ABSTRACT
Salmonella (S.) Infantis is the most common serovar in broilers and broiler meat in the European Union. In the field, fast-growing broilers are reported to be more affected than slow-growing and layer birds. The present study investigated the infection dynamics and immunological response of four chicken lines in the course of a S. Infantis infection. Two commercial chicken lines, Ross 308 and Hubbard ISA-JA-757, and two experimental chicken lines, specific pathogen free (SPF) layers and broilers, were infected at 2 days of age. Investigations focused on faecal shedding, bacterial colonization, humoral and cellular immune response. Ross and SPF broilers proved mainly as high shedders followed by Hubbard. SPF layers showed the least shedding. This is in agreement with the caecal colonization; SPF layers harboured significantly less bacteria. Systemic spread of S. Infantis to liver and spleen was highest in Ross broilers compared to the other lines. Spread of infection to in-contact birds, was noticed 5 days post infection in every line. Antibody response occurred in every chicken line from 21 days of age onwards. In contrast to the other chicken lines, significant differences in T cell subsets and monocytes/macrophages were found between infected and negative Hubbard birds at 7 days of age. Uninfected SPF birds had significantly higher immune cell counts than uninfected commercial birds, a fact important for future experimental settings. The results illustrate that the infection dynamics of S. Infantis is influenced by the chicken line resulting in a higher risk of transmission to humans from fast-growing broilers.
RESEARCH HIGHLIGHTS
Infection dynamics of Salmonella Infantis differs between chicken lines.
Layers showed less faecal shedding and caecal colonization compared to broilers.
Fast-growing broilers proved more susceptible than slow-growing broilers.
Introduction
Poultry are the focus for transmitting Salmonella (S.) enterica subspecies enterica via the food chain to humans causing human salmonellosis. In the European Union, 70% of all serotyped Salmonella isolates reported from food and animal sources originated from broilers (EFSA, Citation2021). In this regard, S. Infantis has experienced an emerging relevance in the last decade accounting for 29.7% of human infections from food-animal sources, followed by S. Enteritidis (6.9%), monophasic variant of S. Typhimurium (4.5%), S. Typhimurium (3.9%) and S. Derby (3.7%) (EFSA, Citation2021). Currently, 36.3% and 49.1% of all serotyped isolates from broilers and broiler meat, respectively, were found to be S. Infantis. Previous studies revealed that this serovar comprises a heterogeneous pheno- and genotypic population of isolates (Nógrády et al., Citation2012; Sakano et al., Citation2013; Montoro-Dasi et al., Citation2021; Tyson et al., Citation2021). The occurrence of such variants characterized by different susceptibilities to standard cleaning and disinfection procedures is of high relevance for persistence of the bacteria on the farm (Drauch et al., Citation2020). The increasing prevalence of multi-resistant S. Infantis isolates is a serious concern in the public health area (Alba et al., Citation2020, García-Soto et al., Citation2020). In this regard, it was revealed that the presence of the pESI-like plasmid is not only involved in transmitting antimicrobial resistance, but also carries several virulence factors (Aviv et al., Citation2014, García-Soto et al., Citation2020). Previous studies showed that S. Infantis isolates harbouring this plasmid have an increased virulence characterized by higher shedding, higher colonization rates of internal organs, and stronger humoral immune response (Aviv et al., Citation2017, Drauch et al., Citation2021).
Interestingly, from the field a higher prevalence of S. Infantis was reported in fast-growing broilers compared to slow-growing lines, keeping in mind that the latter are a minority which might influence the records. Furthermore, less prevalence of this serovar is reported from layer flocks (10.2%) (EFSA, Citation2021). This is in agreement with the outcome of an experimental study in specific pathogen-free (SPF) layer birds demonstrating that S. Infantis has hardly any invasion capabilities compared to S. Enteritidis, S. Typhimurium and S. Hadar (Berndt et al., Citation2007). Therefore, similar to what has been shown for other Salmonella, the chicken line itself might influence the susceptibility of birds to S. Infantis which might also be reflected in differences in the immune response (Qureshi et al., Citation1986; Bumstead & Barrow, Citation1993; Wigley et al., Citation2002, Citation2006).
This animal trial was set up to investigate two commercial broiler lines used in the field with available SPF layers and broilers. Elaboration of the infection dynamics included shedding behaviour, and the ability to colonize caeca, liver and spleen. In order to investigate the innate or adaptive immune response the frequency of T cells (CD4+, CD8α+ and TCRδγ+), monocytes/macrophages and B cells in peripheral blood was assessed by flow cytometry. An ELISA based upon S. Infantis bacteria was used to determine homologous humoral antibodies.
Material and methods
Birds and housing
The animal trial was approved by the institutional ethics committee and licensed by the national authority according to the Austrian law for animal experiments (license number GZ.: 68.205/0157-V/3b/2019).
Four different chicken breeds were used in the study to compare the infection dynamics of S. Infantis: two SPF breeds (SPF layers (Valo BioMedia GmbH, Osterholz-Scharmbeck, Germany) and SPF broilers (Animal Health Service, Deventer, The Netherlands)) and two commercial broiler breeds (fast-growing breed Ross 308 and slow-growing breed Hubbard ISA-JA-757 (Brueterei Schulz, Lassnitzhoehe, Austria)). SPF layers and SPF broilers were hatched at the Clinic. Ross 308 and Hubbard ISA-JA-757 birds were obtained from a commercial hatchery as 1-day-old chicks. Forty chicks per line were randomly divided into experimentally infected groups each comprising 25 birds and negative control groups each containing 15 birds (). Each group was housed separately in an isolator (Montair HM2500, Montair Environmental Solutions B.V., Kronenberg, The Netherlands) with water and feed supply ad libitum. For identification chicks were individually marked with a tag starting subcutaneously in the neck.
Table 1. Design of the experiment.
Bacterial strain
For infection the well-defined S. Infantis strain MRS-16/01939 was used which was already described in a previous study (Drauch et al., Citation2021). Briefly, the strain harbours the plasmid of Emerging Salmonella enterica Infantis (pESI plasmid) carrying the following genes irp2, ipf, klf, ccdB/ccdA and two plasmid-encoded fimbrial operons, pef and sta. It was submitted to the NCBI database under the accession number SAMN19328299. The strain was grown overnight at 37°C in Luria-Bertani-Broth (LB, Invitrogen, Vienna, Austria) in a shaking incubator (250 rpm). Colony-forming-unit (CFU) count was performed by plating serial dilutions (1:10) on MacConkey agar in duplicates. The mean value was calculated. The infection dose was adjusted to a concentration of 108 CFU/ml.
Experimental design and sampling
In the infection groups 15 out of 25 birds were infected orally with 1 ml each of the S. Infantis suspension at 2 days of age via a crop tube. One ml phosphate buffered saline (PBS, GIBCO, Paisley, UK) was administered to the remaining ten birds, of each infection group serving as in-contact birds and to negative control birds.
The clinical status of the birds and technical parameters of the isolators were determined daily. Blood samples (V. basilica) and cloacal swabs in duplicate (Copan, Stoelzle-Oberglas GmbH, Vienna, Austria) were taken once before infection from five birds and weekly after infection from every bird. Five birds from the infected group (three directly infected and two in-contact birds) and three birds from the negative control group were euthanized weekly with the first killing day 5 days post-infection (7 days of age) until 35 days of age. This was done by injecting a combination (1:1) of Sedaxylan® (20 mg/ml, Dechra Pharmaceuticals, Dornbirn, Austria) and Narketan® (100 mg/ml, Vetoquinol, Vienna, Austria) into the breast muscle followed by bleeding (V. jugularis). Necropsy was performed according to a standard protocol, body weight as well as the weights of the liver and spleen were recorded for each bird. Samples of caecum, liver and spleen were taken for bacteriological examination, and blood was collected (V. jugularis) for flow cytometry analyses.
Bacteriology
Shedding of S. Infantis was analysed by investigating the cloacal swabs taken in duplicate. For this, one cloacal swab was streaked on xylose-lysine-deoxycholate agar (XLD, Merck, Vienna, Austria) and incubated at 37°C for 24 h, and the other swab was kept at 4°C and (in case of a negative result by direct plating) used for an enrichment procedure according to EN ISO 6579-1:2017. In case of a positive result by direct plating, the excretion of S. Infantis was defined as “high shedding”. S. Infantis-positive samples based on the enrichment were referred to as “low shedding”. If a swab was negative by direct plating and by enrichment procedure, the sample was evaluated as “negative”.
Quantification of bacterial load in caecum (tissue and content), liver and spleen was determined by homogenizing 1 g of the organ in PBS (vol. 1:1) using the T 10 basic ULTRA TURRAX (IKA, Staufen, Germany). From the homogenates serial dilutions in PBS (1:10) were prepared. These dilutions were plated in duplicate on XLD, incubated aerobically at 37°C for 24 h. Afterwards, the colonies were counted and the mean value determined. Finally, the quantity of bacteria was calculated as CFU/g per organ. In parallel, homogenates were kept at 4°C until the results of direct plating were available. If direct plating revealed a negative result, homogenates were investigated by enrichment according EN ISO 6579-1:2017 in the same way as the cloacal swabs.
Serology
Weekly blood samples were centrifuged, serum was collected and frozen at −20°C. Samples were gathered and analysed all together at the end of the infection trial. The indirect ELISA was prepared according to the procedure used in a former trial and explained by Drauch et al. (Citation2021).
Briefly, 96-well plates (Nunc Medisorb; Thermo Scientific, Roskilde, Denmark) were coated with 100 μl of S. Infantis suspension per well with a concentration of 109 CFU per ml and incubated overnight at 4°C. On the following day the plates were incubated at 52°C till the liquid was dried completely and 200 μl of blocking buffer (StartingBlock™ PBS Blocking Buffer, Thermo Fisher Scientific, Vienna, Austria) was added for 1 h. Wells were then washed with PBS (containing 0.1% Tween 20) and serum was added in a dilution of 1:200, all performed in duplicate. After incubation for 1 h at room temperature, plates were washed and treated with 100 μl of Goat-Anti Chicken IgY (H + L)-HRP (Southern Biotechnology, Birmingham, AL, USA) in a dilution of 1:5000 and again incubated at room temperature for 1 h. After a final washing step, 100 μl of tetramethylbenzidine substrate (Calbiochem, Darmstadt, Germany) was added to each well and plates were incubated in the dark. After 12 min wells were treated with 100 μl of 0.5M H2SO4 to stop the colour reaction and the optical density (OD) was determined at a wavelength of 450 nm with an ELISA reader (Sunrise-Basic; Tecan, Groedig, Austria). Individual results are given as mean of the performed duplicates.
Flow cytometry analyses
For the separation of peripheral blood mononuclear cells (PBMCs), the protocol described by Mitra et al. (Citation2017) was applied with some modifications. Antibodies and antibody combinations used to determine immune cell populations are presented in . Precisely, blood from three birds per group (directly infected and negative control) per breed was investigated. The amount of blood taken increased with the age of the birds starting with 1 ml for 1-week-old birds, and reaching 6.5 ml for 5-week-old birds.
Table 2. Table of antibodies and antibody combinations used to determine immune cell populations of chickens by flow cytometry.
In a first step, the blood was mixed with 100 µl heparin (Serva, Heidelberg, Germany) per ml to prevent coagulation. PBS with 2% foetal bovine serum (FBS) (ThermoFisher Scientific, Vienna, Austria) was added to the blood in an equal amount. To enable density gradient centrifugation this mixture was overlaid twice the volume with Histopaque®-1077 (Sigma-Aldrich, Vienna, Austria) and centrifuged at 400×g for 20 min at room temperature without brake. Cells were then collected from the interface and washed three times with PBS + 2% FBS by centrifugation at 350×g for 5 min at 4°C. Before starting the immunophenotyping, the viability of cells was verified via Cellometer® X2 fluorescent viability cell counter system (Nexcelom Bioscience, Manchester, UK) and adjusted to a concentration of 2 × 107 cells/ml.
Staining of cells was performed by adding 25 μl of the adjusted cell mixture together with the primary antibodies into 96-well microtitre plates (Sarstedt, Nümbrecht, Germany) before incubation at 4°C for 20 min. Additionally, as a technical control, samples without staining and isotype controls were included for every group. Following incubation, the cells were washed twice with PBS + 2% FBS by centrifugation at 450×g for 4 min at 4°C. The secondary antibody BV421 was added to cells of the antibody panel “a” and 2.5 ul of Lide/Dead Dye (BD Horizon™ Fixable Viability Stain 780) were added to cells of both antibody penels. Subsequently, the plate was pulse-shaken and incubated for 30 min at 4°C. Afterwards, the cells were washed again by centrifugation before the pellets were re-suspended in 100 µl of PBS + 2% FBS for further analysis. From each bird, at least 20,000 leukocytes were recorded with a FACSCanto II (BD Biosciences, San Jose, CA) flow cytometer using three lasers (405, 488 and 633 nm). After doublet discrimination, live cells were detected before gating the cell population for leukocytes (CD45+). Two different CD45+ gating strategies were applied to target lymphocytes and monocytes/macrophages. From leukocytes, subsequent cell populations targeting CD4+ T cells, CD8α+ T cells, TCRδγ+ T cells, B cells (Bu1) and monocytes/macrophages (Kul01) were gated (Additional File 1). The data were analysed by FACSDiva Software version 9.0 (BD Biosciences). The absolute cell count for each cell population was calculated according to Mitra et al. (Citation2017) before proceeding to statistical analysis.
Statistical analysis
Statistical analysis was performed in R Core Team by using three packages (nortest, ggplot2 and ggpubr). After exploratory data analysis, normality of the results was assessed by the Anderson–Darling test. In the case of non-normally distributed data, logarithmic transformation was used. Linear models were implemented to test for significant differences between bodyweight, weight of liver and spleen, CFU results, OD values and flow cytometry results of directly infected, in-contact and negative control birds. Shedding profiles were analysed with a generalized linear model with a specification for a binominal distribution of data.
Results
Clinical signs and necropsy
No clinical signs were observed in any of the groups throughout the duration of the trial. Neither the body weights, nor the weights of liver and spleen revealed any significant differences between infected and negative control groups within each breed. No gross pathological lesions were found during necropsy. One SPF layer bird was found dead in the night before the second sampling day (14 days of age) due to cannibalism. The bird was necropsied and sampled according to the described scheme. As the CFU results of this bird were in agreement with the results of the birds killed at the sampling day these data were included into the analysis. But, serological analysis was not feasible. Therefore, ELISA data for SPF layers on 14 days of age comprise 19 instead of 20 samples.
Bacteriology
Samples taken before the infection as well as from birds of the negative control groups were negative for S. Infantis throughout the trial until termination.
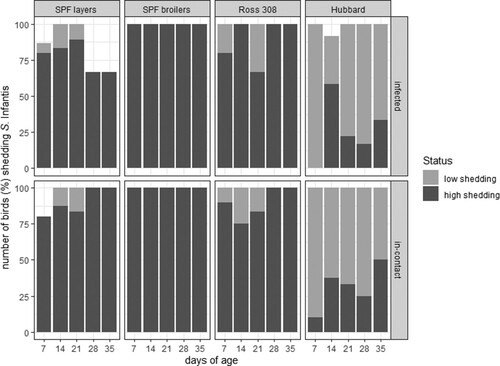
Cloacal swabs revealed shedding of S. Infantis in all breeds from 7 days of age onwards (). A rapid spread of S. Infantis was detected as in-contact birds were positive for shedding from 7 days of age onwards with no significant difference between directly infected and in-contact birds in regards of the shedding profile ().
Figure 1. Shedding of S. Infantis in four different chicken breeds (directly infected and in-contact birds). Shedding profile of Hubbard birds was significantly different (P < 0.05) from the three other breeds.
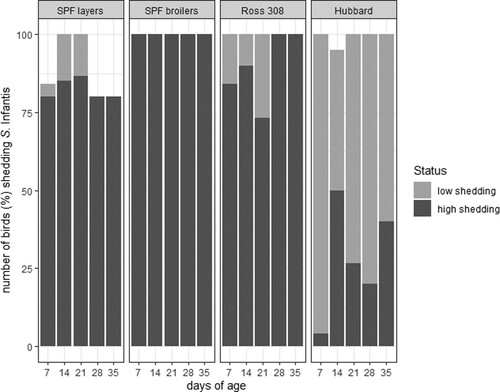
Figure 2. Shedding of S. Infantis in four different chicken lines separating directly infected (n = 15 birds per line) from in-contact birds (n = 10 bird per line).
SPF layers (directly infected and in-contact birds) mainly appeared as high shedders (in total 62 from 75 samples, 82.7%) and seldom as low-shedders (in total six samples, 8%). At three sampling time-points (days 7, 28 and 35) some birds were not shedding S. Infantis (in total seven samples, 9.3%).
At each sampling point 100% of the SPF broilers and the Ross 308 birds, independent of whether they were directly infected or in-contact birds, were shedding S. Infantis. The group of SPF broilers contained only high-shedding birds, whereas Ross 308 birds also showed low shedding at the first three sampling time-points (days 7, 14 and 21; in total 10 out of 75 samples, 13.3%).
All Hubbard birds (directly infected and in-contact) shed S. Infantis during the sampling point with the exception of one bird (directly infected) on day 14 that did not shed at all. Hubbard birds showed mainly low shedding (in total 55 out of 75 samples, 73.3%) and less high shedding (in total 19 samples, 25.3%) being statistically significant compared to the three other breeds (P < 0.05).
The colonization of the caecum detected by direct plating was independent of infection (directly or in-contact) (). The three broiler breeds gave similar results, except for 21 days of age at which SPF broilers harboured significantly less S. Infantis ((a)). The counts ranged from 1 × 108–1.2 × 1011 CFU/g. In comparison to the three broiler breeds, a lower colonization rate was found in SPF layers (range 2 × 106–6 × 1010 CFU/g) being significant at 7, 28 and 35 days of age ((a)).
Figure 3. Colonization of S. Infantis in caecum, liver and spleen of birds from four different breeds detected by enrichment or direct plating.
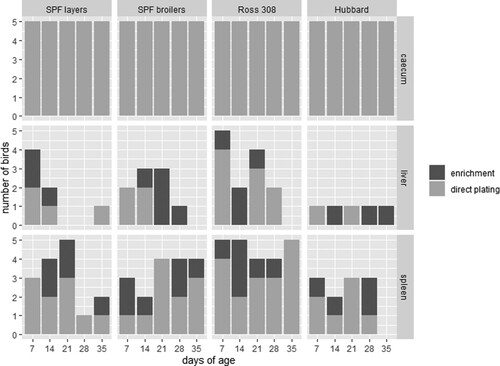
Figure 4. Comparison of the colonization of S. Infantis in caecum (a), liver (b) and spleen (c) between four different chicken lines (n = 5 birds per line and per killing day). Logarithmic transformation of CFU counts were used. Significant differences between the two groups are marked with **P < 0.01 or ***P < 0.001.
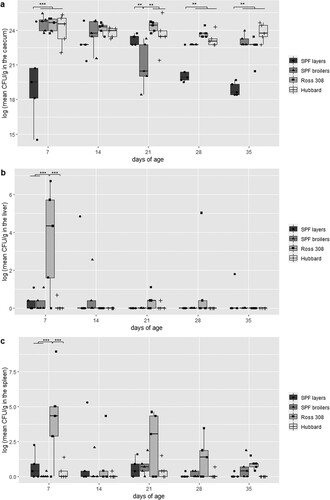
In general, S. Infantis was re-isolated more often from liver samples from Ross 308 birds (n = 13/25), mainly by direct plating (). Furthermore, at 7 days of age a significantly higher CFU count was found in Ross 308 compared to the three other breeds, namely 1–1.1 × 103 CFU/g in Ross 308, 1–12 CFU/g in SPF broilers, 1–2 × 102 CFU/g in SPF layers and 1–2 CFU/g in Hubbard birds ((b)). In total, nine, seven and five liver samples were positive for S. Infantis from SPF broilers, SPF layers and Hubbard birds, respectively. Here, approximately equal re-isolation with direct plating and enrichment was observed. With the exception of Hubbard birds which had only a single bird positive at each sampling time-point, all other breeds showed a decrease in positive birds throughout sampling with broiler breeds being negative at 35 days of age ( and (b)).
The S. Infantis colonization in spleens showed a similar tendency as obtained from livers albeit positive birds were noticed with higher frequency and already by direct plating (). S. Infantis was re-isolated from all spleen samples derived from Ross 308 birds with CFU counts ranging from 1–1 × 104 CFU/g. The CFU was even significantly higher compared to the three other breeds at 7 days of age ((c)). In total, S. Infantis was re-isolated from 17 spleen samples from SPF broilers, followed by SPF layers with 15 positive samples. The lowest colonization rate was again found in Hubbard birds resulting in 11 positve samples (). The counts ranged from 1–9 CFU/g for SPF broilers, 1–3 × 102 CFU/g for SPF layers and 1–7 CFU/g for Hubbard birds ((c)).
Serology
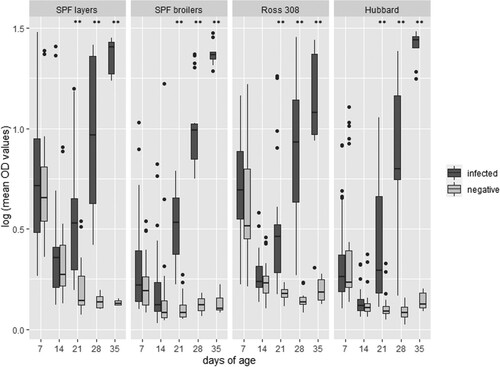
Independent of the breed, a significant (P < 0.01) increase of humoral antibodies in the infected birds (directly infected and in-contact) was observed from 21 days of age onwards compared to their negative controls with a stable increase until the end of the study (). In-contact birds also presented a significant rise of antibodies from 21 days of age onwards compared to the negative controls, and therefore the figure represents directly infected and in-contact birds as one group. No inter-breed differences were found.
Figure 5. S. Infantis ELISA results (logarithmic transformation of mean OD values) of four different lines comparing infected (directly infected and in-contact birds) against negative birds (n = 25 birds on the first sampling day per breed with reduction of five birds per day). Significant differences between the two groups are marked with **P < 0.01.
Flow cytometry analyses
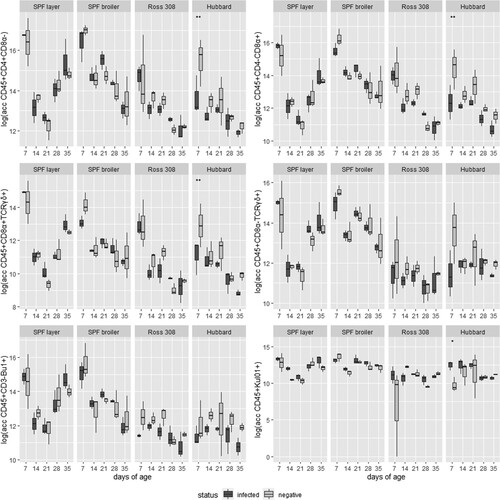
No substantial differences were found in the absolute cell counts of different immune cell populations between directly infected and negative birds in SPF layers, SPF broilers and Ross 308 (data not shown). In Hubbard birds at 7 days of age significantly lower CD45+CD4+CD8α−, CD45+CD4-CD8α+ and CD45+CD8α+TCRδγ+ counts were found in the PBMCs of infected birds. At the same time, the amount of macrophages/monocytes (CD45+KUL01+) in infected Hubbard birds was significantly increased in the blood compared to the negative control (). For CD45+CD8α-TCRδγ+ cells and B cells no significant differences were detected in Hubbard birds (data not shown).
Figure 6. Absolute cell counts in logarithmic transformation of six immune cell populations in four different lines, infected and controls, at five sampling days (n = 3 birds per group per sampling day). Significant differences between infected and negative birds are marked with *P < 0.05 or **P < 0.01.
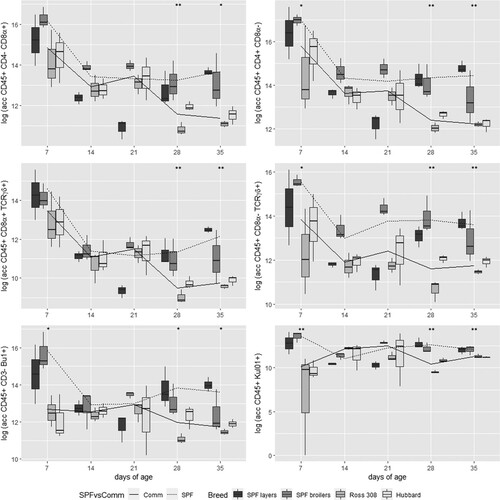
Interestingly, uninfected birds from both SPF breeds (layers and broilers) revealed a generally higher number of immune cells compared to uninfected birds from commercial broilers (Ross 308 and Hubbard ISA-JA-757). Therefore, the results of SPF birds were merged as well as the results of commercial broiler birds, revealing a significantly higher number of CD45+CD4−CD8α+ and macrophages/monocytes (KUL01) in SPF birds compared to commercial birds at 7 days, and for all analysed populations at 28 and 35 days ().
Figure 7. Comparison of absolute cell counts in logarithmic transformation of six different immune cell populations at five sampling days between four different lines represented in boxplots and between combined data of SPF lines (including results of SPF layers and SPF broilers) and commercial (Comm) lines (including results of Ross 308 and Hubbard) in lines. Significant differences between the two combined groups (SPF and Comm) are indicated with *P < 0.05 or **P < 0.01.
Discussion
S. Infantis is among the top 10 Salmonella serovars causing human illness in the EU, and poultry are the most common source (EFSA, Citation2021). Especially broiler birds have a central role in distribution of this serovar, indicating a certain affinity to this type of bird. Despite Salmonella Control Programmes according to Regulation (EC) No. 2160/2003, together with biosecurity measures characterized by strict cleaning and disinfection protocols, the prevalence of S. Infantis in broiler birds has remained very stable over recent years. This lead to the assumption that an eradication of this serovar is a very difficult task (Drauch et al., Citation2020; Sevilla-Navarro et al., Citation2020). In addition to the overall epidemiology and the prevalence on a farm level, basic knowledge about the infection dynamics of S. Infantis is the centre of interest. Therefore, the present experimental study intended to compare the infection dynamics of S. Infantis in different chicken lines. To determine possible differences in shedding, organ colonization and immune response, two experimental chicken lines (SPF layers and SPF broilers) and two commercial lines (Ross 308 and Hubbard ISA-JA-757) were infected with S. Infantis. For this purpose, a well-defined S. Infantis strain was used harbouring the pESI-like plasmid. This strain was chosen as it showed increased ability to colonize liver and spleen of commercial broilers (Drauch et al., Citation2021). However, it has to be acknowledged that a certain limitation in interpretation of the results might be present by using one strain only keeping the heterogeneity character of S. Infantis in mind.
Shedding of Salmonella via the faecal route plays an important role in transmission within a chicken flock as well as in the contamination of chicken meat (Gast et al., Citation2005, Citation2017; Boubendir et al., Citation2021; Montoro-Dasi et al., Citation2021; Zeng et al., Citation2021). Comparing the four different lines, the greatest similarity of shedding behaviour was found among SPF broilers and Ross 308 where the majority of birds were defined as high-shedders with 100% and 86.7%, respectively. An explanation for this might be that both lines represent fast-growing meat-type chickens and respond in a similar way to infections with S. Infantis. In contrast, predominantly low-shedding birds (73.3%) were detected in the slow-growing line Hubbard ISA-JA-757. Shedding was lowest in SPF layers where 9.3% of the birds were negative for S. Infantis. The present findings are in agreement with reported differences regarding the faecal excretion of Salmonella serotypes including S. Infantis in different chicken lines (Barrow et al., Citation2004). Similar results were reported for infections with C. jejuni proving broilers are more prone to infections than layers (Hankel et al., Citation2018). Interestingly, despite less shedding or fewer infected birds excreting bacteria, the transmission of S. Infantis to in-contact birds in all groups happened within 5 days resulting in no differences among the breeds. Previous studies revealed the importance of different shedding levels within flocks in context of infection dynamics independent from the genetic host population (Matthews et al., Citation2006; Slater et al., Citation2016; Menanteau et al., Citation2018; Kempf et al., Citation2020), a phenomenon which was also found in the present study for S. Infantis.
The favoured location site of Salmonella is the caecum (Snoeyenbos et al., Citation1982; Barrow et al., Citation1988). In this intestinal part, colonization and replication takes place at an early age (Varmuzova et al., Citation2016). This is also reflected by the presented results, as no enrichment was needed to re-isolate S. Infantis. In agreement with previous data, there was no difference between the broiler lines in regard to caecal load of bacteria (Guillot et al., Citation1995). The layer-type birds revealed a significantly lower colonization rate which might likely be in relation to the decreased faecal excretion as previously reported (Barrow et al., Citation2004). An explanation for this difference in broiler and layer lines might be a diverse gene expression response in the intestine as shown for S. Enteritidis (van Hemert et al., Citation2006). It was shown that genes responsible for T-cell activation and for macrophage activity differed significantly between two chicken lines. This was not part of the focus in the present study, but needs to be elucidated in future investigations.
The present study revealed a distribution of S. Infantis into internal organs of all infected birds. In previous studies it was shown for S. Enteritidis and S. Typhimurium that they are able to impair the gut integrity by disruption of tight junctions, leading to an increased permeability and resulting in a translocation to internal organs, e.g. liver and spleen (Awad et al., Citation2017). This could also be an explanation for the present finding on S. Infantis, but needs to be clarified in future investigations as this was not in the scope of the present study. Clearly, Ross 308 birds were more affected by S. Infantis colonization compared to the other breeds. For C. jejuni a correlation between CFU in the gut and intestinal permeability allowing invasion of Campylobacter to other tissues was revealed (Awad et al., Citation2015; Han et al., Citation2016). This cannot be assumed for S. Infantis, as caecal counts were very similar for all broiler chicken lines. A similar finding was reported previously for S. Enteritidis suggesting that the intestinal transcriptional and immunological response may be the underlying process of enhanced systemic spread (Schokker et al., Citation2012).
The breed has been reported to be one of the key factors influencing the host response, and with this the outcome of excretion and colonization of Salmonella reflecting resistance or susceptibility (Wigley et al., Citation2002; van Hemert et al., Citation2006; Wigley et al., Citation2006; Zhou & Lamont, Citation2007; Schokker et al., Citation2012; Li et al., Citation2018). It is well known that the immune response differs among chicken breeds which has to be seen in context with the genetic background (Koenen et al., Citation2002; Schokker et al., Citation2012; Bilková et al., Citation2017; Giles et al., Citation2019; Kaiser et al., Citation2022). Especially genetic selection on body weight and feed conversion was reported as a major influence compromising immune response (Koenen et al., Citation2002; van der Most et al., Citation2011; Borodin et al., Citation2020). Differences in resistance to Salmonella species were already reported among diverse chicken lines (Bumstead & Barrow, Citation1993; Barrow et al., Citation2004; Li et al., Citation2018). In this context it was also shown that genetic selection towards enhanced performance traits negatively influenced the immune system which resulted in lower natural resistance to Salmonella (Cheema et al., Citation2003; Kramer et al., Citation2003; van Hemert et al., Citation2006). These findings are confirmed by this study for S. Infantis and illustrate the high susceptibility of commercial fast growing broilers.
No differences in the humoral antibody response were detected among the investigated chicken lines. It is widely accepted that humoral response to Salmonella is a less important defence mechanism (van Immerseel et al., Citation2005). Although an antibody production against S. Infantis was present and confirmed successful infection, B cells in the blood were not increased indicating no positive correlation.
At 7 days of age monocytes/macrophages were found in significantly higher ratios in infected Hubbard ISA-JA-757 birds compared to their negative controls. This chicken line also had the lowest number of birds being positive in regards to colonization of liver and spleen samples. This goes in line with Ijaz et al. (Citation2021), who found an increase of macrophages during the infection with Salmonella initiating phagocytosis of the bacteria. This specificity of the Hubbard ISA-JA-757 line is in agreement with Qureshi (Citation2003) stating that the ability of macrophages to phagocytose pathogens is breed dependent. Furthermore, a previous study revealed that slow-growing chickens are more resistant to Salmonella compared to fast-growing chickens (van Hemert et al., Citation2006). However, no substantial differences among the infected chicken lines of SPF layers, SPF broilers and Ross 308 compared with non-infected control birds were revealed when investigating different immune cells in the blood. In general, the immune response of chickens infected with S. Infantis was reported to be lower than for other Salmonella serovars (Berndt et al., Citation2007; Setta et al., Citation2012). Moreover, it is possible that changes in the immune response are more prominent in the local organ compared to the changes noticed at systemic level (blood) (Beal et al., Citation2004; Setta et al., Citation2012). Hence, a detailed investigation of immune response at local level would be interesting in future studies.
Interestingly, this study demonstrated significantly higher immune cell counts in uninfected SPF birds irrespective of layer or broiler type, compared to uninfected commercially fast- and slow-growing broilers. This finding emphasizes the need to act with caution when correlating such data from SPF birds with commercial birds in experimental settings.
Overall, the present study revealed significant differences in excretion and colonization patterns of S. Infantis in four different chicken lines. The layer-type and the slow-growing broiler line proved less susceptible to an infection compared to the fast-growing birds. These results are in agreement with field reports where S. Infantis is less frequent being found in layer and slow-growing broiler birds. To date, targeted prophylactic measures against S. Infantis are not available for broiler birds and eradication is a difficult task. In this context, different approaches to achieve reduction of this pathogen comprised treatment of feeds, usage of probiotics, application of bacteriophages and vaccination of breeders (Koyuncu et al., Citation2013; Varmuzova et al., Citation2016; Crouch et al., Citation2020; Sevilla-Navarro et al., Citation2020). Therefore, any contribution in reducing the contamination rate of broiler flocks with respect to the excretion and colonization of inner organs will contribute to safer broiler meat products, and by this help to reduce the spread to humans and subsequently the risk of salmonellosis.
Supplemental Material
Download MS Word (12 KB)Supplemental Material
Download MS Word (780.4 KB)Acknowledgements
The authors thank Delfina Jandreski-Cvetkovic and Claudia Wawra-Ibesich for their excellent technical assistance and Vesna Stanislajevic and Attila Sandor for their help in the experiment. The statistical expertise from Dr. Claus Vogl (Institute of Animal Breeding and Genetics, University of Veterinary Medicine Vienna, Austria) was highly appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alba, P., Leekitcharoenphon, P., Carfora, V., Amoruso, R., Cordaro, G., Di Matteo, P., Ianzano, A., Iurescia, M., Diaconu, E.L., Pedersen, S.K., Guerra, B., Hendriksen, R.S., Franco, A. & Battisti, A. (2020). Molecular epidemiology of Salmonella Infantis in Europe: insights into the success of the bacterial host and its parasitic pESI-like megaplasmid. Microbial Genomics, 6, e000365.
- Aviv, G., Elpers, L., Mikhlin, S., Cohen, H., Vitman Zilber, S., Grassl, G.A., Rahav, G., Hensel, M. & Gal-Mor, O. (2017). The plasmid-encoded Ipf and Klf fimbriae display different expression and varying roles in the virulence of Salmonella enterica serovar Infantis in mouse vs. avian hosts. PLoS Pathogens, 13, e1006559.
- Aviv, G., Tsyba, K., Steck, N., Salmon-Divon, M., Cornelius, A., Rahav, G., Grassl, G.A. & Gal-Mor, O. (2014). A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environmental Microbiology, 16, 977–994.
- Awad, W.A., Hess, C. & Hess, M. (2017). Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins, 9, 60.
- Awad, W.A., Molnár, A., Aschenbach, J.R., Ghareeb, K., Khayal, B., Hess, C., Liebhart, D., Dublecz, K. & Hess, M. (2015). Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immunity, 21, 151–160.
- Barrow, P.A., Bumstead, N., Marston, K., Lovell, M.A. & Wigley, P. (2004). Faecal shedding and intestinal colonization of Salmonella enterica in in-bred chickens: the effect of host-genetic background. Epidemiology and Infection, 132, 117–126.
- Barrow, P.A., Simpson, J.M. & Lovell, M.A. (1988). Intestinal colonisation in the chicken by food-poisoning Salmonella serotypes; microbial characteristics associated with faecal excretion. Avian Pathology, 17, 571–588.
- Beal, R.K., Powers, C., Wigley, P., Barrow, P.A. & Smith, A.L. (2004). Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathology, 33, 25–33.
- Berndt, A., Wilhelm, A., Jugert, C., Pieper, J., Sachse, K. & Methner, U. (2007). Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infection and Immunity, 75, 5993–6007.
- Bílková, B., Bainová, Z., Janda, J., Zita, L. & Vinkler, M. (2017). Different breeds, different blood: cytometric analysis of whole blood cellular composition in chicken breeds. Veterinary Immunology and Immunopathology, 188, 71–77.
- Borodin, АМ, Alekseev, Y.I., Gerasimov, K.E., Konovalova, N.V., Тerentjeva, E.V., Efimov, D.N., Emanuilova, Z.V., Tuchemskiy, L.I., Komarov, A.A. & Fisinin, V.I. (2020). Chickens productivity selection affects immune system genes. Vavilovskii Zhurnal Genetiki i Selektsii, 24, 755–760.
- Boubendir, S., Arsenault, J., Quessy, S., Thibodeau, A., Fravalo, P., ThÉriault, W.P., Fournaise, S. & Gaucher, M.L. (2021). Salmonella contamination of broiler chicken carcasses at critical steps of the slaughter process and in the environment of two slaughter plants: prevalence, genetic profiles, and association with the final carcass status. Journal of Food Protection, 84, 321–332.
- Bumstead, N. & Barrow, P. (1993). Resistance to Salmonella gallinarum, S. pullorum, and S. enteritidis in inbred lines of chickens. Avian Diseases, 37, 189–193.
- Cheema, M.A., Qureshi, M.A. & Havenstein, G.B. (2003). A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poultry Science, 82, 1519–1529.
- Crouch, C.F., Pugh, C., Patel, A., Brink, H., Wharmby, C., Watts, A., van Hulten, M. & de Vries, S. (2020). Reduction in intestinal colonization and invasion of internal organs after challenge by homologous and heterologous serovars of Salmonella enterica following vaccination of chickens with a novel trivalent inactivated Salmonella vaccine. Avian Pathology, 49, 666–677.
- Drauch, V., Ibesich, C., Vogl, C., Hess, M. & Hess, C. (2020). In-vitro testing of bacteriostatic and bactericidal efficacy of commercial disinfectants against Salmonella Infantis reveals substantial differences between products and bacterial strains. International Journal of Food Microbiology, 328, 108660.
- Drauch, V., Kornschober, C., Palmieri, N., Hess, M. & Hess, C. (2021). Infection dynamics of Salmonella Infantis strains displaying different genetic backgrounds - with or without pESI-like plasmid – vary considerably. Emerging Microbes & Infections, 10, 1471–1480.
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). (2021). The European union one health 2020 zoonoses report. EFSA Journal, 19, 6971.
- García-Soto, S., Abdel-Glil, M.Y., Tomaso, H., Linde, J. & Methner, U. (2020). Emergence of multidrug-resistant Salmonella enterica subspecies enterica serovar Infantis of multilocus sequence type 2283 in German broiler farms. Frontiers in Microbiology, 11, 1741.
- Gast, R.K., Guard-Bouldin, J. & Holt, P.S. (2005). The relationship between the duration of fecal shedding and the production of contaminated eggs by laying hens infected with strains of Salmonella Enteritidis and Salmonella Heidelberg. Avian Diseases, 49, 382–386.
- Gast, R.K., Guraya, R., Jones, D.R., Guard, J., Anderson, K.E. & Karcher, D.M. (2017). Frequency and duration of fecal shedding of Salmonella serovars Heidelberg and Typhimurium by experimentally infected laying hens housed in enriched colony cages at different stocking densities. Avian Diseases, 61, 366–371.
- Giles, T., Sakkas, P., Belkhiri, A., Barrow, P., Kyriazakis, I. & Foster, N. (2019). Differential immune response to Eimeria maxima infection in fast- and slow-growing broiler genotypes. Parasite Immunology, 41, e12660.
- Guillot, J.F., Beaumont, C., Bellatif, F., Mouline, C., Lantier, F., Colin, P. & Protais, J. (1995). Comparison of resistance of various poultry lines to infection by Salmonella enteritidis. Veterinary Research, 26, 81–86.
- Han, Z., Willer, T., Pielsticker, C., Gerzova, L., Rychlik, I. & Rautenschlein, S. (2016). Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathogens, 8, 56.
- Hankel, J., Popp, J., Meemken, D., Zeiger, K., Beyerbach, M., Taube, V., Klein, G. & Visscher, C. (2018). Influence of lauric acid on the susceptibility of chickens to an experimental Campylobacter jejuni colonisation. PLoS One, 13, e0204483.
- Ijaz, A., Veldhuizen, E., Broere, F., Rutten, V. & Jansen, C.A. (2021). The interplay between Salmonella and intestinal innate immune cells in chickens. Pathogens, 10, 1512.
- Kaiser, M.G., Hsieh, J., Kaiser, P. & Lamont, S.J. (2022). Differential immunological response detected in mRNA expression profiles among diverse chicken lines in response to Salmonella challenge. Poultry Science, 101, 101605.
- Kempf, F., Menanteau, P., Rychlik, I., Kubasová, T., Trotereau, J., Virlogeux-Payant, I., Schaeffer, S., Schouler, C., Drumo, R., Guitton, E. & Velge, P. (2020). Gut microbiota composition before infection determines the Salmonella super- and low-shedder phenotypes in chicken. Microbial Biotechnology, 13, 1611–1630.
- Koenen, M.E., Boonstra-Blom, A.G. & Jeurissen, S.H. (2002). Immunological differences between layer- and broiler-type chickens. Veterinary Immunology and Immunopathology, 89, 47–56.
- Koyuncu, S., Andersson, M.G., Löfström, C., Skandamis, P.N., Gounadaki, A., Zentek, J. & Häggblom, P. (2013). Organic acids for control of Salmonella in different feed materials. BMC Veterinary Research, 9, 81.
- Kramer, J., Visscher, A.H., Wagenaar, J.A., Cornelissen, J.B. & Jeurissen, S.H. (2003). Comparison of natural resistance in seven genetic groups of meat-type chicken. British Poultry Science, 44, 577–585.
- Li, X., Nie, C., Zhang, Z., Wang, Q., Shao, P., Zhao, Q., Chen, Y., Wang, D., Li, Y., Jiao, W., Li, L., Qin, S., He, L., Jia, Y., Ning, Z. & Qu, L. (2018). Evaluation of genetic resistance to Salmonella Pullorum in three chicken lines. Poultry Science, 97, 764–769.
- Matthews, L., Low, J.C., Gally, D.L., Pearce, M.C., Mellor, D.J., Heesterbeek, J.A., Chase-Topping, M., Naylor, S.W., Shaw, D.J., Reid, S.W., Gunn, G.J. & Woolhouse, M.E. (2006). Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. Proceedings of the National Academy of Sciences of the United States of America, 103, 547–552.
- Menanteau, P., Kempf, F., Trotereau, J., Virlogeux-Payant, I., Gitton, E., Dalifard, J., Gabriel, I., Rychlik, I. & Velge, P. (2018). Role of systemic infection, cross contaminations and super-shedders in Salmonella carrier state in chicken. Environmental Microbiology, 20, 3246–3260.
- Mitra, T., Gerner, W., Kidane, F.A., Wernsdorf, P., Hess, M., Saalmüller, A. & Liebhart, D. (2017). Vaccination against histomonosis limits pronounced changes of B cells and T-cell subsets in turkeys and chickens. Vaccine, 35, 4184–4196.
- Montoro-Dasi, L., Villagra, A., Vega, S. & Marin, C. (2021). Influence of farm management on the dynamics of Salmonella enterica serovar Infantis shedding and antibiotic resistance during the growing period of broiler chickens. The Veterinary Record, 188, e302.
- Nógrády, N., Király, M., Davies, R. & Nagy, B. (2012). Multidrug resistant clones of Salmonella Infantis of broiler origin in Europe. International Journal of Food Microbiology, 157, 108–112.
- Qureshi, M.A. (2003). Avian macrophage and immune response: an overview. Poultry Science, 82, 691–698.
- Qureshi, M.A., Dietert, R.R. & Bacon, L.D. (1986). Genetic variation in the recruitment and activation of chicken peritoneal macrophages. Proceedings of the Society for Experimental Biology and Medicine, 181, 560–568.
- Sakano, C., Kuroda, M., Sekizuka, T., Ishioka, T., Morita, Y., Ryo, A., Tsukagoshi, H., Kawai, Y., Inoue, N., Takada, H., Ogaswara, Y., Nishina, A., Shimoda, M.A., Kozawa, K., Oishi, K. & Kimura, H. (2013). Genetic analysis of non-hydrogen sulfide-producing Salmonella enterica serovar typhimurium and S. enterica serovar infantis isolates in Japan. Journal of Clinical Microbiology, 51, 328–330.
- Schokker, D., Peters, T.H., Hoekman, A.J., Rebel, J.M. & Smits, M.A. (2012). Differences in the early response of hatchlings of different chicken breeding lines to Salmonella enterica serovar Enteritidis infection. Poultry Science, 91, 346–353.
- Setta, A.M., Barrow, P.A., Kaiser, P. & Jones, M.A. (2012). Early immune dynamics following infection with Salmonella enterica serovars Enteritidis, Infantis, Pullorum and Gallinarum: cytokine and chemokine gene expression profile and cellular changes of chicken cecal tonsils. Comparative Immunology, Microbiology and Infectious Diseases, 35, 397–410.
- Sevilla-Navarro, S., Catalá-Gregori, P., García, C., Cortés, V. & Marin, C. (2020). Salmonella Infantis and Salmonella Enteritidis specific bacteriophages isolated form poultry faeces as a complementary tool for cleaning and disinfection against Salmonella. Comparative Immunology, Microbiology and Infectious Diseases, 68, 101405.
- Slater, N., Mitchell, R.M., Whitlock, R.H., Fyock, T., Pradhan, A.K., Knupfer, E., Schukken, Y.H. & Louzoun, Y. (2016). Impact of the shedding level on transmission of persistent infections in Mycobacterium avium subspecies paratuberculosis (MAP). Veterinary Research, 47, 38.
- Snoeyenbos, G.H., Soerjadi, A.S. & Weinack, O.M. (1982). Gastrointestinal colonization by salmonellae and pathogenic Escherichia coli in monoxenic and holoxenic chicks and poults. Avian Diseases, 26, 566–575.
- Tyson, G.H., Li, C., Harrison, L.B., Martin, G., Hsu, C.H., Tate, H., Tran, T.T., Strain, E. & Zhao, S. (2021). A multidrug-resistant Salmonella Infantis clone is spreading and recombining in the United States. Microbial Drug Resistance (Larchmont, N.Y.), 27, 792–799.
- van Hemert, S., Hoekman, A.J., Smits, M.A. & Rebel, J.M. (2006). Gene expression responses to a Salmonella infection in the chicken intestine differ between lines. Veterinary Immunology and Immunopathology, 114, 247–258.
- van Immerseel, F., Methner, U., Rychlik, I., Nagy, B., Velge, P., Martin, G., Foster, N., Ducatelle, R. & Barrow, P.A. (2005). Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: exploitation of innate immunity and microbial activity. Epidemiology and Infection, 133, 959–978.
- van der Most, P.J., de Jong, B., Parmentier, H.K. & Verhulst, S. (2011). Trade-off between growth and immune function: a meta-analysis of selection experiments. Functional Ecology, 25, 74–80.
- Varmuzova, K., Kubasova, T., Davidova-Gerzova, L., Sisak, F., Havlickova, H., Sebkova, A., Faldynova, M. & Rychlik, I. (2016). Composition of gut microbiota influences resistance of newly hatched chickens to Salmonella Enteritidis infection. Frontiers in Microbiology, 7, 957.
- Wigley, P., Hulme, S.D., Bumstead, N. & Barrow, P.A. (2002). In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes and Infection, 4, 1111–1120.
- Wigley, P., Hulme, S., Rothwell, L., Bumstead, N., Kaiser, P. & Barrow, P. (2006). Macrophages isolated from chickens genetically resistant or susceptible to systemic salmonellosis show magnitudinal and temporal differential expression of cytokines and chemokines following Salmonella enterica challenge. Infection and Immunity, 74, 1425–1430.
- Zeng, H., De Reu, K., Gabriël, S., Mattheus, W., De Zutter, L. & Rasschaert, G. (2021). Salmonella prevalence and persistence in industrialized poultry slaughterhouses. Poultry Science, 100, 100991.
- Zhou, H. & Lamont, S.J. (2007). Global gene expression profile after Salmonella enterica serovar enteritidis challenge in two F8 advanced intercross chicken lines. Cytogenetic and Genome Research, 117, 131–138.
