Abstract
Results of a cladistic analysis of the species of the African Xylocopa caffra (Linnaeus, 1767) species-group (Hymenoptera: Apidae, genus Xylocopa Latreille, 1802) are presented. Thirty-five external adult morphological characters were coded for the twelve species in this group plus two outgroup taxa. Cladistic analysis with the computer program “TNT” recovered a single most parsimonious tree of 45 steps. This cladogram provides insights into the evolution of aposematic dorsal color patterns in females of species in this group. Females in each of three monophyletic lineages have distinctive black and yellow, white, or orange color patterns: In the first lineage, females have white pubescence on the head and varying amounts of yellow pubescence on the mesosoma and metasoma; in the second lineage, females of all but one species have banded black and yellow or white pubescence; and in the third lineage, the mesosoma in females is predominantly or entirely covered in yellow or orange pubescence, except for a central black glabrous area.
Introduction
Large carpenter bees, members of the genus Xylocopa Latreille, 1802, represent a major global lineage within the family Apidae (Insecta: Hymenoptera), with over 700 species-level taxa described worldwide (Michener Citation2007). Species of Xylocopa are important pollinators in agricultural systems (Keasar Citation2010) and also in terrestrial ecosystems around the world (Janzen Citation1966; Mawdsley et al. Citation2016; Mawdsley Citation2017a). Adults of many species of these bees excavate nests in dead wood and may occasionally become minor pests when the nests are excavated in human structures (Mawdsley Citation2017b).
Studies of the biology, life history, and pollination ecology of species in the genus Xylocopa have been impeded by significant outstanding taxonomic problems, particularly within certain highly diverse tropical and subtropical lineages (as noted and discussed by Hurd and Moure Citation1963 and Hurd 1978). Taxonomists in the nineteenth and early twentieth centuries relied primarily on characters of coloration, particularly the vestiture and wing iridescence, when diagnosing and describing new species of Xylocopa (Hurd and Moure Citation1963). Although many species of Xylocopa can be accurately identified using these characters, certain polymorphic species vary greatly in coloration (Hurd Citation1978; Eardley Citation1983, Citation1987), and thus, these characters are not always reliable for species-level diagnoses. To add further confusion, many species of Xylocopa are extremely similar in coloration, which is thought to be due to Müllerian mimicry, evolutionary convergence on common aposematic color patterns (Mawdsley Citation2015); these similarities increase the difficulty of identifying certain species of Xylocopa on the basis of color characteristics alone. Finally, males and females in certain lineages within Xylocopa such as the New World subgenus Neoxylocopa Michener, 1954, and the Old World subgenus Koptortosoma Gribodo, 1894, are extremely dissimilar in body shape, coloration, and vestiture, so much so that the males and females of these bees were often described as separate species by earlier taxonomists (Hurd and Moure Citation1963; Hurd Citation1978; Mawdsley Citation2017b). Resolving these taxonomic problems requires information about the association of the sexes that can only be obtained by rearing bees from nests, by observing reproductive behaviors in the field, or by application of newer methods such as DNA barcoding (Hurd and Moure Citation1963; Mawdsley Citation2017c).
Perhaps the most problematic group of species within Xylocopa from a taxonomic perspective is the very large African and Australasian subgenus Koptortosoma (Hurd and Moure Citation1963). During the late nineteenth and early twentieth centuries, entomologists described 192 nominal “species” in this group (listed by Hurd and Moure Citation1963, p. 273–275). In most cases, these descriptions were based on very limited specimen material and were written with minimal or no comparisons to the type specimens of taxa which had already described by other authors (Hurd and Moure Citation1963; Eardley Citation1983). Among the 83 valid African species-level taxa included in the subgenus Koptortosoma by Hurd and Moure (Citation1963), 46 were described on the basis of female specimens alone, 27 were described on the basis of male specimens alone, and a mere ten were described on the basis of material from both sexes (and in several cases, these male and female specimens were incorrectly associated, according to Eardley Citation1987).
Eardley (Citation1983) revised the southern African species of subgenus Koptortosoma and the other southern African lineages of Xylocopa, placing many of the doubtful taxa into synonymy and also providing information about many of the central and east African species of this genus as well. Eardley (Citation1987) subsequently published a comprehensive annotated catalogue of the African species of Xylocopa, which incorporated a number of taxonomic changes in this genus based on the direct examination of primary type specimens.
Eardley (Citation1983) proposed the Xylocopa caffra (Linnaeus, 1767) species-group for a small group of valid southern African species within the subgenus Koptortosoma which share several diagnostic features, including an enlarged mite chamber on the first metasomal tergite in females, distinct bands or patterns of pale pubescence in the female (particularly a broad band across the first metasomal tergite), flattened pale yellow pubescence in the male, and a characteristic arrangement of the reproductive structures in the male. The species included in this group by Eardley (Citation1983) are important pollinators and floral visitors throughout much of sub-Saharan Africa (Skaife Citation1952; Watmough Citation1974; Eardley Citation1983; Eardley Citation1987; Mawdsley et al. Citation2016) and thus are of broader interest to botanists, agriculturalists, pollination biologists, conservationists, and national park managers across the African continent (Mawdsley et al. Citation2016). This study applies the methods of morphological cladistic analysis to identify patterns of evolutionary relationships among the species of the Xylocopa caffra species-group as defined by Eardley (Citation1983), including additional valid species from central, east, and west Africa which also belong to this group as indicated by Eardley (Citation1983, Citation1987).
Materials and methods
This study is based on the examination of pinned, mounted specimens of species in the genus Xylocopa in the collections of the United States National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (USNM). The collection of Xylocopa specimens in USNM is one of the largest and most comprehensive in the world, having been assembled over several decades by the late P. D. Hurd who used this material in his own major revisionary studies of the genus (e.g. Hurd and Moure Citation1963; Hurd Citation1978). The collection is particularly rich in African species of Xylocopa, including numerous primary and secondary type specimens, as well as non-type specimens which have been authoritatively identified by H. Friese, N. LeVeque, T. D. A. Cockerell, T. C. Maa, and P. D. Hurd, among other workers who have published on this genus (Mawdsley Citation2017a,Citationb). It also includes material collected by the author during surveys of species of Xylocopa and other pollinator taxa in the Kruger National Park, Republic of South Africa (Mawdsley et al. Citation2016).
Eardley (Citation1983) included the following six southern African species in the Xylocopa caffra species-group: X. caffra Linnaeus, 1767, X. calens Lepeletier, 1841, X. lateritia Smith, 1854, X. senior Vachal, Citation1899, X. somalica Magretti, 1895, and X. watmoughi Eardley, Citation1983. In addition to these six species, I also include six closely related central and west African species in this analysis, based on statements by Eardley (Citation1983, Citation1987) which indicate that these species are closely related to one or more of the southern African species included by Eardley (Citation1983) in the Xylocopa caffra species-group, as well as my own direct examination of specimens of these species. These additional species are X. apicalis Smith, 1854, X. incerta Pérez, 1901, X. isabellae Hurd, 1959, X. modesta Smith, 1854, X. olivacea Fabricius, 1778, and X. schoana Enderlein, 1903. I also examined specimens of all other species of Xylocopa, subgenus Koptortosoma, that were present in the USNM collection in order to confirm that these species lacked the particular combination of external morphological features which had been used by Eardley (Citation1983) to diagnose the Xylocopa caffra species-group.
Two outgroup taxa were selected based on information about species relationships provided by Eardley (Citation1983, Citation1987): the relatively close outgroup taxon Xylocopa scioensis Gribodo, 1884, and the more distant outgroup taxon Xylocopa cloti Vachal, Citation1898. Xylocopa scioensis was included in the subgenus Koptortosoma by Hurd and Moure (Citation1963) but was excluded from the Xylocopa caffra species-group by Eardley (Citation1983) based on the absence of several important morphological features, most notably the lack of a mite chamber on the first metasomal tergite of the female. Xylocopa cloti belongs to a small, well-defined group of African species which were placed by Hurd and Moure (Citation1963) in a separate subgenus, Afroxylocopa Hurd & Moure, Citation1963. Following Minckley (Citation1998), most subsequent authors have considered Afroxylocopa to be a synonym of Koptortosoma, and preliminary molecular phylogenetic studies (Blaimer et al. Citation2016) suggest that it may be the most basal lineage within this subgenus.
To identify external adult morphological characters for this analysis, taxonomic revisionary works treating some or all of the African species of Xylocopa (e.g. Vachal Citation1898, Citation1899; Hurd and Moure Citation1963; Eardley Citation1983, Citation1987) were consulted for information on discrete morphological characters that might provide phylogenetically informative information on the relationships between species in the Xylocopa caffra species-group. Additional adult morphological characters were identified through direct examination of pinned, mounted specimens in the USNM collection. A total of thirty-five discrete adult morphological characters were identified, and these characters were then scored for all species in the group as well as the two outgroups based on direct examination of specimens. In cases where more than a single character state was present within a species, the standard polymorphism coding “?” was used (Farris Citation1989; Lipscomb Citation1998). Following the standard format used in computerized cladistics analysis (Lipscomb Citation1998), the numbering of the characters in this analysis starts with “0.”
Characters of the female:
Upper surface of mandible lacking a distinct tubercle (0), with distinct tubercle (1).
Median tubercle of frons narrow, linear, and slightly elevated, not acutely pointed (0), frons with an elevated and acutely pointed median tubercle (1).
Vertex of frons not strongly raised above top of eyes, length of frons above top of eye margins twice the ocellar diameter or less (0), vertex of frons distinctly raised above top of eyes, length of frons above top of eye margins between two and three times the ocellar diameter (1).
Vertex of frons as wide as median eye width (0), narrower than median eye width (1).
Gena as wide as median eye width (0), narrower than median eye width (1).
Pubescence of frons partly or completely white (0), black (1).
Pubescence of gena partly or completely white (0), black (1).
Outer margin of procoxae curved (0), strongly emarginate (1).
Pubescence of anterior portion of mesonotum forward of the tegulae black (0), light-colored (white, yellow, or red-orange) (1).
Pubescence of posterior portion of mesonotum black (0), light-colored (white, yellow, or red-orange) (1).
Pubescence interrupted medially on base of mesonotum (0), continuous across base of mesonotum (1).
Dorsal surface of mesoscutellum with black pubescence (0), with light-colored pubescence (white, yellow, or red-orange) (1).
Posterior face of mesoscutellum with black or dark-brown pubescence (0), with yellow or orange patches of pubescence laterally (1).
Pubescence of mesopleurae light-colored (white, yellow, or red-orange) (0), black (1).
Lateral metatibial process unidentate at apex (0), bidentate (1).
Anterior face of first metasomal tergite lacking well-developed mite chamber (0), with well-developed mite chamber (1).
Dorsal surface of first metasomal tergite with black pubescence (0); with light-colored (white, yellow, or red-orange) pubescence (1).
Fifth and sixth metasomal tergites lacking a well-defined linear median carina (0), with a well-defined linear median carina (1).
Sixth metasomal sternite not glabrous medially, sculpturing more or less uniform (0), with a well-defined linear median glabrous area, sculpturing not at all uniform (1).
Wings strongly infuscated on basal field (0), weakly infuscated on basal field (1).
Wings strongly infuscated on apical field (0), weakly infuscated on apical field (1).
Wings weakly iridescent (0), with strong blue and/or violet iridescence (1).Characters of the male:
Vertex of frons raised above top of eyes by at least twice the ocellar diameter (0), only slightly raised above top of eyes by one ocellar diameter or less (1).
Third antennomere elongate, longer than antennomeres four, five, and six taken together (0), only slightly elongated, approximately equal in length to antennomeres four, five, and six together (1).
Integument orange-brown (0), integument black (1).
Vestiture elongate, suberect (0), strongly appressed to body (1).
Vestiture red-orange or reddish-brown (0), pale yellow (1).
Eye color pale yellow in live individuals (0), blue (1).
Anterior surface of first metasomal segment strongly concave (0), nearly flat, not strongly concave (1).
Apex of projection at distal end of metatibia strongly rounded (0), sharply acuminate (1).
Apices of parameres with two distinct lobes (0) with one lobe (1).
Parameres rounded in cross-section, not at all flattened or broadly expanded (0), parameres flattened and broadly expanded (1).
Ventral lobe of parameres broadly rounded at apex (0), sharply angulate (1).
Wings lacking iridescence (0), with coppery-violet iridescence (1).
Metasomal tergites more or less concolorous (0), with lateral yellow markings (1).
Cladistic analysis of the resulting taxon-character matrix () was performed using version 1.5 of the computer program “TNT” which was provided by the Willi Hennig Society (Goloboff and Catalano Citation2016). The “implicit enumeration” function was used to search for most parsimonious trees (Farris Citation1989); this function is guaranteed to find the most parsimonious tree(s), although searches may be time-consuming if the matrix is large or if there is extensive homoplasy in the dataset (Lipscomb Citation1994).
Table 1 Taxon-character matrix for cladistic analysis of species in the Xylocopa caffra species-group.
Results
Cladistic analysis of the matrix in with version 1.5 of the software program TNT recovered a single most parsimonious tree with length 45 steps, consistency index of 77, and retention index of 82. This tree is illustrated in with all of the unambiguous character state transformations mapped to the branches, using the software package “Winclada” (Nixon Citation2002).
Figure 1 Most parsimonious tree resulting from cladistic analysis of the taxon-character matrix for species of the Xylocopa caffra species-group (), with unambiguous character state transformations mapped to the tree and female dorsal color patterns, including color variants, illustrated schematically for each species.
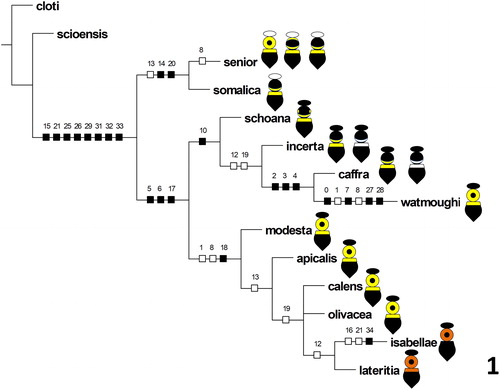
The Xylocopa caffra species-group is monophyletic in this cladogram, with eight unambiguous synapomorphies uniting the group and separating its species from the two outgroup taxa. Beginning at the base of the cladogram, the Xylocopa caffra species-group divides into two monophyletic groups, each of which is supported by at least two unambiguous character state transformations. The first of these groups includes two species with white pubescence on the head of the female, X. senior and X. somalica, both of which are found in east Africa. The second group contains the balance of the species in the species-group. The second group also divides into two monophyletic groups, the first of which includes all of the species with black heads and black and yellow or white banded females plus X. watmoughi, and the second of which includes the remaining species with black heads and yellow or orange pubescence on the mesosoma. Although the tree in is nearly completely resolved, morphological character support for most of the nodes in this cladogram is relatively weak, with relatively few non-homoplasious character state transformations. Only one of the ten resolved nodes in this cladogram is supported by more than three character state transformations, four of the ten nodes in this cladogram are supported by exactly three character state transformations, and the remaining five nodes in this cladogram are supported by one or two character state transformations, and 83% of the transformations supporting these five nodes are homoplasious.
Discussion
The cladogram presented here () resolves all but one node within the Xylocopa caffra species-group, thereby illustrating the value and utility of adult external morphological characters for cladistic analysis and phylogenetic reconstruction within the genus Xylocopa, and in particular within the taxonomically difficult subgenus Koptortosoma. In the tree presented here, the Xylocopa caffra species-group is monophyletic with respect to the outgroup taxa from two other species-groups within subgenus Koptortosoma, suggesting that the Xylocopa caffra species-group may represent a distinct and monophyletic lineage. It should be noted, however, that much of the resolution in the cladogram shown in is only weakly supported by morphological character information, with nine of the ten nodes supported by three or fewer character state transformations. Given the strong morphological similarities among the species in this group, further advances in our understanding of the phylogenetic relationships among these species will likely require additional data sources, such as molecular sequence data.
This analysis does provide some initial insights into the evolutionary history of dorsal color patterns in this group. Females of species in the Xylocopa caffra species-group have distinctive black and yellow dorsal coloration, which serves an aposematic function in warning potential predators of the potent sting of these bees (Hermann and Mullen Citation1974; Piek Citation1986; Mawdsley Citation2015, Citation2017b). Three distinct groupings or sets of dorsal color patterns are present in females of the species in this group, as illustrated schematically in . In the first set of color patterns, the head is covered in white pubescence, while the mesosoma has varying amounts of yellow pubescence. In the second set of color patterns, the head is black and most of the mesosomal disc is covered in black or dark brown pubescence, except for a transverse band of yellow pubescence across the base of the mesosoma. In the third pattern, the pubescence of the head is black, while most of the mesosomal disc is covered in yellow or orange pubescence except for a central glabrous area. Photographs of pinned specimens of species with each of these color patterns were provided by Eardley (Citation1983) and Blaimer et al. (Citation2016). The dorsal color patterns of the females of each species are illustrated schematically in , including each of the variant color patterns present in the museum material that was examined for each species.
The cladogram presented here in indicates that these color patterns appear to be correlated at least in part with patterns of phylogenetic relationships within this species-group. Only one set of color pattern characteristics (those related to the white pubescence of the head) clearly diagnoses a monophyletic lineage within the Xylocopa caffra species-group: the small clade containing X. senior and X. somalica. The banded species with black heads all belong to the single clade containing X. caffra, while all but one of the species with black pubescence on the head and yellow or orange pubescence on the mesosoma belong to the clade with X. apicalis. The major exception to this general correlation is X. watmoughi, which groups with X. caffra and relatives. Females of X. watmoughi are similar in dorsal coloration and general appearance to X. apicalis and X. calens, but X. watmoughi actually shares multiple derived morphological character states with X. caffra and its relatives. Further studies are needed to investigate the origin and possible function of the anomalous female dorsal color pattern in X. watmoughi. Possible explanations might include Müllerian mimicry of other species of Hymenoptera (Mawdsley Citation2015) or independent convergence on a similar dorsal color pattern. It should be noted that mimicry of other species of the genus Xylocopa appears unlikely, as X. watmoughi is distributed at present in the Cape region of the Republic of South Africa, while the other similarly patterned species in this genus occur in central and east Africa (Eardley Citation1983, Citation1987).
Acknowledgements
For permission to examine pinned specimens of species of Xylocopa for this study, I thank Seán Brady and Brian Harris of the Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA. For providing the computer software program “TNT” for use in these analyses, I thank the Willi Hennig Society. For discussions about Xylocopa phylogeny and the evolution of color patterns in this group, I thank Bonnie Blaimer and Seán Brady of the Smithsonian Institution and Sam Droege of the United States Geological Survey’s Bee Inventory and Monitoring Laboratory. For permission to collect specimens of species of Xylocopa in the Kruger National Park, Republic of South Africa, I thank Dr. Freek Venter and South African National Parks. For field and research support in South Africa, I thank Hendrik Sithole, Guin Zambatis, Samantha Mabuza, Patricia Khoza, Obert Mathebula, Adolf Manganyi, Onicca Sithole, Moffat Mambane, and Velly Ndlovu of South African National Parks, James Harrison of the University of the Witwatersrand, Sam Droege of the United States Geological Survey, and Ralph and Alice Mawdsley of Cleveland State University.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Blaimer BB, Lloyd MW, Guillory WX, Brady S. 2016. Sequence capture and phylogenetic utility of genomic ultraconserved elements obtained from pinned insect specimens. PloS One. 11:e0161531.
- Eardley CD. 1983. A taxonomic revision of the genus Xylocopa Latreille (Hymenoptera: Anthophoridae) in southern Africa. Entomology Memoir, Department of Agriculture, Republic of South Africa. 58(i–iii):1–67.
- Eardley CD. 1987. Catalogue of Apoidea (Hymenoptera) in Africa south of the Sahara, Part I, The genus Xylocopa Latreille (Anthophoridae). Entomology Memoir, Department of Agriculture and Water Supply, Republic of South Africa. 70(i-iii):1–20.
- Farris JS. 1989. Hennig86: a PC-DOS program for phylogenetic analysis. Cladistics. 5:163.
- Goloboff PA, Catalano SA. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 32(3):221–238.
- Hermann HR, Mullen MA. 1974. The hymenopterous poison apparatus. XI. Xylocopa virginica (Hymenoptera: Xylocopidae). Journal of the Georgia Entomological Society. 9:246–252.
- Hurd PD. 1978. An annotated catalog of the carpenter bees (genus Xylocopa Latreille) of the Western Hemisphere (Hymenoptera: Anthophoridae). Washington, DC: Smithsonian Institution Press. 106 p.
- Hurd PD, Moure JS. 1963. A classification of the large carpenter bees (Xylocopini) (Hymenoptera: Apoidea). University of California Publications in Entomology. 29(i–vi):1–365.
- Janzen D. 1966. Notes on the behavior of the carpenter bee Xylocopa fimbriata in Mexico. Journal of the Kansas Entomological Society. 39:633–641.
- Keasar T. 2010. Large carpenter bees as agricultural pollinators. Psyche. 2010:1–7.
- Lipscomb DL. 1994. Cladistic analysis using Hennig86. [Internet]. [Cited 5 July 2018]. Available from: https://biology.columbian.gwu.edu/sites/g/files/zaxdzs1961/f/downloads/hennig86.pdf
- Lipscomb DL. 1998. Basics of cladistic analysis. [Internet]. [Cited 5 July 2018]. Available from: https://www2.gwu.edu/∼clade/faculty/lipscomb/Cladistics.pdf (accessed July 5, 2018).
- Mawdsley JR. 2015. An annotated checklist of the large carpenter bees, genus Xylocopa Latreille (Hymenoptera: Apidae), from the Philippine Islands. Oriental Insects. 49(1–2):47-67.
- Mawdsley JR. 2017a. Taxonomy of the African large carpenter bees of the genus Xylocopa Latreille, 1802, subgenus Xenoxylocopa Hurd & Moure, 1963 (Hymenoptera, Apidae). Zookeys. 655:131–139.
- Mawdsley JR. 2017b. Large carpenter bees: A guide to the species of Xylocopa (Neoxylocopa) from North and Central America. University Park, MD: Pineway Press. 64 p.
- Mawdsley JR. 2017c. Giant carpenter bees: Taxonomy and identification of the species of the Xylocopa (Mesotrichia) group. University Park, MD: Pineway Press. 68 p.
- Mawdsley JR, Harrison J, Sithole H. 2016. Natural history of a South African insect pollinator assemblage (Insecta: Coleoptera, Diptera, Hymenoptera, Lepidoptera): diagnostic notes, food web analysis and conservation recommendations. Journal of Natural History. 50:2849–2879.
- Michener CD. 2007. The bees of the world, second edition. Baltimore, MD: Johns Hopkins University Press. XVI +953 p.
- Minckley RL. 1998. A cladistic analysis and classification of the subgenera and genera of the large carpenter bees, tribe Xylocopini (Hymenoptera: Apidae). Scientific Papers, The Natural History Museum, University of Kansas. 9:1–47.
- Nixon KC. 2002. WinClada ver. 1.0000. Ithaca, NY: Published by the author.
- Piek T. 1986. Venoms of the Hymenoptera: Biochemical, pharmacological, and behavioral aspects. London: Academic Press. Chapter 8, Venoms of bumble-bees and carpenter-bees; p. 417–424.
- Skaife SH. 1952. The yellow-banded carpenter bee, Mesotrichia caffra Linn, and its symbiotic mite, Dinogasmus braunsi Vitzthun. Journal of the Entomological Society of Southern Africa. 15:63–76.
- Vachal J. 1898. Materiaux pour une revision des especes africaines du genre Xylocopa Latr. Annales de la Société Entomologique de France. 67:92–99.
- Vachal J. 1899. Essai d’une revision synoptique des especes Europeennes et Africanes du Xylocopa Latr. (Hym.). Miscellanea Entomologica. 7:89–112.
- Watmough RH. 1974. Biology and behaviour of carpenter bees in southern Africa. Journal of the Entomological Society of Southern Africa. 37:261–281.
