Abstract
A quantitative polymerase chain reaction (qPCR) was validated for the detection of Mycoplasma synoviae (PCR equivalents of colony-forming units [CFU eq.]) in chicken joint specimens with time and compared with direct mycoplasma culture. Joint specimens were obtained from 70 layer pullets inoculated intravenously with M. synoviae at 6 weeks of age. Ten control birds were injected intra-articularly with Freund's complete adjuvant. Macroscopic joint lesions were observed in 54 infected birds, of which 11 showed positive M. synoviae culture. The specificity of direct mycoplasma culture was high (100%, 95% confidence interval [CI] = 74 to 100), but its sensitivity low (16%, 95% CI = 8 to 26). Most positive results were obtained during the first 2 weeks after onset of joint swelling using synovial fluid. The qPCR was positive in 26 of 28 synovial fluid samples and in 51 of 70 joint swabs. The sterile joint samples obtained from Freund's complete adjuvant-injected birds were negative in the mycoplasma culture. The specificity and sensitivity of the qPCR for synovial fluid samples were 100% (95% CI = 65 to 100) and 93% (95% CI = 77 to 99); for joint swabs they were 100% (95% CI = 74 to 100) and 73% (95% CI = 61 to 83), respectively. Positive qPCR results (100.3 to 4.6 CFU eq./ml) were found until the end of the experiment (12 weeks post inoculation). At the end of the study, eight out of 16 joint swabs from birds without macroscopic joints lesions were positive in the qPCR (102.0 to 2.8 CFU eq./ml). Under the conditions of this study, the sensitivity of the qPCR was higher than that of direct mycoplasma culture (P< 0.0001) during the acute, subacute and chronic stages of arthritis.
Introduction
Mycoplasma synoviae has been described as the aetiological agent of infectious synovitis, airsacculitis and eggshell apex abnormalities (Kerr & Olson, Citation1970; Kleven et al., Citation1972; Morrow et al., Citation1990; Kleven & Ferguson-Noel, Citation2008; Feberwee et al., Citation2009a,Citationb; Feberwee & Landman, Citation2010) and may cause significant economic losses in commercial poultry production.
The clinical and economic relevance of M. synoviae as the causative agent of infectious synovitis has been extensively documented in the past and present (Olson et al., Citation1954; Wills, Citation1954; Landman & Feberwee, Citation2001; Kang et al., Citation2002; Landman & Feberwee, Citation2004; Landman & Feberwee, Citation2012). Economic losses due to arthropathic M. synoviae strains may be the consequence of growth retardation, culling of lame birds and higher condemnation rates at the slaughterhouse (Stipkovits & Kempf, Citation1996; Kleven & Ferguson-Noel, Citation2008). These losses may be greatly reduced if the early diagnosis of M. synoviae arthritis is made because this will enable timely application of antibiotic treatment. Although antibiotic therapy will not eliminate M. synoviae from infected flocks, clinical signs and subsequent economic damage may be significantly reduced.
Until recently, the diagnosis of infectious synovitis was based on clinical signs and macroscopic lesions in combination with direct mycoplasma culture (Stipkovits & Kempf, Citation1996; Lockaby et al., Citation1998; Narat et al., Citation1998; Kleven & Ferguson-Noel, Citation2008) and/or a qualitative M. synoviae polymerase chain reaction (PCR) of joint specimens (Lockaby et al., Citation1998). M. synoviae serology is of little relevance because not all flocks with antibodies against this mycoplasma species suffer from infectious synovitis.
The sensitivity of direct mycoplasma culture from affected joints is low and often yields negative results, especially in the (sub)chronic stage of the disease (Olson & Kerr, Citation1967; Kawakubo et al., Citation1980; Lockaby et al., Citation1998; Landman & Feberwee, Citation2004; Kleven & Ferguson-Noel, Citation2008). Furthermore, direct mycoplasma culture is laborious, costly and takes up to 28 days before completion.
The rapid diagnosis of M. synoviae infections with PCR tests, which are less laborious, time consuming and costly, has emerged. However, although quantitative M. synoviae PCR tests have been described for trachea samples, they have not yet been validated for joint specimens (Carli & Eyigor, Citation2003; Mekkes & Feberwee, Citation2005). False negative PCR results due to the presence of PCR inhibitors such as heme and/or glycoproteins may occur during the analysis of clinical samples such as synovial fluid (Kuipers et al., Citation1999; Schwaiger et al., Citation2001; Rådström et al., Citation2004; Schneeweiss et al., Citation2007). This study describes the validation of a previously developed quantitative polymerase chain reaction (qPCR) for the detection and quantification of M. synoviae in two types of chicken joint specimens (synovial fluid and joint swabs from synovial fluid and membranes) and its comparison with direct mycoplasma culture. Moreover, its performance was examined in joint specimens collected during the acute, subacute and chronic stages of M. synoviae-induced synovitis.
Materials and Methods
Primers and probes of the M. synoviae quantitative PCR
The primers and probes used were described earlier (Becker et al., Citation2002; Raviv & Kleven, Citation2009). Details regarding primers and probes are outlined in .
Table 1. Primers and probes used for detection of M. synoviae and Synechococcus spp.
Optimization of the M. synoviae quantitative PCR
A multiplex quantitative PCR for the detection of both M. synoviae and Synechococcus spp. (adapted from Becker et al., Citation2002) was used to detect and quantify a 10-fold dilution series of M. synoviae. A culture containing 108.5 colony-forming units (CFU)/ml of an arthropathogenic M. synoviae strain (chicken/NL/Dev/801979Rob/00, further referred to as strain Robbertson) (Landman & Feberwee, Citation2001) was used for the preparation of the 10-fold dilution series. Standard curves were constructed using dilutions ranging from 100.5 to 107.5 CFU/ml. The PCR efficiency was expressed as 10(−1/slope). An inhibition control containing approximately 103 CFU/ml Synechococcus spp. was added to the samples. Checkerboard titrations for both the primers and probes were used to reveal their optimal concentrations.
Briefly, primers and probes (Biolegio, Nijmegen, the Netherlands), Quantifast Probe PCR mix (Qiagen, Hilden, Germany), and water were mixed. The qPCR was performed using the ABI7500 fast system (Applied Biosystems, Foster City, California, USA) under the following conditions: initial denaturation/enzyme activation for 5 min at 95°C, followed by 40 cycles of 8 sec at 95°C and 30 sec at 60°C. Data were analysed using the Applied Biosystems SDS software (Applied Biosystems).
In vitro assessment of specificity and sensitivity
Besides M. synoviae (n = 48 and M. synoviae type strain ATCC 25204), Mycoplasma gallisepticum (n = 21 and M. gallisepticum type strain ATCC 15302), Mycoplasma imitans (n = 2), Mycoplasma gallinaceum (n = 1) and Mycoplasma meleagridis strains (n = 17) and also overnight cultures from other bacteria able to induce joint pathology (Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, and Staphylococcus aureus ATCC 25923) were used for specificity testing. Pooled synovial fluid samples obtained from joints of calves were used as control fluid because it was not possible to obtain sufficient synovial fluid from non-affected chicken joints. Aliquots of synovial fluid were spiked in triplicate with the 10-fold serial dilutions of the arthropathogenic M. synoviae strain prepared as described in the earlier section “Optimization of the M. synoviae quantitative PCR”. DNA extraction was carried out as described in the later section “DNA extraction from synovial fluid and joint swabs” (from synovial fluid and membranes).
Experimental design, inocula, assessment of lameness and sampling of joints
To obtain sterile control joint samples (synovial fluid and joint swabs) 10 birds were injected intra-articularly with 0.25 ml Freund's complete adjuvant (FCA) (Sigma-Aldrich, St Louis, Missouri, USA), which is known to induce sterile arthritis (Landman et al., Citation1998), while another 70 hens each received 1.0 ml containing 108 CFU M. synoviae strain Robbertson intravenously.
The inocula were prepared as described by Landman et al. (Citation2004). Briefly, a frozen vial containing 1 ml ME broth (Avian Mycoplasma Liquid Medium; Mycoplasma Experience, Reigate, UK) with M. synoviae (107 CFU/ml) was thawed, added to 50 ml fresh ME broth and incubated at 37°C until a change of colour was observed. Control of the M. synoviae concentrations was performed retrospectively by means of bacterial counting following the International Standard Organization (ISO7402, Citation1985).
Birds were inspected daily, and if lameness was observed the individual birds were palpated and lameness and/or joint swelling was recorded. These birds were subsequently monitored daily. Birds with lameness and/or swollen joints suggestive of arthritis were stunned using a mixture of CO2 and O2 and were subjected to post-mortem examination at different time points following the onset of lameness (i.e. at 1, 2, 3, 4, 5, 6 or 7 weeks later). The sterile joint specimens were obtained 3 weeks after the injection of FCA.
At post-mortem examination, macroscopic joint lesions suggestive of arthritis were recorded per bird. Thereafter, synovial fluid was collected from overfilled joints (most frequently hock and foot joint) with a sterile needle and syringe after sterilizing the outer surface of the affected joint with a hot scalpel blade. If less than 0.1 to 0.3 ml could be obtained from a joint, fluid from other affected joints was added. The synovial fluid sample was used for the qPCR, direct mycoplasma culture and general bacteriological analysis.
After aspiration of the synovial fluid, the joint was incised with a sterile scalpel blade and sampled (including both synovial fluid and synovial membrane) with one sterile swab (Urethral E.N.T. MW142; Medical Wire & Equipment, Corsham, UK) that was used in the qPCR.
In cases where the obtained volume of synovial fluid was insufficient, two joint swabs were taken of which one was used for the qPCR and the other for direct mycoplasma culture and general bacteriology.
Housing of experimental birds
The experimental chickens originated from M. gallisepticum and M. synoviae free parent stock. Freedom from M. gallisepticum complied with European legislation (2011/214/EU). Birds were reared in a floor pen from day-old until 6 weeks of age. At the start of the experiment they were housed in four separate negative-pressure HEPA-filtered isolators (194 cm long, 95 cm high and 75 cm wide; Beyer and Eggelaar, Utrecht, the Netherlands), one of which harboured the control group treated with FCA. The birds were fed ad libitum with a standard commercial layer rearing pullet feed containing 11.297 MJ/kg metabolizable energy and had free access to drinking water. Lighting was supplied for 23 h at day 1 of age and reduced by 1 hour each day thereafter until a lighting period of 9 h per day was reached.
Serology
The M. synoviae infection status of all birds was assessed before and at the end of the experiment by collecting blood samples for serology, which were tested using the M. synoviae rapid plate agglutination (RPA) test as described previously (Feberwee et al., Citation2005). Briefly, 1:2 diluted sera were tested with the M. synoviae RPA antigen (Nobilis MS antigen; Intervet International B.V., Boxmeer, the Netherlands). In the case of a positive test result (agglutination) the sera were serially diluted from 1:4 to 1:32 in phosphate-buffered saline, pH 7.2, and re-tested in the RPA. If agglutination occurred at dilution 1:8 (log2 titre 3) or higher, the serum sample was considered specifically positive for M. synoviae.
Bacteriology and mycoplasma culture
Fifty microlitres of synovial fluid and/or a joint swab were used per bird for general bacteriology using 5% sheep blood agar (bioTRADING Benelux B.V., Mijdrecht, the Netherlands), while ME agar plates (Avian Mycoplasma Solid Medium, Mycoplasma Experience) were used for direct mycoplasma culture. The latter were incubated at 37°C in a humid environment and examined for colony growth every 2 to 3 days up to 28 days. One separate colony was plated out on fresh ME agar, and from the ME agar with positive clones approximately 2×0.5 cm2 was removed using a sterile scalpel blade and transferred to 5 ml ME broth, which was then incubated at 37°C. Positive mycoplasma cultures were identified as M. synoviae by a SYBR green-based qPCR as described earlier (Mekkes & Feberwee, Citation2005; Feberwee et al., Citation2009b).
DNA extraction from synovial fluid and joint swabs
Synovial fluid samples were centrifuged for 10 min at 10,000×g and supernatant was discarded for DNA extraction. Pellets were resuspended in phosphate-buffered saline and DNA was isolated using the QiaAmp DNA mini kit (Qiagen) following the instructions of the manufacturer. DNA from joint swabs was extracted using the Express Extract DNA kit (Kapa Biosystems, Woburn, Massachusetts, USA), according to the instructions of the manufacturer. DNA extracted from both synovial fluid and joint swabs was tested in the qPCR as described earlier.
Statistical analysis
Kaplan & Meier (Citation1958) survival analysis was used for the graphical representation of the development of macroscopic signs suggestive of M. synoviae arthritis. The sensitivity and the specificity of the direct mycoplasma culture and qPCR for synovial fluid samples and joint swabs were calculated using the binary classification test (Altman & Bland, Citation1994) with RPA as a selected reference test for calculation of specificity and sensitivity. Differences in sensitivity between the direct mycoplasma culture and qPCR of joint fluid and joint swabs were calculated using the Fisher's (Citation1922) exact test. Differences in median M. synoviae qPCR CFU eq. values between birds with and birds without macroscopic joint lesions were calculated using the Mann & Whitney (Citation1947) rank sum test. SigmaPlot 11 (Systat Software, Inc, San Jose, California, USA) was used for all statistical analysis.
Ethical statement
Experiments had been approved by the Animal Experimental Committee, DEC-Consult Foundation according to Dutch law on experimental animals (Wet op de dierproeven).
Results
Optimization of the M. synoviae quantitative PCR
Checkerboard titrations using the 10-fold dilution series of the M. synoviae strain Robbertson showed that a primer concentration of 0.4 µM per primer and a probe concentration of 0.2 µM gave good fluorescent signals and the lowest Ct values. They were therefore selected for the M. synoviae qPCR. The average Ct values obtained from the reaction were plotted against the log10 concentration of the dilution, and the linear equation for the M. synoviae qPCR was y=3.49x+16.15. Using the slope from the linear equation a PCR efficiency of 93.4% was obtained and the quantitative range was at least 6 log10 dilution steps, with coefficients of regression exceeding 0.99. Synechococcus spp. was detected at PCR cycle 32±1.
In vitro assessment of the specificity and sensitivity of the M. synoviae quantitative PCR
All M. synoviae isolates tested positive in the qPCR as shown in . No cross-reactivity with the other tested avian mycoplasma species or other species of bacteria associated with joint pathology was observed. DNA from spiked joint fluid and joint swabs was isolated and tested in the M. synoviae qPCR. Based on the standard curve, the detection limit of the qPCR was 102.5 CFU eq./ml (100 to 1 CFU eq./PCR reaction).
Table 2. Specificity of the quantitative PCR.
Clinical observations and post-mortem examination
At 3 weeks post injection, 70% of the birds treated intra-articularly with FCA showed lameness and joint swelling suggestive of arthritis. The first lame pullets in the M. synoviae inoculated group appeared 3 weeks post inoculation. At 6 weeks post inoculation 59% (41/70) of birds showed lameness and/or swollen hock and/or foot joints, while at the end of the experimental period, which lasted 12 weeks, this was 90% (63/70) as is shown in .
Figure 1. Percentage of birds with M. synoviae-induced macroscopic signs suggestive of arthritis with time.
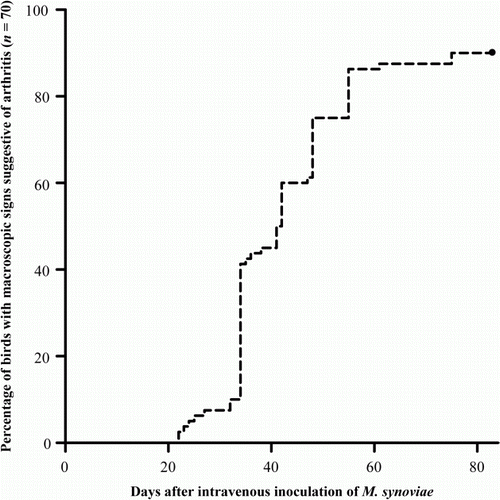
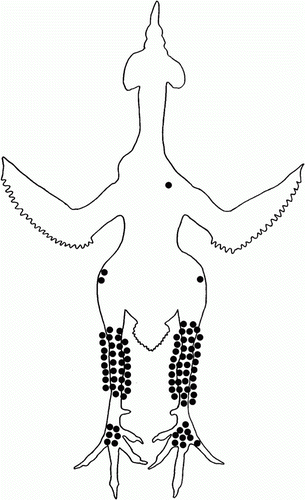
At post-mortem examination, 54/70 (77%) of the M. synoviae inoculated birds showed macroscopic joint lesions. Thirty-nine birds had swollen left and/or right hock joints, 11 birds had swollen left and/or right hock and foot joints, four had swollen left and/or right hock and knee joints and one bird showed a swollen shoulder joint. No macroscopic joint lesions were observed in 16 M. synoviae inoculated birds. The distribution of macroscopic joint lesions of M. synoviae inoculated birds found at post-mortem is shown in .
Figure 2. Ventral view of the distribution of macroscopic joint lesions found post-mortem in birds inoculated intravenously with M. synoviae. Each symbol represents an affected joint.
From 28 M. synoviae inoculated birds with macroscopic joint lesions post-mortem, synovial fluid and joint swabs were obtained. Joint fluid was used for both the qPCR and direct mycoplasma culture, while the joint swab was used for qPCR. From 26 M. synoviae inoculated birds with macroscopic joint lesions post-mortem only two joint swab samples were obtained, one for qPCR and the other for direct mycoplasma culture. From the remaining 16 inoculated birds without macroscopic joint lesions at post-mortem examination, also only two joint swab samples were obtained.
At post-mortem examination, seven out of 10 FCA-injected control birds showed macroscopic joint lesions in the knee and/or hock joint. Synovial fluid and joint swabs were obtained from seven birds. From three birds without macroscopic joint lesions only two joint swabs were taken for qPCR, direct mycoplasma culture and general bacteriology.
Serology
Before the start of the experiment all birds were negative in the RPA test. At the end of the experiment all birds inoculated with M. synoviae (n = 70) showed positive RPA test results, indicating a 100% infection rate, while the FCA-injected control birds (n=10) remained negative.
Mycoplasma culture and general bacteriology of joint samples
General bacteriology and direct mycoplasma culture was performed on 80 joint specimens of all experimental birds. Both synovial fluid and joint swabs were obtained from 28 M. synoviae inoculated birds. However, from the remaining birds of the M. synoviae inoculated group (n = 42) only joint swabs could be obtained. M. synoviae was isolated from 11 samples (once from a joint swab and 10 times from joint fluid). M. synoviae was not cultured from any of the 16 joint swabs from birds without macroscopic joint lesions. Most positive mycoplasma cultures were obtained 6 or 7 weeks post inoculation, corresponding to the first 2 weeks after the onset of lameness (). The specificity and sensitivity of the direct mycoplasma culture were 100% (95% confidence interval [CI] = 74 to 100) and 16% (95% CI = 8 to 26), respectively. No other pathogenic bacteria were isolated from the affected joints. M. synoviae was not isolated from the FCA-injected control birds ().
Table 3. Quantitative PCR and mycoplasma culture results of M. synoviae infected birds (n=70) and FCA-injected controls (n=10) with time after the onset of clinical signs suggestive of arthritis.
M. synoviae quantitative PCR of synovial fluid versus joint swabs
The M. synoviae concentrations found in synovial fluid samples ranged from 100.3 to 104.6 CFU eq./ml and for joint swabs from 101.6 to 104.5 CFU eq./ml (). Joint swab specimens, which were negative in the mycoplasma culture, contained on average 102.5 CFU eq./ml. In joint swab specimens, which were positive in the mycoplasma culture, the concentration was on average 103.4 CFU eq./ml, which is significantly higher (more than 10 times) than concentrations in joint swab specimens that were negative in the direct mycoplasma culture (P=0.032).
The specificity and sensitivity of the qPCR for synovial fluid samples were very high, 100% (95% CI = 65 to 100) and 93% (95% CI = 77 to 99), respectively. The specificity and sensitivity of the qPCR for joint swab specimens were 100% (95% CI = 74 to 100) and 73% (95% CI = 61 to 83), respectively. No M. synoviae was detected in synovial fluid and joint swabs from joints of birds injected with FCA ().
Discussion
PCR assays for the detection of M. synoviae in tracheal samples have been reported earlier (Lauerman et al., Citation1993, Citation1995; Garcia et al., Citation1996; Benčina et al., Citation2001; Hong et al., Citation2004; Mardassi et al., Citation2005; Mekkes & Feberwee, Citation2005; Ramírez et al., Citation2006; Jeffery et al., Citation2007; Hammond et al., Citation2009; Sprygin et al., Citation2010); however, reports on the application of PCR for the analysis of joint specimens are scarce. Lockaby et al. (Citation1998) describe the qualitative use of a M. synoviae PCR (Lauerman et al., Citation1993) in joint samples during the course of a M. synoviae infection after footpath and eyedrop inoculation of 1-day-old broiler chickens.
The sensitivity of PCR for the detection of pathogens in synovial fluid was shown previously to depend greatly on sample preparation (Kuipers et al., Citation1999; Freise et al., Citation2009). Joint fluid from diseased joints may contain inhibitors such as heme or glycoproteins, which may result in false negative results due to inhibition of the PCR (Kuipers et al., Citation1999). In qPCR, inhibition may lead to underestimation of the number of CFU eq. in a sample. An internal amplification control was therefore introduced to identify false negative results (Becker et al., Citation2002) and several procedures for the extraction of DNA from synovial fluid, ranging from centrifugation to labour-intensive procedures applying multiple incubation and treatment steps, have been investigated (Kuipers et al., Citation1999; Freise et al., Citation2009). Centrifugation and subsequent DNA extraction of the pelleted material using a column-based method yielded the best results (data not shown) and was therefore further used in our study.
Control samples for the validation of the qPCR were obtained from FCA-injected birds, while the RPA test was used as a selected reference test. Although the RPA test may give rise to false positive results if undiluted serum samples are used, here it was performed at dilution 1:8, which has been shown to be highly specific (Feberwee et al., Citation2005). Furthermore, all control birds injected with FCA remained serologically negative; that is, false positive results did not occur. Birds of the control group were sampled 3 weeks after the injection of FCA as most birds had developed lameness and/or swollen joints, and at this time sufficient sterile joint material could be harvested.
In the M. synoviae inoculated birds, signs of lameness and/or swollen joints first appeared 22 days post inoculation of M. synoviae, which is in agreement with a previous study (Landman & Feberwee, Citation2004), where the first clinical signs suggestive of arthritis were observed at day 21 post inoculation. At the end of the experiment 90% of the birds belonging to the M. synoviae inoculated group had developed lameness and/or swollen joints, which is also in agreement with earlier research (Landman & Feberwee, Citation2004), where 95% of birds developed arthritis. In contrast, during post-mortem examination the percentage of birds showing macroscopic joint lesions was lower (59%) than in a previous study (Landman & Feberwee, Citation2004). As in the present research individual birds were euthanized at different time-points following the first signs of lameness and/or swollen joints suggestive of arthritis, the severity of the inflammation may have decreased with time leading to the absence of macroscopic joint lesions at post-mortem examination in some birds.
During validation studies no cross-reactivity with other known joint pathogens was found in the qPCR and its sensitivity on spiked samples was very high. Using synovial fluid samples, the specificity and sensitivity of the qPCR were 100% (95% CI = 65 to 100) and 93% (95% CI = 77 to 99), respectively, while for the joint swabs the specificity was 100% (95% CI = 74 to 100) and the sensitivity was 73% (95% CI = 61 to 83). The sensitivity of the qPCR for synovial fluid samples was higher than for joint swabs. However, the difference was not statistically significant (P=0.053), which was attributed to the relatively low number of synovial fluid samples that could be obtained. M. synoviae was detected in joint specimens from infected birds using the qPCR until the end of the experimental period, which lasted 12 weeks.
Similar to work done by other researchers (Lockaby et al., Citation1998), we compared the qPCR results with direct culture. In their study direct mycoplasma culture appeared to be more sensitive than PCR, possibly due to the relatively low sensitivity of their PCR (detection limit 100 CFU) and/or the pooling of samples.
In our hands the sensitivity of the M. synoviae qPCR appeared to be much higher than direct mycoplasma culture (P<0.0001) during the acute, subacute and chronic stages of the disease. However, the sensitivity of the mycoplasma culture might have been higher if ME liquid medium and/or sample dilutions had been used in order to mitigate the effect of inhibitory substances possibly present in joint specimens (J.M. Bradbury, personal communication). Nonetheless, direct culture on mycoplasma agar has some advantages because slow-growing mycoplasma types are not missed, and moreover overgrowth with saprophytes and other mycoplasma species is avoided.
The direct culture of mycoplasma from joint specimens (joint fluid and swabs) yielded a low number of positives. This is in accordance with other studies performed in chickens, where the isolation of M. synoviae was shown to decrease with the chronicity of the infection, few or no mycoplasmas being detected in blood and/or joint samples after 6 to 8 weeks of inoculation (Olson & Kerr, Citation1967; Kawakubo et al., Citation1980; Landman & Feberwee, Citation2004). The low sensitivity of mycoplasma culture found in the present study has been attributed to the length of the time gap between M. synoviae inoculation and the culture of joint specimens. Moreover, as already mentioned, the presence of inhibition factors may also have contributed to the reduced sensitivity of culture.
It was remarkable that eight out of 16 joint swabs from birds with apparently macroscopically normal joints yielded positive qPCR results, while the culture remained negative. These results were attributed to the higher sensitivity of the qPCR in the present study and not due to difference in sample type because a joint swab was used for both the qPCR and direct mycoplasma culture. These birds probably had microscopic mycoplasma lesions, known to persist in M. synoviae infected birds (Lockaby et al., Citation1998), representing a source of live M. synoviae and/or residual M. synoviae DNA, which cannot be differentiated by PCR. However, in practice it is irrelevant whether live M. synoviae or its residual DNA are detected in joints of birds suspected to suffer from infectious synovitis, because both will enable accurate diagnosis by linking joint pathology of unknown aetiology to a specific cause.
As described previously by Raviv and Kleven, the qPCR is highly specific for M. synoviae and here it was successfully used to study the concentrations of M. synoviae in joints after experimental inoculation of chickens by the intravenous route. Owing to the much lower sensitivity of direct mycoplasma culture as compared with qPCR, the latter is the method of choice for the detection of M. synoviae in joint specimens. Synovial fluid is the preferred sample for the detection of M. synoviae by both qPCR and culture as it yielded the highest number of positives. Analysis of joint swabs using the qPCR is the best alternative from a practical point of view. This is also the best choice considering its lower costs, high sensitivity and speed. Culture remains important for cases in which isolation of the micro-organism is needed.
Acknowledgements
This research was funded by the Dutch Commodity Board for Poultry and Eggs. The authors thank C. Reugebrink for her excellent technical assistance.
References
- Altman , D.G. and Bland , J.M. 1994 . Statistics notes: diagnostic tests 1: sensitivity and specificity . BMJ , 308 : 1552 doi: 10.1136/bmj.308.6943.1552
- Becker , S. , Fahrbach , M. , Boger , P. and Ernst , A. 2002 . Quantitative tracing, by Taq nuclease assays, of a Synechococcus ecotype in a highly diversified natural population . Applied and Environmental Microbiology , 68 : 4486 – 4494 . doi: 10.1128/AEM.68.9.4486-4494.2002
- Benčina , D. , Drobnic-Valic , M. , Horvat , S. , Narat , M. , Kleven , S.H. and Dovc , P. 2001 . Molecular basis of the length variation in the N-terminal part of Mycoplasma synoviae hemagglutinin . FEMS Microbiology Letters , 203 : 115 – 123 . doi: 10.1111/j.1574-6968.2001.tb10829.x
- Carli , K.T. and Eyigor , A. 2003 . Real-time polymerase chain reaction for Mycoplasma gallisepticum in chicken trachea . Avian Diseases , 47 : 712 – 717 . doi: 10.1637/6041
- Feberwee , A. , de Wit , J.J. and Landman , W.J.M. 2009a . Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies . Avian Pathology , 38 : 77 – 85 . doi: 10.1080/03079450802662772
- Feberwee , A. and Landman , W.J.M. 2010 . Induction of eggshell apex abnormalities in broiler breeder hens . Avian Pathology , 39 : 133 – 137 . doi: 10.1080/03079451003657637
- Feberwee , A. , Mekkes , D.R. , de Wit , J.J. , Hartman , E.G. and Pijpers , A. 2005 . Comparison of culture, PCR, and different serologic tests for detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections . Avian Diseases , 49 : 260 – 268 . doi: 10.1637/7274-090804R
- Feberwee , A. , Morrow , C.J. , Ghorashi , S.A. , Noormohammadi , A.H. and Landman , W.J.M. 2009b . Effect of a live Mycoplasma synoviae vaccine on the production of eggshell apex abnormalities induced by a M. synoviae infection preceded by an infection with infectious bronchitis virus D1466 . Avian Pathology , 38 : 333 – 340 . doi: 10.1080/03079450903183652
- Fisher , R.A. 1922 . On the interpretation of χ2 from contingency tables, and the calculation of P . Journal of the Royal Statistical Society , 85 : 87 – 94 . doi: 10.2307/2340521
- Freise , J. , Bernau , I. , Meier , S. , Zeidler , H. and Kuipers , J.G. 2009 . Detection of Chlamydia trachomatis-DNA in synovial fluid: evaluation of the sensitivity of different DNA extraction methods and amplification systems . Arthritis Research & Therapy , 11 : R175 doi: 10.1186/ar2864
- Garcia , M. , Jackwood , M.W. , Head , M. , Levisohn , S. and Kleven , S.H. 1996 . Use of species-specific oligonucleotide probes to detect Mycoplasma gallisepticum, M. synoviae, and M. iowae PCR amplification products . Journal of Veterinary Diagnostic Investigation , 8 : 56 – 63 . doi: 10.1177/104063879600800109
- Hammond , P.P. , Ramirez , A.S. , Morrow , C.J. and Bradbury , J.M. 2009 . Development and evaluation of an improved diagnostic PCR for Mycoplasma synoviae using primers located in the haemagglutinin encoding gene vlhA and its value for strain typing . Veterinary Microbiology , 136 : 61 – 68 . doi: 10.1016/j.vetmic.2008.10.011
- Hong , Y. , Garcia , M. , Leiting , V. , Bencina , D. , Dufour-Zavala , L. , Zavala , G. and Kleven , S.H. 2004 . Specific detection and typing of Mycoplasma synoviae strains in poultry with PCR and DNA sequence analysis targeting the hemagglutinin encoding gene vlhA . Avian Diseases , 48 : 606 – 616 . doi: 10.1637/7156-011504R
- ISO7402 1985 Microbiology - General guidance for the enumeration of Enterobacteriaceae without resuscitation - MPN technique and colony count technique, , 1st edn . International Standard Organisation , Geneva
- Jeffery , N. , Gasser , R.B. , Steer , P.A. and Noormohammadi , A.H. 2007 . Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and high-resolution melting-curve analysis of the vlhA gene single-copy region . Microbiology , 153 : 2679 – 2688 . doi: 10.1099/mic.0.2006/005140-0
- Kang , M.S. , Gazdzinski , P. and Kleven , S.H. 2002 . Virulence of recent isolates of Mycoplasma synoviae in turkeys . Avian Diseases , 46 : 102 – 110 . doi: 10.1637/0005-2086(2002)046[0102:VORIOM]2.0.CO;2
- Kaplan , E.L. and Meier , P. 1958 . Nonparametric estimation from incomplete observations . Journal of the American Statistical Association , 53 : 457 – 481 . doi: 10.1080/01621459.1958.10501452
- Kawakubo , Y. , Kume , K. , Yoshioka , M. and Nishiyama , Y. 1980 . Histo- and immuno-pathological studies on experimental Mycoplasma synoviae infection of the chicken . Journal of Comparative Pathology , 90 : 457 – 467 . doi: 10.1016/0021-9975(80)90015-8
- Kerr , K.M. and Olson , N.O. 1970 . Pathology of chickens inoculated experimentally or contact-infected with Mycoplasma synoviae . Avian Diseases , 14 : 291 – 320 . doi: 10.2307/1588474
- Kleven , S.H. and Ferguson-Noel , N. 2008 . “ Mycoplasma synoviae infection ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 845 – 856 . London : Blackwell .
- Kleven , S.H. , King , D.D. and Anderson , D.P. 1972 . Airsacculitis in broilers from Mycoplasma synoviae: effect on air-sac lesions of vaccinating with infectious bronchitis and Newcastle virus . Avian Diseases , 16 : 915 – 924 . doi: 10.2307/1588772
- Kuipers , J.G. , Nietfeld , L. , Dreses-Werringloer , U. , Koehler , L. , Wollenhaupt , J. , Zeidler , H. and Hammer , M. 1999 . Optimised sample preparation of synovial fluid for detection of Chlamydia trachomatis DNA by polymerase chain reaction . Annals of the Rheumatic Diseases , 58 : 103 – 108 . doi: 10.1136/ard.58.2.103
- Landman , W.J.M. , Corbanie , E.A. , Feberwee , A. and Van Eck , J.H.H. 2004 . Aerosolization of Mycoplasma synoviae compared with Mycoplasma gallisepticum and Enterococcus faecalis . Avian Pathology , 33 : 210 – 215 . doi: 10.1080/0307945042000195812
- Landman , W.J.M. and Feberwee , A. 2001 . Field studies on the association between amyloid arthropathy and Mycoplasma synoviae infection, and experimental reproduction of the condition in brown layers . Avian Pathology , 30 : 629 – 639 . doi: 10.1080/03079450120092125
- Landman , W.J.M. and Feberwee , A. 2004 . Aerosol-induced Mycoplasma synoviae arthritis: the synergistic effect of infectious bronchitis virus infection . Avian Pathology , 33 : 591 – 598 . doi: 10.1080/03079450400013170
- Landman , W.J.M. and Feberwee , A. 2012 . Longitudinal field study on the occurrence of Mycoplasma synoviae in Dutch turkey flocks with lameness and experimental induction of the condition . Avian Pathology , 41 : 141 – 149 . doi: 10.1080/03079457.2011.652595
- Landman , W.J.M. , V.d. Bogaard , A.E.J.M. , Doornenbal , P. , Tooten , P.C.J. , Elbers , A.R.W. and Gruys , E. 1998 . The role of various agents in chicken amyloid arthropathy . Amyloid , 5 : 266 – 278 . doi: 10.3109/13506129809007300
- Lauerman , L.H. , Chilina , A.R. , Closser , J.A. and Johansen , D. 1995 . Avian mycoplasma identification using polymerase chain reaction amplicon and restriction fragment length polymorphism analysis . Avian Diseases , 39 : 804 – 811 . doi: 10.2307/1592417
- Lauerman , L.H. , Hoerr , F.J. , Sharpton , A.R. , Shah , S.M. , van Santen , V.L. , Chilina , A.R. , Closser , J.A. and Johansen , D. 1993 . Development and application of a polymerase chain reaction assay for Mycoplasma synoviae . Avian Diseases , 37 : 829 – 834 . doi: 10.2307/1592037
- Lockaby , S.B. , Hoerr , F.J. , Lauerman , L.H. and Kleven , S.H. 1998 . Pathogenicity of Mycoplasma synoviae in broiler chickens . Veterinary Pathology , 35 : 178 – 190 . doi: 10.1177/030098589803500303
- Mann , H.B. and Whitney , D.R. 1947 . On a test of whether one of two random variables is stochastically larger than the other . Annals of Mathematical Statistics , 18 : 50 – 60 . doi: 10.1214/aoms/1177730491
- Mardassi , B.B. , Mohamed , R.B. , Gueriri , I. , Boughattas , S. and Mlik , B. 2005 . Duplex PCR to differentiate between Mycoplasma synoviae and Mycoplasma gallisepticum on the basis of conserved species-specific sequences of their hemagglutinin genes . Journal of Clinical Microbiology , 43 : 948 – 958 . doi: 10.1128/JCM.43.2.948-950.2005
- Mekkes , D.R. and Feberwee , A. 2005 . Real-time polymerase chain reaction for the qualitative and quantitative detection of Mycoplasma gallisepticum . Avian Pathology , 34 : 348 – 354 . doi: 10.1080/03079450500179954
- Morrow , C.J. , Bell , I.G. , Walker , S.B. , Markham , P.F. , Thorp , B.H. and Whithear , K.G. 1990 . Isolation of Mycoplasma synoviae from infectious synovitis of chickens . Australian Veterinary Journal , 67 : 121 – 124 . doi: 10.1111/j.1751-0813.1990.tb07726.x
- Narat , M. , Benčina , D. , Kleven , S.H. and Habe , F. 1998 . The hemagglutination-positive phenotype of Mycoplasma synoviae induces experimental infectious synovitis in chickens more frequently than does the hemagglutination-negative phenotype . Infection and Immunity , 66 : 6004 – 6009 .
- Olson , N.O. , Bletner , J.K. , Shelton , D.C. , Munro , D.A. & Anderson , G.C. 1954 . Enlarged joint condition in poultry caused by an infectious agent . Poultry Science, 33, 1075 .
- Olson , N.O. and Kerr , K.M. 1967 . The duration and distribution of synovitis-producing agents in chickens . Avian Diseases , 11 : 578 – 585 . doi: 10.2307/1588298
- Rådström , P. , Knutsson , R. , Wolffs , P. , Løvenklev , M. and Løfstrøm , C. 2004 . Pre-PCR processing: strategies to generate PCR-compatible samples . Molecular Biotechnology , 26 : 133 – 146 . doi: 10.1385/MB:26:2:133
- Ramírez , A.S. , Naylor , C.J. , Hammond , P.P. and Bradbury , J.M. 2006 . Development and evaluation of a diagnostic PCR for Mycoplasma synoviae using primers located in the intergenic spacer region and the 23S rRNA gene . Veterinary Microbiology , 118 : 76 – 82 . doi: 10.1016/j.vetmic.2006.06.021
- Raviv , Z. and Kleven , S.H. 2009 . The development of diagnostic real-time TaqMan PCRs for the four pathogenic avian mycoplasmas . Avian Diseases , 53 : 103 – 107 . doi: 10.1637/8469-091508-Reg.1
- Schneeweiss , W. , Stanek , C. , Wagner , M. and Hein , I. 2007 . Inhibitor-free DNA for real-time PCR analysis of synovial fluid from horses, cattle and pigs . Veterinary Microbiology , 121 : 189 – 193 . doi: 10.1016/j.vetmic.2006.12.004
- Schwaiger , M. , Péter , O. and Cassinotti , P. 2001 . Routine diagnosis of Borrelia burgdorferi (sensu lato) infections using a real-time PCR assay . Clinical Microbiology and Infection , 7 : 461 – 469 . doi: 10.1046/j.1198-743x.2001.00282.x
- Sprygin , A.V. , Andreychuk , D.B. , Kolotilov , A.N. , Volkov , M.S. , Runina , I.A. , Mudrak , N.S. , Borisov , A.V. , Irza , V.N. , Drygin , V.V. and Perevozchikova , N.A. 2010 . Development of a duplex real-time TaqMan PCR assay with an internal control for the detection of Mycoplasma gallisepticum and Mycoplasma synoviae in clinical samples from commercial and backyard poultry . Avian Pathology , 39 : 99 – 109 . doi: 10.1080/03079451003604621
- Stipkovits , L. and Kempf , I. 1996 . Mycoplasmoses in poultry . Revue Scientifique et Technique [International Office of Epizootics] , 15 : 1495 – 1525 .
- Wills , F.K. 1954 . Preliminary Report on Transmission of an Agent Producing Arthritis in Chickens. (Texas Agricultural Experiment Station Progress Report 1674 & 1723) , College Station , Texas : Texas Agricultural and Mechanical University .