Abstract
Clubroot, caused by the protist pathogen Plasmodiophora brassicae, causes substantial yield and quality losses in Brassica vegetable crops around the world. Cultivars of several vegetable and oilseed Brassica crops with resistance to clubroot have been developed recently. This study compared clubroot incidence and severity on resistant and standard (susceptible) cultivars of broccoli (Brassica oleracea var. italica), Brussels sprouts (B. oleracea var. gemmifera), Shanghai pak choy (B. rapa var. communis) and napa cabbage (B. rapa spp. pekinensis) grown at field sites naturally infested with pathotype 6 of P. brassicae (P6) in Ontario and pathotypes 3 (P3) and 5 (P5) in Alberta. In Ontario, all of the susceptible cultivars had 100% clubroot incidence and very high severity. The resistant cultivars of Shanghai pak choy and Brussels sprouts had no symptoms of clubroot, and disease severity was low in resistant broccoli. For each crop species, yield of the resistant cultivar(s) was higher than in the standard cultivar(s) under moderate to high disease pressure. In Alberta, the standard cultivars of each Brassica crop were highly susceptible to P3, except for napa cabbage ‘Bilko’. The clubroot reaction to pathotypes 2, 3, 5 and 6 of the cultivars grown in the field trials in Ontario was assessed under controlled conditions. The reaction to P6 was consistent with the field assessments, but several of the cultivars exhibited a differential reaction to pathotypes 2, 3 and 5. No symptoms were observed on Tillage Radish® (Raphanus sativus var. longipinnatus) in an infested (P5) field trial at Bassano, Alberta, but some clubroot symptoms (9%) developed on roots under controlled conditions.
Résumé
La hernie, causée par le protiste pathogène Plasmodiophora brassicae, provoque des pertes substantielles de rendement et de qualité dans les cultures de légumes-feuille et de légumes-fleur partout dans le monde. Des cultivars de plusieurs légumes et oléagineux de la famille des Brassicacées, résistants à la hernie, ont été développés récemment. Cette étude compare l'incidence de la hernie et sa gravité relativement aux cultivars résistants et courants (réceptifs) de brocoli (Brassica oleracea var. italica), de chou de Bruxelles (B. oleracea var. gemmifera), de pak-choï (B. rapa var. communis) et de petsaï (B. rapa spp. pekinensis) cultivés dans des champs naturellement infestés par le pathotype 6 de P. brassicae (P6) en Ontario et les pathotypes 3 (P3) et 5 (P5) en Alberta. En Ontario, tous les cultivars réceptifs affichaient un taux d'incidence de la hernie de 100 % et une extrême gravité. Les cultivars résistants de pak-choï et de chou de Bruxelles n'affichaient aucun symptôme de la hernie et la gravité de la maladie chez les cultivars résistants de brocoli était faible. Pour chaque espèce, le rendement du ou des cultivars résistants était plus élevé que chez les cultivars subissant des pressions de maladie variant de modérées à élevées. En Alberta, les cultivars courants de chaque plante de la famille des Brassicacées étaient fortement réceptifs au pathotype P3, sauf le petsaï ‘Bilko’. La réaction des cultivars aux pathotypes 2, 3, 5 et 6 de la hernie lors des essais en champ en Ontario a été évaluée dans des conditions contrôlées. La réaction à P6 était compatible avec les évaluations faites en champ, mais plusieurs des cultivars ont affiché une réaction différentielle aux pathotypes 2, 3 et 5. Aucun symptôme n'a été observé chez Tillage RadishMD (Raphanus sativus var. longipinnatus) dans un champ infesté (P5) à Bassano, en Alberta, mais quelques symptômes (9 %) de la hernie sont apparus sur les racines dans des conditions contrôlées.
Introduction
Clubroot, caused by the soil-borne protist pathogen Plasmodiophora brassicae (Woronin), has been a major threat to crops belonging to the Brassicae family in many parts of the world for centuries (Howard et al., Citation2010). The pathogen has a complex life cycle comprised of three main stages: survival in soil as resting spores, primary infection of root hairs, and secondary infection and development within the root cortex, which results in the production of clubroot symptoms and new resting spores (Ingram & Tommerup, Citation1972; Kageyama & Asno, Citation2009). Disruption of tissue organization in the roots affects the capacity for water and nutrient uptake and transport (Mithen & Magrath, Citation1992). As a result, growth of the above-ground parts of the hosts become stunted, discoloured and wilted, and crop yield and quality are reduced (Wallenhammar, 1996; Diederichsen et al., Citation2009; Donald & Porter, Citation2009).
Clubroot causes significant economic losses in many Brassica crops worldwide, and occurs in more than 60 countries, with annual losses estimated at 10–15% (Dixon, Citation2009). In Canada, the most significant outbreaks of the disease on Brassica vegetables have occurred in British Columbia, Ontario, Alberta and Quebec (Howard et al., Citation2010). The major Brassica vegetable crops grown in these regions are cabbage (Brassica oleracea L. var. capitata L.), broccoli (B. oleracea L. var. italica Plenck), cauliflower (B. oleracea L. var. botrytis L.), Brussels sprouts (B. oleracea L. var. gemmifera DC.) and some Asian vegetables including napa cabbage (B. rapa L. subsp. pekinensis (Lour) Hanlet), Shanghai pak choy (B. rapa L. subsp. chinensis (Rupr.) var. communis Tsen and Lee) and Chinese flowering cabbage (B. rapa L. subsp. chinensis (Rupr.) var. utilis Tsen and Lee). A relatively small number of growers produce these crops in small, fragmented fields with minimal crop rotation. This has contributed to the build-up of the resting spore populations in soil over time (McDonald & Westerveld, Citation2008; Gossen et al., Citation2013).
Several strategies have been recommended for managing clubroot in infested fields, including the application of fungicides (Suzuki et al., Citation1995), liming to increase soil pH above 7.2 (Tremblay et al., Citation2005), use of biofungicides (Cheal et al., Citation2000; Peng et al., Citation2011), manipulation of seeding date to avoid conditions that are highly conducive for infection and development (Gossen et al., Citation2012a , Citation2012b ), and crop rotation with non-hosts and bait crops to reduce populations of resting spores (Ikegami et al., Citation1981; Murakami et al., Citation2000; Gossen et al., Citation2013). However, successful reduction of clubroot has been limited under field conditions, especially when levels of resting spores in soil are high (Hwang et al., Citation2012a , Citation2012b ). Effective use of crop rotation to reduce clubroot is complicated by the fact that the resting spores can survive in soil for many years (Wallenhammar, 1996), and that many common weed species also act as hosts for the pathogen (Feng et al., Citation2012).
Effective clubroot management in Brassica vegetables will largely depend on incorporation of genetic resistance into clubroot-resistant cultivars, which greatly reduces clubroot development and build-up of the resting spore population in infested fields (Gossen et al., Citation2013). Development of clubroot-resistant cultivars is complicated by the occurrence of many pathotypes of P. brassicae, because most sources of resistance are pathotype-specific (Diederichsen et al., Citation2009). However, clubroot-resistant cultivars of B. napus, B. oleracea and B. rapa have been developed in recent years (Donald et al., Citation2006; Piao et al., Citation2009; Diederichsen et al., Citation2009). The clubroot-resistant white cabbage cultivars ‘Tekila’, ‘Kilaton’ and ‘Kilaxy’, and cauliflower ‘Clapton’, were released in the UK and Europe in 2005. Two oilseed rape cultivars (‘Mendel’ and ‘Tosca’) with resistance to a number of P. brassicae isolates were released in 2003 and both are known to have race-specific clubroot resistance (Diederichsen et al., Citation2009). There are reports of serious clubroot outbreaks in ‘Mendel’, which indicates that the genes for resistance in these crops were not durable (Diederichsen et al., Citation2009). Pathotype composition of P. brassicae can shift rapidly in response to selection pressure, and genetic resistance can quickly break down (Diederichsen et al., Citation2009; LeBoldus et al., Citation2012).
Tillage Radish® (Raphanus sativus var. longipinnatus), also known as ‘daikon’ radish, is an autumn/winter cover crop that is relatively new to Canada. It has potential for grazing, soil improvement, weed control and erosion control, and this type of radish is also used as a vegetable in many Asian cuisines (Williams & Weil, Citation2004; Weil & Kremen, Citation2007; Dean & Weil, Citation2009). Tillage Radish® has a dense canopy and a straight tap root that can grow more than 1 m into the soil to break hardpans or other compacted soil. Since Tillage Radish® belongs to the Brassicae family, it might be a host of P. brassicae, but its reaction to clubroot is not known.
The predominant pathotypes of P. brassicae in Canada are 2, 3, 5 and 6, based on the differential set of Williams and confirmed using the European Clubroot Differential (ECD) set (Strelkov et al., Citation2006, Citation2007; Xue et al., Citation2008; Cao et al., Citation2009). Pathotype 6 (equivalent to ECD 16/0/14) is the predominant pathotype in Ontario and British Columbia (Reyes et al., Citation1974; Strelkov et al., Citation2006). Pathotype 2 (ECD 16/15/31) is present in Quebec (Williams, Citation1966; Strelkov et al., Citation2006). Pathotype 3 (ECD 16/15/12) is predominant in Alberta, but pathotype 5 (ECD 16/15/0) was identified at the sites in Edmonton and Bassano (Strelkov et al., Citation2006, Citation2007; Xue et al., Citation2008; Cao et al., Citation2009). Pathotype 3 is also the dominant pathotype in Nova Scotia (Hildebrand & McRae, Citation1998). However, pathotypes 2, 3, 6 and 8 were identified in Alberta based on typing of single spore, pathotypes 3, 5 and 8 were identified in Ontario, and pathotype 6 in British Columbia (Xue et al., Citation2008).
Recently, the clubroot reaction of five cultivars of cabbage (‘Kilaton’, ‘Tekila’, ‘Kilaxy’, ‘Bronco’, ‘Kilaherb’) and seven cultivars of napa cabbage (‘Yuki’, ‘Bilko’, ‘Deneko’, ‘Mirako’, ‘Yuki’, ‘China Gold’, ‘Emiko’) to pathotype 6 of P. brassicae was assessed at field sites in Ontario (Adhikari et al., Citation2012; Saude et al., Citation2012). However, the reaction of these cultivars to the other important pathotypes of P. brassicae in Canada is not known. For successful management of clubroot, it is important to assess resistance to the pathotypes prevalent in the regions where that resistance will eventually be deployed. Therefore, this study was conducted to evaluate the reaction of selected Brassica vegetable crops and cultivars to the predominant Canadian pathotypes of P. brassicae, and to determine the reaction of Tillage Radish® to pathotypes 3 and 5.
Materials and methods
Field trials
Field trials were conducted on a muck soil (Typic Hemic-Histosoil, ∼74% organic matter, pH 6.4) at the Muck Crops Research Station, University of Guelph, on the Holland/Bradford Marsh, Ontario (ON) and on mineral soil sites at Crop Diversification Centre North at Edmonton, and in commercial fields at Leduc and Bassano, Alberta. The predominant pathotype of P. brassicae in Ontario was pathotype 6, at the sites at Edmonton and Bassano was pathotype 5, and at Leduc was pathotype 3, based on the differential set of Williams (Citation1966) (Strelkov et al., Citation2006; Cao et al., Citation2009). The list of Brassica crops and cultivars that were assessed at each location in each year are listed in . Napa cabbage ‘Granaat’ was included in trials in Bassano and Shanghai pak choy ‘Mei Qing Choi’ was included at the Ontario site to serve as susceptible checks.
Table 1. Cultivar and seed source for the Brassica crops assessed and the predominant pathotype (P) of Plasmodiophora brassicae at each site (Muck Crops Research Station (MCRS), Crop Diversification Centre North (CDCN), Leduc, AB, and Bassano, AB)
Muck Crops Research Station
Field trials to compare the clubroot reaction of standard commercial cultivars of broccoli, Brussels sprouts, napa cabbage and Shanghai pak choy with that of cultivars promoted as resistant to clubroot were conducted at a naturally infested site, the Muck Crops Research Station, in 2011 and 2012. The plot was cultivated at right angle directions each year, prior to seeding, to reduce variation in soil characteristics and inoculum concentration. Seeds of each crop were placed into 128-cell plug trays containing soil-less mix (Sunshine mix # 4, Sun Gro Horticulture Canada Ltd, Spruce Grove, AB), thinned to one seedling per plug, and grown in a greenhouse at 25/20 °C (day/night) temperature with 75% relative humidity and a 16-h photoperiod for one month. The seedlings were then hand-transplanted into the field. The trial was laid out in a randomized complete block design with four replications (20 plants per replication). Plants were hand-transplanted into three 7.5 m rows, 55 cm apart with 30 cm in-row spacing (pak choy, broccoli and napa cabbage), or two 7.5 m rows 86 cm apart with 45 cm in-row spacing (cabbage and Brussels sprouts) on 6 June of each year.
Plants were uprooted and washed when each crop species reached marketable maturity, and then assessed for clubroot incidence and severity, and marketable yield. Clubroot incidence (CI) was assessed as the proportion of plants (%) with visual symptoms of root galling. To determine clubroot severity, each plant was rated based on a 0–3 scale, where: 0 = no clubbing, 1 < 1/3 of root clubbed, 2 = 1/3–2/3 of root clubbed, and 3 > 2/3 of roots clubbed (Kuginuki et al., Citation1999). A disease severity index (DSI) was calculated using the following equation:
Response to P. brassicae was classified based on the mean DSI value for each cultivar, as follows: (i) susceptible = 68–100 DSI, (ii) intermediate = 34–67 DSI, and (iii) resistant = 0–33 DSI.
In 2012, green cabbage ‘Kilaton’ and ‘Kilaxy’ were removed from the study because they had the same response to the pathogen and similar yield as ‘Kilaherb’, which confirmed a previous report (Saude et al., Citation2012), and napa cabbage ‘Bilko’ and ‘Mirako’ were added. Also, two repetitions of the trial were conducted at this location, one at a site with high disease pressure and the second at a site with low disease pressure.
The concentration of resting spores of P. brassicae at each study site was assessed using the method of Dhingra & Sinclair (Citation1985). Soil samples were collected in a W-shaped sampling pattern [five samples per replication (four replications in total)] from the upper 5 cm of soil. An aliquot of the bulked sample was added to 2% sodium hexametaphosphate (NaPO3)6 and shaken vigorously. The suspension was held overnight and filtered through several layers of cheesecloth. The filtrate was run through three cycles of centrifugation at 1000 g to separate fine particles (spores) from coarse particles (soil). A suspension of the fine particles was centrifuged, the resulting pellet was suspended in 40% sucrose, and the suspension was held for two days at 4 °C. The supernatant was poured off and replaced with distilled water, and the suspension was centrifuged at 1000 g for 1 h. The supernatant was discarded and the pellet was washed twice and then resuspended in 5 mL of water. Spore concentration was estimated using a haemocytometer. The concentration of P. brassicae resting spores at the high disease site was 7.0 × 106 g−1 (standard error (SE) = 4.9) in 2011 and 9.0 × 106 g−1 (SE = 3.5) in 2012, as compared to 5.0 × 104 g−1 (SE = 5.0) at the low disease site in 2012.
Edmonton and Leduc
Field trials were conducted on naturally infested mineral soil sites near Edmonton, AB, at the Crop Diversification Centre North (CDCN) of Alberta Agriculture, Food and Rural Development in 2005 and a commercial field (Leduc) in 2007. The clubroot reaction of standard cultivars of selected Brassica vegetables was assessed in a split-plot design with crop species as the main plot treatments, cultivars as subplots, and four replicates per treatment. Each subplot consisted of two 5-m-long rows, with 0.3 m spacing between rows and 2 m between replicates. Seeding was done at the rate of 3.6 kg ha−1 using a six-row seed planter on 20 June. On 27 September, 100 plants per replication were sampled and rated for clubroot symptoms (0–3 scale, as above).
Bassano
A field trial to assess the clubroot reaction of yellow mustard ‘Pennant’, canola ‘9551’, and Chinese cabbage ‘Graanat’ was conducted on a naturally infested commercial field near Bassano in southern Alberta in 2008. The study was laid out in a randomized complete block design with four replications. Each plot consisted of four 5-m-long rows, with 30 cm between rows and 2 m between replications. The seeding rate was 10 kg ha−1 for mustard and canola, respectively and 1 kg ha−1 for Chinese cabbage. The trial was sown on 19 June, and clubroot symptoms were rated on 28 July and 13 August 2008.
A field trial to assess the clubroot reaction of Tillage Radish® was conducted in the same infested field near Bassano, AB in 2012. The study was laid out as four 40-m-long plots, each with four rows, 30 cm between rows, and 60 cm between plots. Tillage Radish® was planted on 1 June 2012 at 10 kg ha−1. On August 27, 10 plants at each of 10 sites per plot were sampled and rated for clubroot symptoms (0–3 scale as above).
Growth room trial
Broccoli ‘Emerald Jewel’ (resistant, R) and ‘Diplomat’ (susceptible standard, S); Brussels sprouts ‘Crispus’(R) and ‘Jade Cross E’ (S); napa cabbage ‘Bilko’ (R) and ‘Mirako’ (S), bok choy ‘Bejo 2834 F1’ (R), and Shanghai pak choy ‘Mei Qing Choi’ (S) were included in each component of the study ().
Primary and secondary infection
Seedlings were grown in sterile sand in 24-cell tissue culture plates (Sigma Aldrich, Toronto, ON) at 25/20 °C (day/night) temperature with 75% relative humidity and a 16-h photoperiod. Two pathotypes of P. brassicae (P5 (ECD 16/15/0) and P6 (ECD 16/0/14)) collected from clubbed roots of canola grown in Alberta, and Ontario, respectively, were used. Spores were extracted by thawing the frozen clubs at room temperature, then homogenizing ∼3 g in 100 mL water at high speed for 2 min and straining the resulting spore suspension through eight layers of cheesecloth. The spore concentration was determined using a haemocytometer and adjusted to 3 × 106 resting spores mL−1. Freshly prepared inoculum was used for each inoculation. Ten-day-old plants were inoculated by pipetting 0.5 mL of resting spore suspension (3 × 106 spores mL−1) around the base of each seedling. Control plants were grown in separate plates and mock-inoculated with deionized water. Each plant was assessed for primary and secondary infection on the top 2 cm of root at six days after inoculation (Feng et al., Citation2012). In each of five fields of view (20× objective) per root, the incidence of root hairs with primary infection (%), and the number of secondary plasmodia in the root cortex were counted using the methods of Sharma et al. (Citation2011) and Feng et al. (Citation2012). The trial was laid out in a randomized complete block design with four replications (plants) per treatment. There were two repetitions of the trial.
Clubroot incidence and severity
Four pathotypes of P. brassicae (2 (ECD 16/15/31), 3 (ECD 16/15/12), 5 (ECD 16/15/0) and 6 (ECD 16/0/14)) collected from clubbed roots of canola grown in Quebec, central Alberta, southern Alberta, and Ontario, respectively, were used in this study. They were distinguished based on pathogenicity on the differential set of Williams and/or using the ECD set (Buczacki et al., Citation1975).
Plants were grown in tall plastic pots (3.8 cm dia. × 21 cm Containers, Stuewe and Sons Inc., Corvallis, OR) filled with soil-less mix (Sunshine mix # 4, Sun Gro. Horticulture Canada Ltd, Spruce Grove, AB). Two seeds were planted per pot, thinned to one 5 days after germination, and maintained at 25/20 °C (day/night), 75% relative humidity and a 16-h photoperiod. Plants were watered daily with demineralized water adjusted to pH 6.3 using 5% acetic acid, and fertilized weekly with a nutrient solution composed of 0.025% each of NPK (20:20:20) (Plant Products Co. Ltd., ON). The layout of the trial and the inoculation procedure were as described for the primary and secondary infection study, except that there were 10 plants per experimental unit and each plant was inoculated with 5 mL of resting spore suspension (3 × 106 spores mL−1). The plants were harvested six weeks after inoculation and the roots were washed and assessed for clubroot incidence and severity as described above. There were two repetitions of the trial.
Tillage Radish®
Seeds of the Tillage Radish® cultivar, four commercial vegetable radish cultivars and two canola cultivars were planted in clubroot-infested (P5) (ECD 16/15/0) soil collected in 2009 from an infested commercial field near Bassano, AB, and in non-infested sifted soil from a site near Brooks, AB. The soil was dispensed into seven 3.8 cm × 21 cm plastic pots (Economy Stubby Supper Cell Conetainers, Stuewe & Sons Inc., Tangent, OR) per treatment. The plant trays were placed in a growth chamber (23 /18 °C (day/night) with 75% relative humidity and a 16-h photoperiod) and fertilized weekly with a nutrient solution composed of 0.025% of NPK (20:20:20) (Plant Products Co. Ltd., ON). The radish seeds were planted 1.3 cm below the soil surface and the canola seeds were sowed 0.5 cm below the soil surface to facilitate proper germination. This trial was repeated using clubroot-infested soil (pathotype 3) collected from a market garden in the Leduc area of central Alberta in 2008. A commercial horticultural growing medium (Sunshine Professional Peat-Lite Mix no. 4 Aggregate, Sun Gro Horticulture Canada Ltd., Vilna, AB) was mixed with clubroot resting spores obtained from dried and ground canola root gall material (1 × 104 spores g−1) obtained in 2011. The trial was laid out in a randomized complete block design with six replications per treatment. The plants were removed from the Conetainers 8 wk after seeding and assessed for clubroot symptoms and severity as described above.
Data analysis
Treatment effects were assessed using analysis of variance (PROC GLM, SAS software version 9.2, SAS Institute Inc., 2010). The dataset for each trial was tested for normality using the Shapiro–Wilk test of residuals. Prior to analysis, percentages were arcsine transformed when necessary to improve normality and homogeneity of variance, but non-transformed means are presented for uniformity of presentation. No outliers were found in any dataset based on Lund's test of standardized residuals (Lund, Citation1975). There was no effect of repetition or repetition × treatment, so the data were pooled across repetitions for analysis. Means were separated using Tukey's test. The relationship between growth room and field trial results for P6 in Ontario was examined using Pearson's correlation coefficient. Significance levels were at P ≤ 0.05 unless otherwise noted.
Results
Field trials
Muck Crops Research Station
There were differences among the Brassica crops and cultivars between years and sites, so the results from each site-year are presented separately (). At the site with high disease pressure, clubroot incidence and DSI were similar in 2011 and 2012, except that incidence of clubroot on broccoli ‘BC 7540’ was lower in 2011 than in 2012 (43 vs. 78%). There was no block effect for any of the site-years. Across both years, clubroot severity was very high (100 DSI) in Shanghai pak choy ‘Mei Qing Choi’, broccoli ‘Diplomat’, Brussels sprouts ‘Jade Cross’, green cabbage ‘Bronco’ and napa cabbage ‘Mirako’; intermediate in broccoli ‘BC 7540’ and bok choy ‘Bejo 2834’, and very low (0 DSI) in Brussels sprouts ‘Crispus’, green cabbage ‘Kilaherb’, ‘Kilaton’ and ‘Kilaxy’, and napa cabbage ‘Bilko’. The resistant napa cabbage ‘Bilko’ did not develop clubroot symptoms in 2011, but it developed minor clubroot symptoms in 2012 (mean 0 vs. 3%, DSI). Marketable yield was substantially higher in those cultivars promoted as clubroot-resistant relative to the standard (susceptible) cultivars in both years ().
Table 2. Clubroot incidence (CI) and disease severity index (DSI), and marketable yield of Brassica crops and cultivars at the Muck Crops Research Station, ON (P6) (ECD 16/0/14) at sites with high and low levels of clubroot in 2011 and 2012
As expected, clubroot incidence and severity were higher at the site with high disease pressure compared with the low disease pressure site (mean 59 vs. 28% CI, 51 vs. 13 DSI) and yield was lower in the high disease pressure site (mean 1.12 vs. 1.83 kg/plant) (). Broccoli ‘Diplomat’ was highly susceptible (50 DSI), Brussels sprouts ‘Jade Cross’ (24 DSI) and green cabbage ‘Bronco’ (26 DSI) were intermediate, and several crops and cultivars were resistant: bok choy ‘Bejo 2834’ (0 DSI) and pak choy ‘Mei Qing Choi’ (4 DSI), broccoli ‘BC 7540’ (8 DSI), Brussels sprout ‘Crispus’ (0 DSI), green cabbage ‘Kilaherb’ (0 DSI), and napa cabbage ‘Bilko’ (2 DSI) and ‘Mirako’ (10 DSI). Marketable yield of the susceptible lines of each crop were substantially higher at low disease pressure than at the high disease pressure site. Susceptible cultivars of broccoli, green cabbage and napa cabbage performed as well as resistant cultivars when grown under low disease pressure. The resistant lines of bok choy ‘Bejo 2834’ and green cabbage ‘Kilaherb’ also had higher marketable yield at the low disease site than at high disease site ().
Edmonton and Leduc
Poor weather conditions and flooding in 2005 contributed to poor seedling establishment and uneven stands at the CDCN site near Edmonton. All of the broccoli and Brussels sprout lines were lost, and no clubroot symptoms developed in any of the cabbage cultivars or the Shanghai pak choy ‘Feng Quing Choi’. Only low levels of clubroot symptoms were found in napa cabbage, but ‘Bilko’ developed numerically fewer symptoms than ‘Mirako’ (0 vs. 8 DSI). Clubroot incidence on the other cultivars in the study was low, and there were no statistical differences among cultivars (data not shown).
At the commercial field site near Leduc in 2007, weather conditions were favourable for both clubroot development and plant growth. Clubroot levels were moderate to high on each of the Shanghai pak choy, Brussels sprout, green cabbage and broccoli cultivars assessed (). Napa cabbage ‘Bilko’ was less susceptible (12% CI, 6 DSI) than the other six cultivars evaluated (range 90–100% CI, 87–99 DSI).
Table 3. Clubroot incidence (CI) and disease severity index (DSI) of selected Brassica vegetables at Leduc, AB (pathotype 3) (ECD 16/15/12) in 2007
Table 4. Primary (%) and secondary infection (number of secondary plasmodia) of Brassica crops and cultivars by pathotypes 5 (P5) (ECD 16/15/0) and 6 (P6) (ECD 16/0/14) of Plasmodiophora brassicae under controlled conditions
Bassano
Clubroot levels were high for all three crop lines in the trial in 2008, and there were no differences among the lines on either rating date. Clubroot severity at the final rating date was 64 DSI for canola ‘9551’, 74 DSI for yellow mustard ‘Pennant’, 86 DSI for napa cabbage ‘Graanat’. In contrast, no clubroot symptoms were observed on the roots of Tillage Radish® in a trial carried out at this site in 2012 (data not shown).
Growth room trial
Primary and secondary infection
Primary and secondary stages of P. brassicae developed in each of the treatments, regardless of pathotype. Overall, inoculation with pathotype 6 (P6) produced higher levels of primary and secondary infection than P5 (). However, there was a cultivar × pathotype interaction (P value = 0.0001), so the reaction of each cultivar was investigated in more detail.
For primary infection, some of the cultivars showed a differential response to pathotypes (). The incidence of primary infection by P5 and P6 was generally lowest in napa cabbage ‘Bilko’ and highest in Shanghai pak choy ‘Mei Qing Choi’. The two exceptions were napa cabbage ‘Mirako’, where primary infection by P5 was intermediate (73%) but highest for P6 (79%), and broccoli ‘BC 7540’, where P6 was intermediate (67%) and P5 was highest (81%). The incidence of primary infection in bok choy ‘Bejo 2834’, broccoli ‘Diplomat’, and Brussels sprout ‘Crispus’ and ‘Jade Cross’ was intermediate for both pathotypes ().
Counts of secondary plasmodia were used to estimate the incidence of secondary infection. Shanghai pak choy ‘Mei Qing Choi’ and Brussels sprout ‘Jade Cross’ had the highest incidence of secondary infection for both pathotypes, napa cabbage ‘Mirako’ was intermediate, and napa cabbage ‘Bilko’ had the fewest plasmodia (). Bok choy ‘Bejo 2834’ and broccoli ‘Diplomat’ had higher numbers of secondary plasmodia of pathotype 6, but were intermediate for pathotype 5 ().
Clubroot incidence and severity
Clubroot incidence and severity of each cultivar showed a similar pattern of response (). There were no differences in mean clubroot severity among P5 (57 DSI), P3 (54 DSI) and P6 (53 DSI), but severity was lower for P2 (36 ± 2.1, DSI). Based on mean severity, napa cabbage ‘Bilko’(0 DSI), bok choy ‘Bejo 2834’(0 DSI), and Brussels sprout ‘Crispus’(9 DSI) were resistant, broccoli ‘BC 7540’(35 DSI) was intermediate, and broccoli ‘Diplomat’(82 DSI), napa cabbage ‘Mirako’(83 DSI), Brussels sprout ‘Jade Cross’ (93 DSI) and Shanghai pak choy ‘Mei Qing Choi’(100 DSI) were susceptible. However, there was a cultivar × pathotype interaction in analysis of variance (Pvalue = 0.001), so the reaction of each cultivar to the individual pathotypes was investigated in more detail ().
Fig. 1. Clubroot incidence (%) and severity (disease severity index) of the Brassica vegetables inoculated with field collected Plasmodiophora brassicae pathotypes 2 (ECD 16/15/31), 3 (ECD 16/15/12), 5 (ECD 16/15/0) and 6 (ECD 16/0/14) under controlled environment. Bars with the same letter above do not differ based on Tukey's test at P < 0.05. Capped lines represent ± SE.
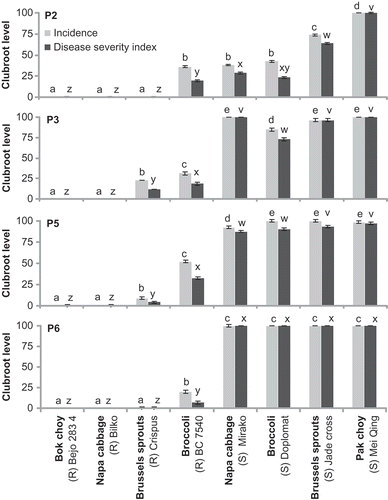
Several of the cultivars produced a consistent disease reaction across pathotypes: Shanghai pak choy ‘Mei Qing Choi’ was consistently highly susceptible (100 DSI), and bok choy ‘Bejo 2834’ (0 DSI), napa cabbage ‘Bilko’ (0 DSI), and Brussels sprout ‘Crispus’ (0–12 DSI) were resistant ( and ). Others showed a differential reaction to pathotype: napa cabbage ‘Mirako’ and Brussels sprout ‘Jade Cross’ were highly susceptible to P3, P5 and P6 (88–100 DSI), but intermediate to P2 (29 and 64 DSI, respectively). Broccoli ‘Diplomat’ was susceptible to P3, P5 and P6 (73–100 DSI), but quite resistant to P2 (23 DSI). Susceptible cultivars developed typical large clubs, but clubroot symptoms did not develop in resistant cultivars ().
Fig. 2. Clubroot reaction of cultivars of broccoli, Pak/Bok choy, Brussels sprouts and napa cabbage inoculated with Plasmodiophora brassicae pathotype 6 (ECD 16/0/14) under controlled environmental conditions.

There was a strong positive correlation between the DSI of cultivars inoculated with P6 under controlled conditions and at the high disease field site at the Muck Crops Field Station (P6) in 2011 (r = 0.88, P < 0.0001) and 2012 (r = 0.91, P < 0.0001).
Tillage Radish®
Clubroot incidence and DSI were highest in susceptible canola ‘45H26’ and lowest in Tillage Radish® in soil from the site at Bassano. Similarly, CI and DSI were higher in canola ‘45H26’ than in the resistant ‘45H29’ or the five radish cultivars in soil from the Leduc area market garden, but differences were not statistically significant (). When grown in soil-less mix, CI and DSI were highest in canola ‘45H26’, lowest in Tillage Radish®, and intermediate in the resistant canola ‘45H29’ and the five radish cultivars (). No clubroot symptoms developed in the non-infested soil.
Table 5. Clubroot incidence (CI) and disease index severity (DSI) of canola and radish cultivars grown in clubroot-infested soil from two sites near Edmonton, AB (CDCN, pathotype 5 (P5) (ECD 16/15/0); Leduc, P3(ECD 16/15/12)) and soil-less mix inoculated with resting spores of P3 under controlled environmental conditions
Discussion
All of the standard cultivars of Shanghai pak choy, broccoli, green cabbage and napa cabbage were susceptible to clubroot at the Muck Crops Research Station in Ontario (where pathotype 6 (P6) predominated), at Bassano, AB (P5) and at Leduc, AB (P3). The cultivars marketed as being clubroot-resistant showed a differential reaction to clubroot at field sites in Ontario and Alberta, and to artificial inoculation under controlled conditions with the four pathotypes of P. brassicae that are predominant in Canada. Under high disease pressure, yield was substantially higher in resistant than in susceptible cultivars of each crop, but under low disease pressure, susceptible cultivars performed as well as resistant cultivars. Although none of the resistant cultivars were completely immune at each field site, napa cabbage ‘Bilko’ had very few symptoms of clubroot and broccoli ‘Surveyor’ and ‘Coronado’ were moderately resistant at Leduc, AB. Finally, no clubroot symptoms were observed on Tillage Radish® in a naturally infested field at Bassano, AB (P5). This is the first report of the reaction of Tillage Radish® to clubroot.
Under high disease pressure at the Muck Crops Research Station (P6), broccoli ‘Diplomat’, Shanghai pak choy ‘Mei Qing Choi’, Brussels sprouts ‘Jade Cross’, green cabbage ‘Bronco’ and napa cabbage ‘Mirako’ were highly susceptible to clubroot, ‘Broccoli ‘BC 7540’ was moderately resistant, and bok choy ‘Bejo 2834’, Brussels sprout ‘Crispus’, green cabbage ‘Kilaherb’, ‘Kilaton’ and ‘Kilaxy’, and napa cabbage ‘Bilko’ were resistant. The green cabbage ‘Kilaton’, ‘Tekila’, ‘Kilaxy’ and ‘Kilaherb’ had previously been reported to be resistant to P6, and ‘Bronco’ was susceptible (Saude et al., Citation2012). Similarly, Shanghai pak choy ‘Mei Qing Choi’ and napa cabbage ‘Bilko’ were resistant to P6 (Adhikari et al., Citation2012). At Leduc, where standard cultivars of several crops were assessed for reaction to P3, most of the cultivars were highly susceptible, broccoli ‘Surveyor’ and ‘Coronado’ were intermediate, and napa cabbage ‘Bilko’ was resistant but not immune.
The lower disease incidence and severity at the low disease site at the Muck Crops Research Station in 2012 are likely due to the lower inoculum pressure compared with the high disease site. The concentration of resting spores in soil is an important factor in clubroot incidence and severity (Hildebrand & McRae, Citation1998). The concentration of P. brassicae resting spores at the high disease site was 7.0 × 106 g−1 in 2011 and 9.0 × 106 g−1 in 2012, vs. 5.0 × 104 g−1 at the low disease site in 2012, based on counts of spores extracted from soil. This is well above the minimum threshold level of 103 spores g−1 required for clubroot symptom development (Donald & Porter, Citation2009; Faggian & Stelkov, Citation2009).
Under high clubroot disease pressure at the Muck Crops Research Station, marketable yield was substantially higher in resistant than in susceptible cultivars of each crop, and consistent with previous reports for that region (Saude et al., Citation2012). Also, yield of each crop at the high disease site was quite consistent in 2011 and 2012, except that yield of Brussels sprouts was higher in 2011 than 2012.
In 2012, yield for each of the cultivars (standard and resistant) at the Muck Crops Research Station was consistently higher at the low disease site than at the high disease site. This may indicate that increasing inoculum pressure can reduce marketable yield in resistant cultivars of these vegetable crops and resistance to the pathogen comes as a physiological cost to the resistant cultivars.
Under controlled growing conditions, high levels of primary and secondary infection were observed on the resistant cultivars of each crop, but few or no visible symptoms developed on roots (). These results indicate that resistance is expressed during secondary colonization, but prior to symptom development. However, the role of root hair infection in subsequent infection success and development of P. brassicae is not well understood. Previous studies indicated that non-host recognition of infection likely occurs during primary infection (Feng et al., Citation2012) and suggest that P. brassicae uses the primary infection phase to overcome the basal resistance of the plant to cortical infection and disease development (Feng et al., Citation2013).
The current study indicates that there is a cost to yield with the expression of clubroot resistance in each of the resistant cultivars assessed. This supports previous reports of a reduction in plant height and development in canola inoculated with P. brassicae in susceptible, intermediate and resistant cultivars (Deora et al., Citation2012) and that increasing inoculum density reduced seedling emergence, plant height, and seed yield of a resistant canola cultivar (Hwang et al., Citation2012a , Citation2012b ).
The clubroot reaction of crops and cultivars to pathotype 6 under controlled environment conditions was strongly and positively correlated with field results at the Muck Crops Research Station. This response is consistent with a previous assessment of response across a range of Brassica hosts (Sharma et al., Citation2013), and indicates that testing under controlled conditions is highly reproducible and representative of results under field conditions. Bok choy ‘Bejo 2834’ and napa cabbage ‘Bilko’ were immune and Shanghai pak choy ‘Mei Qing Choi’ was highly susceptible to each of the pathotypes assessed under controlled environment conditions. Brassica vegetables that showed a differential reaction to pathotype were generally moderately resistant. It is interesting to note that canola genotypes with resistance to pathotypes 3 or 5 were generally also resistant to the other pathotypes present in Canada (Strelkov et al., Citation2006; Deora et al., Citation2012, Citation2013). Also, pathotypes 3, 5 and 6 produced similar levels of symptoms, but severity was lower with pathotype 2 in the current experiment. In previous studies, pathotype 3 produced slightly more severe symptoms than pathotype 6 (Strelkov et al., Citation2006; Gossen et al., Citation2012b ). This likely indicates that differences in aggressiveness among the pathotypes are more strongly associated with host cultivar than intrinsic differences in the pathotype itself. Additional research is required to confirm the differential reaction of these resistant vegetable cultivars under field conditions.
The results of the current study demonstrate that selection of clubroot-resistant cultivars can be an effective approach for managing clubroot in various Brassica vegetable crops. The green cabbage ‘Kilaherb’, ‘Kilaton’ and ‘Tekila’, and napa cabbage ‘Bilko’ were resistant to P6. The source of genetic resistance in these cultivars is proprietary information. Knowing the source of resistance would allow producers to pair the specific resistance gene(s) in a cultivar with the pathotype(s) in their field to not only influence clubroot levels in the current season, but also improve the durability of resistance. Clubroot resistance in B. oleracea is based on genes derived initially from turnip (B. rapa) (Hirai et al., Citation2003; Hirai, Citation2006). Clubroot resistance from this source is generally race-specific and breakdown of resistance has already been reported (Manzanares-Dauleux et al., Citation2001; Dixon, Citation2006; Diederichsen et al., Citation2009; Piao et al., Citation2009). Examples of crops in which resistance has already broken down include green cabbage ‘Kilaton’ and ‘Tekila’ (resistant in the present study). Soon after these cultivars were introduced in Germany, Poland and France in 2005, there were reports of medium to severe clubroot severity at some sites (Diederichsen et al., Citation2009). Similarly, clubroot control failures on Chinese cabbage ‘Yuki’ were reported in Australia (Donald & Porter, Citation2009).
No clubroot symptoms developed on Tillage Radish® in a field at Bassano, AB (P5) in 2012. Similarly, CI and DSI were very low under controlled environmental conditions, irrespective of pathotype. The advantage of Tillage Radish® as a cover crop is that it can break hardpan layers in soil and reduce soil compaction, which facilitates moisture infiltration and root development in subsequent crops. The results of the current study indicate that Tillage Radish® is not highly susceptible to the predominant pathotypes of clubroot in Alberta and so might be considered as a cover crop even in areas where clubroot occurs. However, additional field testing is required to confirm this initial indication.
In summary, the current study examined the reaction of selected Brassica vegetable crops to Canadian pathotypes of P. brassicae under controlled environment conditions and in the field. The standard cultivars were generally very susceptible to clubroot at each field site. Several of the new clubroot-resistant cultivars exhibited a differential reaction to pathotypes: Brussels sprout ‘Crispus’ was immune to P2 and P6 but developed some symptoms when challenged with P3 and P5. However, bok choy ‘Bejo 2834’ and napa cabbage ‘Bilko’ were highly resistant to all of the pathotypes assessed. The reaction of Brassica vegetables to P6 under field and controlled environment conditions was consistent. Marketable yield was substantially higher in those cultivars promoted as clubroot-resistant relative to the standard (susceptible) cultivars in both years at the Muck Crop Research Station. As expected, all of the vegetables had higher yields at the site with low disease pressure, as compared with the high disease pressure site. Very low levels of clubroot developed on Tillage Radish®. Production of resistant cultivars represents an effective clubroot management strategy, but genetic resistance should be used as part of an integrated pest management strategy to ensure the durability of these sources of resistance.
Acknowledgements
We thank L. Riches, Anne-Miet Van Den Nieuwelaar, X. Junzhong (visiting fellow, University of Guelph), and the field crew at the Muck Crops Research Station, as well as S. Lisowski, D. Burke and C. Pugh, Alberta Agriculture and Rural Development, Brooks, AB, for technical assistance, and the Clubroot Risk Mitigation Initiative of Agriculture and Agri-Food Canada, Canola Agronomic Research Program (Alberta Canola Producers Commission) and the Alberta Crop Industry Development Fund for funding of the project.
References
- Adhikari , K.K.C. , Gossen , B.D. and Mcdonald , M.R. 2012 . Reaction to Plasmodiophora brassicae pathotype 6 in lines of Brassica vegetables, Wisconsin Fast Plants, and canola . HortScience , 47 : 374 – 377 .
- Buczacki , S.T. , Toxopeus , H. , Mattusch , P. , Johnston , T.D. , Dixon , G.R. and Hobolth , L.A. 1975 . Study of physiologic specialization in Plasmodiophora brassicae: proposals for attempted rationalization through an international approach . Trans. Br. Mycol. Soc , 65 : 295 – 303 .
- Cao , T. , Manolli , V.P. , Hwang , S.F. , Howard , R.J. and Strelkov , S.E. 2009 . Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada . Can. J. Plant Pathol , 31 : 321 – 329 .
- Cheal , L.H. , Veerakone , S. and Kent , G. 2000 . Biological control of clubroot on cauliflower with Trichoderma and Streptomyces spp . N. Z. Plant Prot. Soc , 53 : 18 – 21 .
- Dean , J.E. and Weil , R.R. 2009 . Brassica cover crops for nitrogen retention in the Mid-Atlantic coastal plain . J. Env. Qual , 38 : 520 – 528 .
- Dhingra , O.D. and Sinclair , J.B. 1985 . “ Detection and estimation of inoculum ” . In Basic Plant Pathology Methods , Edited by: Dhingra , O.D. and Sinclair , J.B. 67 – 117 . Boca Raton , FL : CRC Press .
- Diederichsen , E. , Frauen , M. , Linders , E.G.A. , Hatakeyama , K. and Hirai , M. 2009 . Status and perspectives of clubroot resistance breeding in crucifer crops . J. Plant Growth Regul , 28 : 265 – 281 .
- Dixon , G.R. 2006 . The biology of Plasmodiophora brassicae Wor. – a review of recent advances . Acta Hort , 706 : 271 – 282 .
- Dixon , G.R. 2009 . Plasmodiophora brassicae in its environment . J. Plant Growth Regul , 28 : 212 – 228 .
- Donald , C. and Porter , I. 2009 . Integrated control of clubroot . J. Plant Growth Regul , 28 : 289 – 303 .
- Donald , E.C. , Porter , I.J. , Faggian , R. and Lancaster , R.A. 2006 . An integrated approach to the control of clubroot in vegetable Brassica crops . Acta Hort , 706 : 283 – 300 .
- Deora , A. , Gossen , B.D. and Mcdonald , M.R. 2012 . Infection and development of Plasmodiophora brassicae in resistant and susceptible canola cultivars . Can. J. Plant Pathol , 34 : 239 – 247 .
- Deora , A. , Gossen , B.D. and Mcdonald , M.R. 2013 . Cytology of infection, development, and expression of resistance to Plasmodiophora brassicae in canola . Ann. Appl. Biol , xx : xxx – xxx . In press
- Faggian , R. and Strelkov , S.E. 2009 . Detection and measurement of Plasmodiophora brassicae . J. Plant Growth Regul , 28 : 282 – 288 .
- Feng , J. , Xiao , Q. , Hwang , S.F. , Strelkov , S.E. and Gossen , B.D. 2012 . Infection of canola by secondary zoospores of Plasmodiophora brassicae produced on a nonhost . Eur. J. Plant Pathol , 132 : 309 – 315 .
- Feng , J. , Hwang , S.F. and Strelkov , S.E. 2013 . Studies into primary and secondary infection processes by Plasmodiophora brassicae on canola . Plant Pathol , In press. Doi: 10.1111/j.1365-3059.2012.02612.x
- Gossen , B.D. , Adhikari , K.K.C. and Mcdonald , M.R. 2012a . Effects of temperature on infection and subsequent development of clubroot under controlled conditions . Plant Pathol , 61 : 593 – 599 .
- Gossen , B.D. , Adhikari , K.K.C. and Mcdonald , M.R. 2012b . Effect of seeding date on development of clubroot in vegetable Brassica crops . Can. J. Plant Pathol , 34 : 516 – 523 .
- Gossen , B.D. , Mcdonald , M.R. , Hwang , S.F. , Strelkov , S.E. and Peng , G. 2013 . Comparison of clubroot (Plasmodiophora brassicae) development and management on canola and Brassica vegetables . Can. J. Plant Pathol , 35 : 175 – 191 .
- Hildebrand , P.D. and Mcrae , K.B. 1998 . Control of clubroot caused by Plasmodiophora brassicae with non-ionic surfactants . Can. J. Plant Pathol , 20 : 1 – 11 .
- Hirai , M. 2006 . Genetic analysis of clubroot resistance in Brassica crops . Breed. Sci , 56 : 223 – 229 .
- Hirai , M. , Haruda , T. , Kubo , Tsukada , N. , Suwabe , K. and Matsumoto , S. 2003 . A novel locus for clubroot resistance in Brassica rapa and its linkage markers . Theor. Appl. Genet, , 108 : 639 – 643 .
- Howard , R.J. , Strelkov , S.E. and Harding , M.W. 2010 . Clubroot of cruciferous crops – New perspective on an old disease . Can. J. Plant Pathol, , 32 : 43 – 57 .
- Hwang , S.F. , Ahmed , H.U. , Zhou , Q. , Strelkov , S.E. , Gossen , B.D. , Peng , G. and Turnbull , G.D. 2012a . Assessment of the impact of resistant and susceptible canola on Plasmodiophora brassicae inoculum potential . Plant Pathol , 61 : 945 – 952 .
- Hwang , S.F. , Ahmed , H.U. , Zhou , Q. , Rashid , A. , Strelkov , S. E. , Gossen , B.D. , Peng , G. and Turnbull , G.D. 2012b . Effect of susceptible and resistant canola plants on Plasmodiophora brassicae resting spore populations in the soil . Plant Pathol , DOI: 10.1111/j.1365-3059.2012.02636.x
- Ikegami , H. , Ito , T. , Imuro , Y. and Naiki , T. 1981 . Growth of Plasmodiophora brassicae in the root and callus of Chinese cabbage , Taiwan : Asian Vegetable Research and Development Center .
- Ingram , D.S. and Tommerup , C. 1972 . The life history of Plasmodiophora brassicae Woron . Proc. R. Soc. Lond. Ser. B , 180 : 103 – 112 .
- Kageyama , K. and Asano , T. 2009 . Life cycle of Plasmodiophora brassicae . J. Plant Growth Regul, , 28 : 203 – 211 .
- Kuginuki , Y. , Yoshikawa , H. and Hirai , M. 1999 . Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinensis) . Eur. J. Plant Pathol , 105 : 327 – 332 .
- LeBoldus , J.M. , Manolii , V.P. , Turkington , T.K. and Strelkov , S.E. 2012 . Adaptation to brassica host genotypes by a single-spore isolate and population of Plasmodiophora brassicae (clubroot) . Plant Dis , 96 : 833 – 838 .
- Lund , R.E. 1975 . Tables for an approximate test for outliers in linear models . Techometrics , 17 : 473 – 476 .
- Manzanares-Dauleux , M.J. , Divaret , L. , Baron , F. and Thomas , G. 2001 . Assessment of biological and molecular variability between field isolates of Plasmodiophora brassicae . Plant Pathol , 50 : 165 – 173 .
- Mcdonald , M.R. and Westerveld , S.M. 2008 . Temperature prior to harvest influences the incidence and severity of clubroot on two Asian Brassica vegetables . HortScience , 43 : 1509 – 1513 .
- Mithen , R. and Magrath , R. 1992 . A contribution to the life history of Plasmodiophora brassicae: secondary plasmodia development in root galls of Arabidopsis thaliana . Mycol. Res , 96 : 877 – 885 .
- Murakami , H. , Tsushima , S. , Akimoto , T. , Murakami , K. , Goto , I. and Shishido , Y. 2000 . Effects of growing leafy daikon (Raphanus sativus) on populations of Plasmodiophora brassicae (clubroot) . Plant Pathol , 49 : 584 – 589 .
- Peng , G. , Mcgregor , L. , Lahlali , R. , Gossen , B.G. , Hwang , S.F. , Adhikari , K.K. , Strelkov , S.E. and Mcdonald , M.R. 2011 . Potential biocontrol of clubroot on canola crucifer vegetable crops . Plant Pathol , 60 : 566 – 574 .
- Piao , Z. , Ramchiary , N. and Lim , Y.P. 2009 . Genetics of clubroot resistance in Brassica species . J. Plant Growth Regul , 28 : 252 – 264 .
- Reyes , A.A. , Davidson , T.R. and Marks , C.F. 1974 . Races, pathogenicity and chemical control of Plasmodiophora brassicae in Ontario . Phytopathology , 64 : 173 – 177 .
- Saude , C. , Mckeown , A. , Gossen , B.D. and Mcdonald , MR. 2012 . Effect of host resistance and fungicide application on clubroot pathotype 6 in Green cabbage and napa cabbage . HortTechnology , 22 : 311 – 319 .
- Sharma , K. , Gossen , B.D. and Mcdonald , M. R. 2011 . Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms . Plant Pathol , 60 : 830 – 838 .
- Sharma , K. , Gossen , B.D. , Greenshields , D. , Selvaraj , G. , Strelkov , S.E. and Mcdonald , M.R. 2013 . Reaction of lines of the Rapid Cycling Brassica Collection and Arabidopsis thaliana to selected pathotypes of Plasmodiophora brassicae . Plant Dis , 97 : 720 – 727 .
- Strelkov , S.E. , Tewari , J.P. , Smith , E. and Smith-Degenhardt , E. 2006 . Characterization of Plasmodiophora brassicae populations from Alberta, Canada . Can. J. Plant Pathol , 28 : 467 – 474 .
- Strelkov , S.E. , Manolii , V.P. , Cao , T. , Xue , S. and Hwang , S.F. 2007 . Pathotype classification of Plasmodiophora brassicae and its occurrence in Brassica napus in Alberta, Canada . J. Phytopathol , 155 : 706 – 712 .
- Suzuki , K. , Sugimoto , K. , Hayashi , H. and Komyoji , T. 1995 . Biological mode of action of fluazinam, a new fungicide for Chinese cabbage clubroot . Phytopathol. Soc. Jpn , 61 : 395 – 398 .
- Tremblay , N. , Belec , C. , Coulombe , J. and Godin , C. 2005 . Evaluation of calcium cyanamide and liming for control of clubroot disease in cauliflower . Crop Prot , 24 : 798 – 803 .
- 1996 . Plant Pathol , 45 Wallenhammar, A.C. (). Prevalence of Plasmodiophora brassicae in a spring oilseed rape in central Sweden and factors influencing soil infestation levels. 710–719.
- Weil , R. and Kremen , A. 2007 . Prospectives: thinking across and beyond disciplines to make cover crops pay . J. Sci. Food Agric , 87 : 551 – 557 .
- Williams , P.H. 1966 . A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga . Phytopathology , 56 : 521 – 524 .
- Williams , S. and Weil , R.R. 2004 . Brassica cover crop root channels may alleviate soil compaction effects on subsequent soybean crop . Soil Sci. Soc. Am. J , 68 : 1403 – 1409 .
- Xue , S. , Cao , T. , Howard , R.J. , Hwang , S.F. and Strelkov , S.E. 2008 . Isolation and variation in virulence of single-spore isolates of Plasmodiophora brassicae from Canada . Plant Dis , 92 : 456 – 462 .