealand. Phytotherapy Research. 14:623–629. doi: 10.1002/1099-1573(200012)14:8<623::AID-PTR763>3.0.CO;2-Z
- Lloyd RC. 1960. Growth study of regenerated kauri and podocarps in Russell Forest. New Zealand Journal of Forestry. 8:355–361.
- Maroyi A. 2019. Phytochemical and ethnopharmacological review of Heteropyxis natalensis. Asian Journal of Pharmaceutical and Clinical Research. 12:8–15. doi: 10.22159/ajpcr.2019.v12i3.29375
- Massaro CF, Katouli M, Grkovic T, Vu H, Quinn RJ, Heard TA, Carvalho C, Manley-Harris M, Wallace HM, Brooks P. 2014. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia. 95:247–257. doi: 10.1016/j.fitote.2014.03.024
- Matsuura S. 1957. The structure of cryptostrobin and strobopinin, the flavanones from the heartwood of Pinus strobus. Pharmaceutical Bulletin. 5:195–198. doi: 10.1248/cpb1953.5.195
- Mayer R. 1990. Flavonoids from Leptospermum scoparium. Phytochemistry. 29:1340–1342. doi: 10.1016/0031-9422(90)85462-O
- Miao J, Cai M, Dong X, Liu L, Lin D, Zhang C, Pang Z, Liu X. 2016. Resistance assessment for oxathiapiprolin in Phytophthora capsici and the detection of a point mutation (G769W) in PcORP1 that confers resistance. Frontiers in Microbiology. 7:615. doi: 10.3389/fmicb.2016.00615
- Morris PF, Ward EWB. 1992. Chemoattraction of zoospores of the soybean pathogen, Phytophthora sojae, by isoflavones. Physiological and Molecular Plant Pathology. 40:17–22. doi: 10.1016/0885-5765(92)90067-6
- Mustafa KA, Perry NB, Weavers RT. 2005. Lipophilic C-methylflavonoids with no B-ring oxygenation in Metrosideros species (Myrtaceae). Biochemical Systematics and Ecology. 33:1049–1059. doi: 10.1016/j.bse.2005.02.003
- Oliver RP, Hewitt HG. 2014. Plant pathology and plant pathogens. Fungicides in Crop protection. CABI Publishing.
- Parra G, Ristaino JB. 2001. Resistance to mefenoxam and metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper. Plant Disease. 85:1069–1075. doi: 10.1094/PDIS.2001.85.10.1069
- Perry NB, van Klink JW, Brennan NJ, Harris W, Anderson RE, Douglas MH, Smallfield BM. 1997. Essential oils from New Zealand manuka and kanuka: chemotaxonomy of Kunzea. Phytochemistry. 45:1606–1612. doi: 10.1016/S0031-9422(97)00203-3
- Porter NG, Wilkins AL. 1999. Chemical, physical and antimicrobial properties of essential oils of Leptospermum scoparium and Kunzea ericoides. Phytochemistry. 50(3):407–415. doi: 10.1016/S0031-9422(98)00548-2
- Prosser JA, Woods RR, Horswell J, Robinson BH. 2016. The potential in-situ antimicrobial ability of Myrtaceae plant species on pathogens in soil. Soil Biology and Biochemistry. 96:1–3. doi: 10.1016/j.soilbio.2015.12.007
- Pusztahelyi T, Holb IJ, Pocsi I. 2015. Secondary metabolites in fungus-plant interactions. Frontiers in Plant Science. 6. doi:10.3389/fpls.2015.00573.
- Rivera-Vargas LI, Schmitthenner AF, Graham TL. 1993. Soybean flavonoid effects on and metabolism by Phytophthora sojae. Phytochemistry. 32:851–857. doi: 10.1016/0031-9422(93)85219-H
- Sá FAS, Borges LL, Paula JAM, Sampaio BL, Ferri PH, Paula JR. 2012. Essential oils in aerial parts of Myrcia tomentosa: composition and variability. Brazilian Journal of Pharmacognosy. 22:1233–1240. doi: 10.1590/S0102-695X2012005000120
- Samanta A, Das G, Das S. 2011. Roles of flavonoids in plants. International Journal of Pharmaceutical Science and Technology. 6:12–35.
- Saslis-Lagoudakis CH, Savolainen V, Williamson EM, Forest F, Wagstaff SJ, Baral SR, Watson MF, Pendry CA, Hawkins JA. 2012. Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proceedings of the National Academy of Sciences. 109:15835–15840. doi: 10.1073/pnas.1202242109
- Scott P, Williams N. 2014. Phytophthora diseases in New Zealand forests. New Zealand Journal of Forestry. 59:14–21.
- Seleem D, Pardi V, Murata RM. 2017. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Archives of Oral Biology. 76:76–83. doi: 10.1016/j.archoralbio.2016.08.030
- Srivarangkul P, Yuttithamnon W, Suroengrit A, Pankaew S, Hengphasatporn K, Rungrotmongkol T, Phuwapraisirisan P, Ruxrungtham K, Boonyasuppayakorn S. 2018. A novel flavanone derivative inhibits dengue virus fusion and infectivity. Antiviral Research. 151:27–38. doi: 10.1016/j.antiviral.2018.01.010
- Steward GA, Beveridge AE. 2010. A review of New Zealand kauri (Agathis australis (D.Don) Lindl.): its ecology, history, growth and potential for management for timber. New Zealand Journal of Forestry Science. 40:33–59.
- Subramanian S, Graham MY, Yu O, Graham TL. 2005. RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae. Plant Physiology. 137:1345–1353. doi: 10.1104/pp.104.057257
- Tyler BM. 2002. Molecular basis of recognition between Phytophthora pathogens and their hosts. Annual Review of Phytopathology. 40:137–167. doi: 10.1146/annurev.phyto.40.120601.125310
- Tyler BM, Wu M, Wang J, Cheung W, Morris PF. 1996. Chemotactic preferences and strain variation in the response of Phytophthora sojae zoospores to host isoflavones. Applied and Environmental Microbiology. 62:2811–2817.
- Waipara NWH, Hill S, Hill LMW, Hough EG, Horner IJ. 2013. Surveillance methods to determine tree health, distribution of kauri dieback disease and associated pathogens. New Zealand Plant Protection. 66:235–241. doi: 10.30843/nzpp.2013.66.5671
- Waitangi Tribunal Report. 2011. Ko Aotearoa tēnei: a report into claims concerning New Zealand law and policy affecting Māori culture and identify: Te taumata tuatahi. Wellington.
- Weir BS, Paderes EP, Anand N, Uchida JY, Pennycook SR, Bellgard SE, Beever RE. 2015. A taxonomic revision of Phytophthora Clade 5 including two new species, Phytophthora agathidicida and P. cocois. Phytotaxa. 205:21–38. doi: 10.11646/phytotaxa.205.1.2
- Wyse SV, Burns BR, Wright SD. 2014. Distinctive vegetation communities are associated with the long-lived conifer Agathis australis (New Zealand kauri, Araucariaceae) in New Zealand rainforests. Australian Journal of Ecology. 39:388–400. doi: 10.1111/aec.12089
- Wyse SV, Macinnis-Ng CM, Burns BR, Clearwater MJ, Schwendenmann L. 2013. Species assemblage patterns around a dominant emergent tree are associated with drought resistance. Tree Physiology. 33:1269–1283. doi: 10.1093/treephys/tpt095