Abstract
Phytophthora blight (Phytophthora capsici) affecting greenhouse vegetable production in Canada is reported for the first time. Aspects of the epidemiology and management of P. capsici in relation to greenhouse and field vegetable production in Ontario are discussed. Identification of the pathogen was based on cultural, morphological and molecular approaches, the latter using primer set PC1/2. The pathogen caused 10% plant population losses in a greenhouse pepper operation in Leamington, ON in 2006–2007, and 5%, 5% and 10% losses in commercial greenhouse tomato operations at sites in 2007, 2008 and 2011, respectively, with 1000 greenhouse tomato plants affected in 2012. Phytophthora blight occurred in 2006 in Haldimand-Norfolk and Essex counties on field cucumber and pepper, respectively, and in Essex county in 2007 on field pepper, squash and tomato. The disease was not observed on field pepper, tomato or cucumber plants in fungicide efficacy trials near Harrow, ON in 2005–2006 or near St. Williams, ON in 2007 where the disease had occurred previously. Symptom development was most rapid on greenhouse cucumber and least rapid on greenhouse tomato. Virkon® disinfectant at 0.25%, 0.5%, 1% and 2% commercially formulated product was 100% effective in preventing zoospore germination of P. capsici while Chemprocide™ disinfectant was not fully effective at 0.04%, 0.4% and 4%, and Virucidal Extra® was intermediate in efficacy. The fungicides fluazinam, mandipropamid, cyazofamid and fluopicolide were effective in controlling P. capsici on greenhouse tomato, cucumber, and pepper plants for at least a 14-day period. Metalaxyl was less effective, possibly due to partial resistance in the fungus to this chemical.
Résumé
On rapporte pour la première fois la pourriture des fruits (Phytophthora capsici) touchant des légumes produits en serre au Canada. Nous discutons dans cet article des aspects de l’épidémiologie et de la gestion de P. capsici relativement à la production de légumes de serre et de plein champ en Ontario. L’identification de l’agent pathogène a été basée sur les approches culturale, morphologique et moléculaire, la dernière faisant appel à l’utilisation d’un jeu d’amorces PC1/2. En 2006–2007, à Leamington en Ontario, l’agent pathogène a été responsable de 10% des pertes de plants de poivron cultivés en serre, ainsi que de 5%, de 5% et de 10% des pertes dans des serres commerciales où l’on a cultivé la tomate en 2007, 2008 et 2011, respectivement. De plus, 1000 plants de tomate de serre ont été touchés en 2012. La pourriture des fruits est apparue pour la première fois en 2006 dans les comtés d’Haldimand-Norfolk et d’Essex sur les concombres et les poivrons cultivés en champ, respectivement, et en 2007 dans le comté d’Essex sur les poivrons, les courges et les tomates cultivés en champ. La maladie n’a pas été observée sur les plants de poivron, de tomate ou de concombre au cours d’essais d’efficacité sur les fongicides près de Harrow, en Ontario, en 2005–2006, ou près de St. Williams en 2007, où la maladie s’était manifestée auparavant. Les symptômes se sont développés très rapidement sur les concombres de serre et plus lentement sur les tomates de serre. À des concentrations de 0.25%, 0.5%, 1% et 2%, le VirkonMD, un désinfectant commercialement formulé, a été efficace à 100% pour ce qui est de prévenir la germination des zoospores de P. capsici, tandis que le désinfectant ChemprocidMC n’a pas été entièrement efficace à des concentrations de 0.04%, 0.4% et 4%. Quant à l’efficacité de Virucidal ExtraMD, elle était moyenne. Les fongicides fluaziname, mandipropamide, cyazofamide, et fluopicolide permettaient de gérer efficacement P. capsici sur les tomates, les concombres et les poivrons cultivés en serre pendant au moins 14 jours. Le métalaxyl était moins efficace, probablement à cause d’une résistance partielle à ce produit de synthèse chez le champignon.
Introduction
Phytophthora blight, caused by the soilborne pathogen Phytophthora capsici Leonian, is a devastating disease on field pepper (Capsicum annuum L.), tomato (Solanum lycopersicum L.) (Cerkauskas Citation2004a, Citation2004b), cucumber (Cucumis sativus L.) and many other crops (Erwin & Ribeiro Citation1996). It has been reported from various states in the USA (Ristaino & Johnston Citation1999; Babadoost Citation2000; Hausbeck & Lamour Citation2004) and it was reported for the first time in Canada from Essex county, ON in 1994–95 where it significantly reduced yield of commercial sweet peppers and butternut squash (Cucurbita moschata (Duch.) Duch. ex Poir.) (Anderson & Garton Citation2000). The disease appeared again in 2004 in two commercial sweet pepper fields and one processing tomato field in Essex county (J. LeBouef, pers. comm.). It was also reported on field cucumbers and sweet peppers in Quebec (Gilbert et al. Citation2001), and from British Columbia on field cucurbits (Sholberg et al. Citation2007). In Leamington, where the majority of Ontario greenhouse vegetable and processing tomato production occurs, symptoms similar to phytophthora blight were observed in 2006–2007 in two commercial greenhouse pepper operations where losses of 10% were noted. In 2007–2008 and 2011 in greenhouse tomato operations, losses of 5%, 5% and 10% plant population, respectively were attributed to the disease, and in 2012 about 1000 greenhouse tomato plants were affected by the disease. In Ontario, this disease has the potential to cause major losses in greenhouse pepper, tomato and cucumber crops which in 2011 had a market value of $189.1, $292.4, and $216.8 million, respectively (OMAFRA Citation2012), making Ontario the principal region of greenhouse vegetable production in Canada.
This research was initiated to: (i) determine the extent of occurrence of P. capsici in processing tomato and pepper fields, and in processing cucumber fields in southern Ontario; (ii) to initiate field trials for chemical and non-chemical control of phytophthora blight at selected sites where the disease had been identified previously; (iii) to identify the pathogen and compare the occurrence of the disease in greenhouse vegetable production; and (iv) to investigate various methods for disease management in the greenhouse in Ontario.
Materials and methods
Diseased plant samples
Diseased plant samples were obtained in 2006 from commercial greenhouse pepper and tomato operations near Leamington, ON, and from chili pepper plants in a commercial field near AAFC-GPCRC where severe symptoms of phytophthora blight were evident. The plant tissue pieces, about 5 mm long, were cut from the inner crown area and then surface-disinfested in 70% ethanol for 3–5 s and subsequently in 10% Clorox (0.5% NaOCl) for 3 min. The pieces were placed onto V8® juice agar, acidified potato dextrose agar (aPDA) or lima bean agar plates (Dhingra & Sinclair Citation1995) and the plates were incubated under fluorescent light at room temperature.
Pathogen characterization – cultural and morphological tests
The methodology of Islam et al. (Citation2005) was followed to study colony characteristics. Seven-mm diameter mycelial plugs were taken aseptically using a cork-borer from the edge of 4- to 5-day-old coenocytic cultures suspected to be P. capsici after examination at 40× to 100× by sub-stage illumination of culture plates using a Leica MS 5 stereoscope. The plugs were transferred to PDA plates that were incubated on a lab bench at 22°C for 7 days under continuous fluorescent illumination and culture characteristics were observed.
The effect of temperature on growth response was determined by placing a 7-mm diameter mycelial plug in the centre of a PDA plate. The inoculated plates were incubated in the dark in a completely randomized design in incubators set at temperatures of 32, 36, 37 and 38°C. Each isolate had six replicates. Colony diameter was measured after 3, 5 and 7 days of incubation.
Morphological features were examined using PDA plates that were incubated on a lab bench at 22°C for 6 days under continuous fluorescent illumination. The length, width and pedicel length of 50 sporangia were determined using a Wild Leitz Dialux 20 compound microscope at 400×, and the length/width ratios of sporangia were calculated. The isolates were identified by comparing cultural and morphological data with taxonomic descriptions of P. capsici (Erwin & Ribeiro Citation1996).
Oospore and chlamydospore formation was studied following the methodology of Islam et al. (Citation2005) using isolates DT-1A-A, −1B-C, −1D-A, −6-B from greenhouse tomato, and isolates Carr-1 – 4 from field pepper. One 7-mm plug of mycelium was transferred from the margins of 4-day-old V8 agar plates that had been incubated in darkness at 24°C into each of two flasks (300 mL) that contained 25 mL of a modified cleared V8-CaCO3 liquid medium (V8 juice, 100 mL; filtered 2% CaCO3, 100 mL; and distilled water, 800 mL) and submerged in the medium. The flasks were incubated in darkness for 24 h at 24°C. Each flask then was shaken by hand (30 short strokes) to fragment hyphae that had grown out from the inoculum plug and the flasks were incubated as noted previously for 6 days. After this time period, 100 mL of sterile distilled water was added to each flask and they were incubated in darkness at 18°C for 10 weeks. Five samples of mycelium were randomly selected from each flask and transferred using forceps onto separate microscope slides, a cover slip was added to each slide, and examined for presence of chlamydospores and oospores. Five oospores were randomly sampled from each slide and oospore diameter and wall thickness were measured at 630× using a Wild Leitz Dialux 20 compound microscope.
Pathogen characterization – pathogenicity tests
‘Trust’ tomato, ‘Corona’ cucumber, and ‘Cubico’ pepper plants were initiated from seeds placed in rock wool plugs in the greenhouse, then transplanted into 7.6 × 7.2 × 6.5 cm rock wool blocks (Fibrgro Horticultural Products, Sarnia, ON) that were subsequently placed onto rock wool slabs, 100 cm long × 24 cm wide, in a glass greenhouse. Plants were fertilized as per nutrient film technique regime and maintained under recommended growing conditions for each greenhouse vegetable (Growing greenhouse… Citation2005). Tomato, cucumber and pepper plants were 28–37, 22–28 and 49–56 days old, respectively, at the time of inoculation.
The isolates described above were used in subsequent pathogenicity trials. They were cultured on V8 juice agar under continuous fluorescent illumination at room temperature for 6–22 days to promote production of sporangia before use. The plates were examined for production of sporangia using a Wild Leitz stereoscope with sub-stage illumination and areas of sporangial production marked on the plate undersurface. The zoospore suspension was prepared by transferring one to two 8-mm-diameter plugs from areas of the culture plate where sporangia were abundant to a test tube containing sterile distilled water at 4°C for 1 h. The plugs were left undisturbed in the test tube to allow zoospore release from the sporangia. After re-warming at 22°C for 1 h, the zoospore concentration was estimated using a hemocytometer slide and 2 mL of 5 × 103 zoospores mL−1 was pipetted around the base of each plant. Two mL of sterile distilled water was pipetted around the base of each control plant and a plant that was untreated also served as a control. Depending upon the trial, there were 3–6 replicates for each isolate and the isolates were randomly arranged with each row in the greenhouse containing all isolates. Disease symptoms were examined after about 10 days. The pathogenicity test was repeated at least once for each greenhouse vegetable.
Pathogen characterization – molecular identification
Four putative P. capsici isolates (DT-6B, DT 1B-C, DT 1D-A, DT 1A-A) from greenhouse tomato and pepper, and two isolates of P. capsici obtained from the American Type Culture Collection (ATCC-15399, ATCC-15427) were used for DNA extraction. A small portion of agar from each cultured P. capsici isolate was removed under aseptic conditions and DNA was extracted using liquid nitrogen and following the standard protocol from Qiagen DNeasy (Qiagen Inc. Canada, Toronto, ON) as follows. The agar was crushed in a 1.5 mL Eppendorf tube using a sterile pestle and dissolved using buffer QG from Qiagen QIAquick Gel Extraction Kit at 3:1 v/w of agar. A sterile pipette tip was also used to disrupt the agar. The material was agitated using a Mini Vortexer (VWR International, Mississauga, ON) until the agar was fully dissolved. Each tube was filled with ice-cold 100% ethanol and centrifuged at 14 000 g for 15 min in an Eppendorf Micro-centrifuge 5417C (Fisher, Ottawa, ON) and the supernatant disposed. Buffer QG was added at 1:1 v/w to the remaining pellet based on the original weight of agar in order to dissolve any residual agar and leave only the fungal mycelium. The material was mixed using a Mini Vortexer and the pellet was re-suspended in Buffer QG at a volume of 1:1 v/w using the original agar weight and 100% ethanol at 1.5× the volume of Buffer QG. The material was centrifuged at 14 000 g for 15 min and the supernatant was discarded. The remaining fluid was pipetted out without disturbing the mycelium pellet. The Eppendorf tube was fully submerged into liquid nitrogen and the sample was crushed while frozen. Immediately afterwards, 400 µL of buffer AP1 and 4 µL of RNase A from the Qiagen DNeasy Kit were added. The remaining steps followed the standard protocol from Qiagen DNeasy (Qiagen Inc. Canada, Toronto, ON).
The DNA concentration of each isolate was determined using IMPLEN P330 Nanophotometer™ (MBI Montreal, QC). This was used to calculate the volume of genomic DNA required to obtain approximately 60 ηg of template DNA for each PCR reaction. One µL of each 10 µM primer, PC-1: 5ʹ GTCTTGTACCCTATCATGGCG 3ʹ and PC-2: 5ʹ CGCCACAGCAGGAAAAGCATT 3ʹ(Zhang et al. Citation2006) were used with Illustra PuReTaq Ready 2 Go PCR bead tubes (GE Healthcare, Baie d’Urfe, QC) and brought to a total volume of 25 µL using ddH2O. PCR tubes were gently agitated using a Mini Vortexer until the solution was clear. Tubes were then spun quickly to ensure no bubbles were present and placed in the Techne Genius Thermocycler PCR machine (Techne Inc., Princeton, NJ) with the following PCR conditions: (1) 94°C for 3 min, (2) 94°C for 35 s, 62.1°C for 30 s, 72°C for 45 s and (3) 72°C for 10 min. Step 2 was repeated 30 times. Gel electrophoresis was then conducted using a 1% agarose (Bio-Rad, Hercules, CA), TBE (Tris-borate-EDTA) gel (Sambrook & Russell Citation2001) stained with 10 µL of Invitrogen SYBR safe (Carlsbad, CA). Two μL of loading dye was added to each 25 μL reaction tube and 20 μL from each tube were loaded onto the gel. In addition to the previously mentioned isolates, a negative control containing template DNA of Fusarium oxysporum f. sp. radicis-lycopersici W.R. Jarvis & Shoemaker was used. The gel was run at 90 V for 145 min and viewed with a Transilluminator using Quantam Capture software (MBI Montreal, QC).
To further confirm the identity of the putative isolates, DNA samples displaying the expected 560 bp band in the agarose gel were sequenced. These samples were prepared by purification using the QIAgen Gel Extraction kit (standard protocol) (Qiagen Inc. Canada, Toronto, ON). DNA concentration of each sample was determined using an IMPLEN P330 Nanophotometer™. Any samples less than 20 ηg µL−1 were amplified once again using PCR as described previously. A 10 µL subsample of each of the putative Phytophthora capsici samples was sent to UHN Gene Profiling Facility at Princess Margaret Hospital, Toronto, ON for DNA sequencing. Sample sequences were clipped from the 5ʹ and the 3ʹ ends, leaving a 515 bp sequence in order to exclude the inaccurate beginning and end of the sequencing reaction. All sample results were compared using ClustalW multiple sequence alignment software (Larkin et al. Citation2007) and analysed using BLASTn (Madden Citation2002).
Prevalence of phytophthora blight in southwestern Ontario
A survey for phytophthora blight was conducted in September 2005, August–October 2006 and August–September 2008 of 12, 19 and 6 commercial pepper fields in Essex and Kent counties, ON representing 143, 237 and 20 ha of production, respectively. In August 2007, two fields in Haldimand-Norfolk county, ON consisting of 12 ha of commercial peppers were also surveyed. In all cases, about 30 plants were examined in each field by walking in a diagonal and selecting plants equidistant from each other. Any low spots in fields were also carefully scrutinized.
Samples of diseased greenhouse tomato and pepper plants with putative P. capsici infection were obtained in 2006 and 2007 from commercial operations representing 4.8 ha and 4 ha of production, respectively, with losses of 5–10% in plant population, and later in 2008 and 2012 from Leamington, ON.
Evaluation of fungicides for control of phytophthora blight under field conditions
Plots were established in 2005 at a commercial field pepper operation, located about 5 km from AAFC-GPCRC, where P. capsici was reported in 2004. The plots were located at the edge of the field where symptoms of phytophthora blight were most severe on pepper plants in 2004. ‘Boynton Bell’ pepper plants were transplanted on 9 June 2005 in rows 1 m apart with 40 cm between plants. Heinz ‘9478’ tomato plants were transplanted as noted above in rows 1 m apart with 50 cm between plants, whereas ‘Fanci Pak’ cucumber plants were transplanted on 14 June at 20 cm apart in rows 50 cm apart. For all plants there were three rows per treatment plot with six plants per row and four replicates per treatment arranged in a randomized complete block design. Dual® (metolachlor) herbicide at 1.14 a.i. kg ha−1 was applied on peppers, and Dual + Sencor® (metribuzin) at 1.25 and 0.375 a.i. kg ha−1, respectively, was applied on tomatoes prior to transplanting for weed control. Hand weeding was conducted thereafter as necessary. Fungicide treatments consisted of the following: dimethomorph (Acrobat® 50 WP, BASF Canada Inc., Mississauga, ON, 1.735 g L−1 at 455 g ha−1), boscalid + pyraclostrobin (Pristine® 38 WG, Mississauga, ON, 2.955 g L−1 at 775 g ha−1), zoxamide + mancozeb (Gavel® 75 DF, Dow AgroSciences Canada Inc., Calgary, AB, 8.78 g L−1 at 2.3 kg ha−1), copper hydroxide (Kocide® 2000 54 DF, Dupont Canada Inc., Mississauga, ON, 6.82 g L−1 at 1.8 kg ha−1) + dimethomorph (Acrobat® 50 WP at the rate as listed previously), copper hydroxide (Kocide® 2000 54 DF) + zoxamide + mancozeb (Gavel® 75 DF at rates as listed previously), potassium salt of phosphorus acid and copper sulphate with citrate as a chelating agent (AG3 phosphonate® (Calirus 150), liquid formulation; 10.45% a.i., supplied by Bromine Compounds Ltd, Beer Sheva, Israel) at 0.09% a.i., and a water control. All treatments except AG3 were applied as a spray using a Solo 425 backpack sprayer (Solo Kleinmotoren GMBH, Sindelfingen 6, Germany) containing size 4 074 757 flat spray nozzle at 276–310 kPa. AG3 phosphonate was poured at the base of the tomato and pepper plants at the time of transplanting on 9 June whereas the other fungicide treatments were sprayed 1 day later. All treatments were applied to cucumber plants at the time of transplanting on 14 June. All treatments except AG3 phosphonate were re-applied at 5 days after transplanting (DAT) onto tomato and pepper plants, and onto cucumber, tomato and pepper plants 45, 50 and 50 DAT, respectively. The incidence and severity of phytophthora blight in each treatment plot for pepper, cucumber and tomato was monitored during the course of the experiment where 0 = no symptoms and 5 = death of the plant. Pepper and tomato fruit from three and two plants, respectively, of the middle row of each treatment plot were counted and weighed 77 DAT and 105 DAT, respectively. Cucumber fruit were harvested from plants in each treatment plot 35 and 41 DAT.
The experiment was repeated at the same site in 2006 with transplanting of pepper and cucumber plants on 19 June, and tomato plants on 16 June. All treatments were applied 4 DAT for pepper and cucumber, and 7 DAT for tomato, and re-applied 40 and 43 DAT using the same method as noted above. The harvest of cucumber, pepper and tomato fruit occurred 46, 73 and 93 DAT, respectively.
In 2007, the plots were situated at the edge of a field at a vegetable farm located near St Williams, ON where symptoms of phytophthora blight were most severe on pepper plants the previous year. The plot was planted to winter wheat following the affected pepper crop of 2006, and sprayed with Roundup® (glyphosate) 2 weeks prior to roto-tilling and installation of the experiment. Pepper, tomato and cucumber plants were transplanted on 24 May and all fungicide treatments, as noted above, were applied immediately afterwards. All fungicide treatments were re-applied 48 DAT, and only on pepper and tomato plants at 88 DAT. Pepper and tomato plants were harvested 103 DAT while the cucumber plants were removed by the co-operating grower because of concerns relating to spread of cucumber downy mildew from some of the affected plants in the plot to a commercial planting nearby. The incidence and severity of phytophthora blight in each treatment plot for pepper, cucumber and tomato was monitored during the course of the experiment.
Evaluation of seed treatments for control of phytophthora blight in field soil contaminated with the pathogen
Soil was obtained from the site where the cucumber, pepper and tomato field trial was conducted in 2005 (field 1), located about 5 km from AAFC-GPCRC, and from two other nearby commercial pepper grower sites (fields 2, 3), located less than one km away, where the disease was also observed during the 2004 growing season. The soil samples were taken from a depth of no more than 22 cm using soil cores from five randomly chosen locations at each of the pepper, cucumber and tomato subplots, and at the two other grower sites. The samples from each field were combined and mixed by shaking the soil in the plastic sampling bag. The soil was uniformly dispensed into cells of 244-cell Plastomer (AMA Plastics Ltd, Kingsville, ON) seedling trays with 50 cells per replicate and four replicates. Seeds of zucchini (Cucurbita pepo L., ‘Dark Green’) obtained from Stokes Seeds (St. David’s, ON) were treated with a slurry of fungicide treatment and allowed to dry overnight before seeding. Fungicide treatments comprised: metalaxyl (Allegiance® FL, Bayer CropScience Inc., Calgary, AB, 0.98 mL kg−1 of seed), mefenoxam (Apron® XL LS, Syngenta Canada Inc., Guelph, ON, 0.42 mL kg−1), potassium salt of phosphorus acid and copper sulphate with citrate as a chelating agent (AG3 phosphonate (see above) at 90 mL L−1 of water), and water (control). The treatments were arranged in a randomized complete block design in a greenhouse. The per cent seed germination and the per cent stand were evaluated after 7 and 12 days, respectively. The experiment was repeated once. The Hartley test for homogeneity of variance where sample variances have the same degrees of freedom was used to determine if results from the two trials could be pooled from each field (1, 2, 3) soil sample (Neter et al. Citation1985).
Evaluation of fungicides for control of phytophthora blight under greenhouse conditions
‘Cubico’ pepper, ‘Corona’ cucumber and ‘Trust’ tomato plants were started in the greenhouse, then transplanted into 7.6 × 7.2 × 6.5 cm rock wool blocks (Fibrgro Horticultural Products, Sarnia, ON) that were subsequently placed onto ‘Fibrgro’ rock wool slabs, 100 cm long × 24 cm wide. Plants were fertilized as per nutrient film technique regime and maintained under recommended growing conditions for each greenhouse vegetable (Growing greenhouse vegetables Citation2005). Generally, pepper plants were at the 5–6 node stage (about 7–8 weeks), cucumber plants at the 3–4 node stage (about 3–4 weeks) and tomato plants at the 4–6 node stage (5–6 weeks) at the time of fungicide application. The treatments were arranged in a randomized complete block design with two or three plants representing a treatment per rock wool slab, and three replicates per treatment in a glass greenhouse. Fungicide treatments (mL L−1) consisted of the following: fluazinam (Allegro® 500F, 0.21) and cyazofamid (Ranman® 400SC, 0.15, 0.25, 0.35) both from ISK Biosciences, London, ON, mandipropamid (Revus® 250SC, 0.3) and metalaxyl (Ridomil® 480 EC, 0.125, 0.14) both from Syngenta Canada Inc., Guelph, ON, and fluopicolide (Presidio® 4SC, 0.3) from Valent Canada Inc., Guelph, ON. Controls consisted of water and no treatment. The fungicide treatments were applied at a rate of 120 mL per plant and were poured around the base of the plant to allow uniform distribution of the material in the rock wool block. One to two days later, the plants were inoculated with 9 mL of zoospore suspension (104 zoospores mL−1) of P. capsici by pouring the suspension near the base of the plant. The zoospore suspension was prepared as described previously by taking one to two 8 mm-diameter plugs from a 10- to 12-day-old culture of the fungus that was grown on V8 agar under continuous fluorescent illumination. The suspension was gently shaken and poured around the base of the plant to ensure uniform application of zoospores onto the plant.
Cucumber and pepper plants were observed about 2 and 2–3 weeks after inoculation, respectively, and tomato plants were observed 1–2 months after inoculation to determine the efficacy of the fungicide treatments. Three disease severity ratings were given to each plant: (1) for overall foliar symptoms, (2) one for overall root health and (3) one for crown decay and discolouration symptoms. The overall plant rating was based upon development of symptoms (Hausbeck & Lamour Citation2004; Cerkauskas Citation2004a, Citation2004b) that were observed in previous greenhouse vegetable experiments using a scale of 0–5 as follows: 0 = no symptoms, 1 = trace symptom occurrence, 2 = some observable symptoms, 3 = moderate symptom appearance, 4 = severe symptom appearance, and 5 = death of plant. Roots were rated from 0 to 5 as follows: 0 = no symptoms, 1 = trace dark root tip discolouration with rock wool block difficult to separate from root mass, and extensive healthy root development, 2 = some roots discoloured with rock wool block somewhat difficult to separate from root mass and with good root development, 3 = large number of dark discoloured roots with rock wool block easily separated from root mass and with poor root development, 4 = most roots with extensive dark discolouration with rock wool block very easily separated from majority of root mass, and 5 = death and rotting of roots with very few remaining roots and rock wool block falls apart from remaining roots. The crown was evaluated by making a vertical cut of the crown region and looking for any discolouration that may be present. Condition of the crown was rated using a 0–5 scale as follows: 0 = no discolouration, 1 = trace brown discolouration of internal crown tissue, 2 = some brown discolouration of internal crown tissue, 3 = moderate amount of crown tissue with dark discolouration, 4 = extensive dark discolouration of crown tissue, 5 = crown tissue with brown to black discolouration and with extensive decay present. After ratings were conducted the crown tissue was placed in labelled plastic bags, stored at 4°C, and subsequently, isolations were conducted from this tissue to confirm that disease symptoms were due to P. capsici. Five tissue sections per plant sample were surface-disinfested as described previously and plated onto aPDA. The culture plates were placed on the lab bench under fluorescent light at room temperature and examined 5–7 days later for cultural and morphological characteristics consistent with those of P. capsici. The greenhouse trials were repeated five, two and four times for greenhouse tomato, cucumber and pepper, respectively.
Evaluation of disinfectants for control of the pathogen in the greenhouse
The cellophane transfer bioassay using zoospores of P. capsici was conducted under aseptic conditions following the procedure of Neely and Himelick (Citation1966). To facilitate the disinfection of the spores, a 12-well, 9 × 11 cm ceramic spot plate was used for every time period of disinfection and for every disinfection treatment being considered. Two wells per plate were used for individual concentrations of the treatments and there was a control well positioned at the base of every set of replicate wells. There were four replicates per spot plate. The time periods for disinfection were 1, 2 and 24 h. A 0.6 cm disc of sterile filter paper (#740-E, Schleicher & Schuell, New Hampshire, USA) was placed at the bottom of every well. The concentrations of disinfectants were then prepared using series dilution with Virucidal Extra® (DiverseyLever Canada, Oakville, ON, potassium hydrogen peroxymonosulphate, 40–70% w/w; sodium dichloroisocyanurate, 3–7% w/w; sulphamic acid, 10–30% w/w; sodium dodecylbenzene sulphonate, 3–7% w/w) at 2.0%, 1.0%, 0.50% and 0.25%, Chemprocide® (Pace Chemicals Ltd, Burnaby BC, didecyldimethyl ammonium chloride, 7.5% w/w; iso-propyl alcohol, 10% w/w; ethanol, 1.5% w/w) at 4.0%, 0.40% and 0.04%, and Virkon® (Vétoquinol Canada Inc., Lavaltrie QC, potassium monopersulphate 21.4% w/w) at 2.0%, 1.0%, 0.5% and 0.25%. The 2.0% Virucidal Extra concentration was heated to 54°C to allow complete dissolving of the crystals. Sterile distilled water was used as the control. Three drops of the treatments were then loaded into the appropriate wells using a sterile Pasteur pipette. There were three plates per disinfectant concentration.
Two, 6-mm diameter sterile cellophane (Research Products International Corp., Mount Prospect, IL) discs were carefully placed onto the moist filter paper that ensured no overlapping occurred. Special attention was taken when placing the cellophane discs as the optical properties cause the cellophane to become difficult to view using the human eye.
A suspension of 2.5 × 105 zoospores mL−1 of P. capsici was prepared, as described previously, from a 7-day-old V8 juice agar culture. It was then examined under a Wild Leitz compound microscope to determine if any pre-treatment zoospore germination was evident. Each cellophane disc in each well was inoculated with 4 µL of 2.5 × 105 zoospores mL−1. Each inoculated ceramic plate was then placed into a separate plastic bag containing three Petri plates filled with moistened cotton to create a moist chamber. There were three moist chambers for every treatment corresponding to 1-h, 2-h and 24-h periods and the chambers were stored at room temperature in a completely random arrangement on the lab bench.
Individual cellophane discs from the 1-h, 2-h and 24-h ceramic spot plates were transferred aseptically to microscope slides. The discs were then treated with lactophenol cotton blue to prevent additional germination and to stain the P. capsici zoospores. The discs were covered with a cover slip and observed at 320× magnification using a Wild Leitz compound microscope. The number of geminated and total zoospores was counted. A range from 25 to greater than 100 zoospores were evaluated for each disc. A zoospore was considered germinated if the germination tube was at least 50% of the total length of the zoospore. Germinated spores appeared thicker, had two flagella, and stained blue while spores that lysed were a lighter blue colour. A disinfectant concentration was considered fungicidal if 99% of the total zoospores remained ungerminated.
The experiment was repeated but the two cellophane discs from the various disinfectant treatments for the 1-h and 2-h periods were removed under aseptic conditions from the ceramic spot plate and transferred to a single culture plate of either V8 juice agar, acidified PDA, PDA or nutrient glucose agar. The culture plates were checked for growth of any P. capsici after 5 days and 15 days. If growth did not occur after 5 days, the disinfectant concentration was considered fungistatic. If growth did not occur after 15 days, the disinfectant concentration was considered fungicidal (Neely & Himelick Citation1966).
Data analysis
Analysis of variance for all field studies was done using the ANOVA procedure of the Statistical Analysis System (SAS Institute Inc. Citation2000) and Duncan’s Multiple Range Test (Steel & Torrie Citation1960). Results were considered significant at P = 0.05.
Results
Pathogen characterization – cultural and morphological tests
Mycelium of field pepper isolates Carr-1 and Carr-4 were petaloid while those obtained from greenhouse tomato samples (DT-1A-A, DT-1B-C, DT-1D-A and DT-6-B) were cottony or petaloid. All isolates grew at 32°C and 36°C; however, at 38°C limited growth occurred with isolates DT-1A-A, −1D-A, −6-B and −1B-C, and no growth with Carr-1, and only growth of 0.2 mm day−1 with Carr-4. Sporangia from greenhouse isolates were easily detached from sporangiophores (i.e. caducous), papillate, ovoid with rounded bases, and with long pedicels. They were 46.3 µm long (± 0.8 µm) with a minimum and maximum of 32.6 µm and 57.7 µm, respectively, and 31.7 µm wide (± 0.4 µm) with a minimum and maximum of 24.5 µm and 39.2 µm, respectively. The length to width ratio was 1.47. The pedicels were 83.3 µm long with a minimum and maximum of 42 µm and 231 µm, respectively. Chlamydospores were not observed while oogonia, antheridia and yellowish-brown oospores were present.
Minimum and maximum oospore diameter ranged from 20.5 to 26.0 µm and 35.4 to 38.9 µm, respectively, with a mean value from 29.3 to 32.0 µm. Minimum and maximum oospore wall thickness ranged from 0.45 to 0.94 µm and 1.3 to 4.95 µm, respectively, with a mean value from 1.1 to 2.2 µm (). The field chili pepper isolates had a greater mean oospore diameter but a thinner oospore wall than the greenhouse tomato isolates.
Table 1. Diameter and wall thickness of oospores from several isolates of Phytophthora capsici.
Pathogen characterization – pathogenicity tests
Healthy, non-inoculated greenhouse ‘Cubico’ pepper had white roots and no visible stem canker () in contrast to P. capsici-infected pepper which had dark brown root discolouration and dark brown canker formation at the base of the stem near the top of the rock wool block () 7 days after inoculation with the fungus. Early symptoms included leaf flagging followed by wilting () in addition to dark cankers visible at the stem base. As the disease progressed, the cankers extended more than 2.5 cm up the stem from the rock wool base. At severe stage of disease development, the central part of the stem was dry and hollow (). The discolouration eventually spread out to almost the entire plant () and the vascular system was affected and started showing dark discolouration. Aside from these symptoms, the leaves started to wilt and curl rapidly and turned brownish-yellow at later stages of disease development (). One month after inoculation, the plants were stunted with dark discoloured fruit () that began to rot.
Early symptoms in affected ‘Corona’ greenhouse cucumber plants included wilting () and a light yellowish-green coloured constriction or shrivelling of the stem, about 2.5 cm in length, at the base of the stem near the top of the rock wool block (). As the disease developed, the stem canker colouration progressed to a yellowish-brown () followed by extensive foliar chlorosis and necrosis in the final stage (). Disease development was more rapid in greenhouse cucumber than in greenhouse pepper, and 8 days after inoculation with P. capsici, there was very poor root development in the former with only a few small roots extending beyond the bottom of the rock wool block and extensive, internal, dark brown discolouration of vascular stem tissue (). The diseased cucumber roots were light brown to tan in colour and most were thin to sparse resulting in easy removal of the rock wool substrate from the remaining root mass. In greenhouse ‘Cubico’ pepper, this state of disease development generally occurred 1 month after introduction of zoospore inoculum at the stem base.
In contrast, greenhouse ‘Trust’ tomato plants did not show any symptoms on stems or foliage 8 days after inoculation. Wilting () appeared several weeks after zoospore inoculation with accompanying light yellowish-green stem canker formation at the base of the stem () followed by increasing dark discolouration of the canker tissue () and brown-black discolouration of internal stem tissue at the stem base and extending upwards several cm (). Diseased root tissue was sparse in the rock wool block but not as severely affected as diseased pepper root tissue at the same period of time.
Pathogen characterization – molecular identification
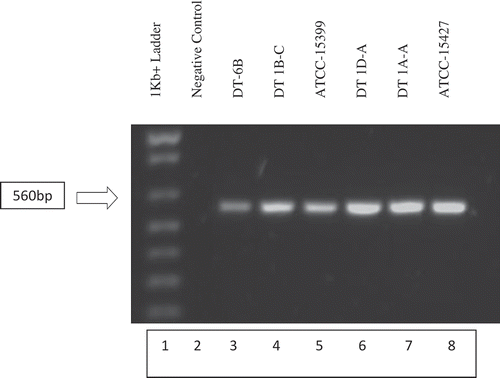
Putative Phytophthora capsici isolates displayed the expected band at 560 bp when amplified using primers PC1/2 and run on an agarose gel (). PCR amplicons of the putative isolates were sequenced to confirm molecular identification as Phytophthora capsici. After removal of the inaccurate beginning and end of the sequence data, the remaining 515 bp region showed >99% similarity between samples, including the known American Type Culture Collection (ATCC-15399, ATCC-15427) isolates. Because all samples contained the same 515 bp sequence, a representative DT-6B sequence was run through NCBI (National Center for Biotechnology Information) BLASTn (Madden Citation2002) to identify the organism in which this sequence is present. NCBI sequence results identified the 515 bp sequence as Phytophthora capsici. The sequence was matched to P. capsici internal transcribed spacer 1, 5.8S ribosomal RNA gene, internal transcribed spacer 2, and 28 s ribosomal RNA gene region as was to be expected with this primer pair (Zhang et al. Citation2006). PCR amplification and the sequencing data confirm the identity of the isolates to be P. capsici.
Fig. 1 (Colour online) Development of P. capsici on greenhouse vegetables at various stages following inoculation. (a) Healthy roots and stem of greenhouse ‘Cubico’ pepper. (b) Early stage dark brown discolouration of roots (arrow) and dark stem canker formation (arrow) on greenhouse ‘Cubico’ pepper 1 week after inoculation (c) Progressive wilting of infected greenhouse ‘Cubico’ pepper plants (arrows). (d) Internal brown–black discolouration within the stem and crown tissue of infected greenhouse ‘Cubico’ pepper. The central part of the stem is also hollow and dried. (e) Severe symptoms on greenhouse ‘Cubico’ pepper 2 months after inoculation. The leaves are necrotic, wilted and yellowish–brown in colour (arrow). The stem and branches are very stiff and hollow, and yellowish-green colour. The plant is dried-up, the fruits are shrivelled and partially rotted. (f) Stem and fruit rot on infected greenhouse ‘Cubico’ pepper plant. The pepper plant was inoculated on the fruit and at the branches. The leaves have wilted and the fruit is decayed and shrivelled. Some white mycelial growth due to P. capsici is visible on the top part of the fruit and on the stem (arrows). (g) Foliar wilt of greenhouse ‘Corona’ cucumber 7 days after inoculation. (h) Decayed stem of infected greenhouse ‘Corona’ cucumber plant. The lower stem is yellowish-green colour and is shrivelled (arrow) compared with the upper healthy part. (i) Later stage of stem canker formation on ‘Corona’ cucumber. Stem canker is yellow-brown (arrow) with oldest leaves now necrotic (not shown). (j) Foliar wilt and necrosis of ‘Corona’ cucumber (arrow) during the late stage of infection. (k) Internal dark brown discolouration of vascular stem tissue (arrow) and dark brown roots during the late stage of infection of ‘Corona’ cucumber. (l) Foliar wilt of greenhouse ‘Trust’ tomato 12 days after inoculation. (m) Initial yellow-brown canker formation (arrow) at the base of infected greenhouse ‘Trust’ tomato and chlorosis of bottom branches (arrow). (n) Dark canker formation (arrow) at stem base during later stage of infection on greenhouse ‘Trust’ tomato. (o) Internal dark brown discolouration of vascular stem tissue (arrow) of infected greenhouse ‘Trust’ tomato.

Fig. 2 Gel electrophoresis of PCR amplified field isolates DT-6B, DT1B-C, DT1D-A, DT1A-A and known P. capsici isolates ATCC-15399 and ATCC-15427. DNA extracts of individual isolates were PCR amplified using PC-1 (5ʹ GTCTTGTACCCTATCATGGCG 3ʹ) and PC-2 (5ʹ CGCCACAGCAGGAAAAGCATT 3ʹ) primers. PCR amplicons were run on a 1% agarose gel containing 10 µL of Invitrogen SYBR safe DNA gel stain. Putative P. capsici field isolates in lanes 3, 4, 6 and 7 as well as the known P. capsici isolates in lanes 5 and 6 displayed the expected 560 bp band (Zhang et al. Citation2006), confirming their identity as P. capsici.
Prevalence of phytophthora blight in southwestern Ontario
Phytophthora blight was not observed in any commercial pepper field that was surveyed in 2005. It was also not observed in the AAFC-Minor Use trial adjacent to the experimental trial located near AAFC-GPCRC where highly susceptible zucchini ‘Select’ and ‘Pulsar’ cantaloupe (Cucumis melo var. cantalupensis Naud.) were grown. This trial plot was located in a portion of the field where phytophthora blight had been reported in 2004 and where water from a nearby irrigation pond had spilled into the plot. Additionally, phytophthora blight was not observed on about 10 ha of commercial field peppers adjacent to the trial plot.
Phytophthora blight occurred in one cucumber field in Haldimand-Norfolk county in September 2006 and in two pepper fields, consisting of 0.4 ha of hot pepper with 80–85% of the plants with stem cankers, crown rot, wilted and/or dead plants, and 20.2 ha of bell peppers with several areas where stem cankers and crown rot were evident in severely affected plants near Harrow, ON. Isolations from these affected plants confirmed the presence of P. capsici.
Phytophthora capsici was observed in three pepper fields and one adjacent field of squash in the Harrow area in September 2007. The first pepper field was 2.4 ha with 100% of plants severely affected with stem cankers, crown rot, wilting or dead, and with numerous fruit symptoms. Similar symptoms were reported in another pepper field located nearby with 80% of plants in 0.4 ha affected, and also on squash and tomato fruit in two other small fields located nearby. No phytophthora symptoms were observed in two fields consisting of 12 ha of pepper in Haldimand-Norfolk county in 2007.
In the greenhouse, the disease was observed in one commercial pepper operation in Leamington, ON in 2006–2007 with 10% losses, and in greenhouse tomatoes at one site each in 2007, 2008 and 2011 with 5%, 5% and 10% losses, respectively, and at another site in 2012 where 1000 greenhouse tomato plants were affected.
Evaluation of fungicides for control of phytophthora blight under field conditions
Phytophthora blight was not observed on the foliage, stem, vine, or fruit of any pepper, cucumber or tomato plants in the experimental plots throughout the growing seasons of 2005–2007. In 2005, there were no significant (P = 0.05) differences among fungicide treatments for marketable number or yield of pepper or tomato fruit. There were significant (P = 0.05) differences among treatments for marketable number and yield of cucumber fruit. The highest marketable yield at 4.28 kg/centre row and number of fruit at 17/centre row was with Pristine and Kocide + Acrobat, respectively. All other treatments showed significantly lower marketable weight while marketable number from Kocide + Acrobat (17.0) was similar to Pristine (16.75), AG3 phosphonate (17.0), Kocide + Gavel (15.75) and Acrobat (15.75) treatments, but not in the water control (14.5) or Gavel (14.25) treatment. There were no significant (P = 0.05) differences among fungicide treatments for marketable number or yield of tomato fruit.
There were no significant (P = 0.05) differences among fungicide treatments for marketable number or yield of pepper, cucumber or tomato fruit in 2006, and for pepper and tomato in 2007.
Evaluation of seed treatments for control of phytophthora blight in field soil contaminated with the pathogen
Symptoms of phytophthora blight were not observed on the foliage or stem of any of the susceptible ‘Dark Green’ zucchini plants grown in soil taken from any of the three commercial fields (1, 2, 3) where P. capsici was reported the previous year. Hartley test results (Neter et al. Citation1985) allowed for combining of trial 1 and 2 data for per cent germination and per cent stand for fields 1 and 2, and for per cent stand for field 3 but not for per cent germination for the latter field (). With field soil 1, there were significant differences in germination among seed treatments but not for stand. In both cases, metalaxyl seed treatment was most effective and water (control) least effective (). Rates of germination were considerably higher than final stand count, indicating the occurrence of post-emergence damping-off. There were no significant differences among treatments in germination or stand in soil from field 2 and in soil from field 3 for germination only. Plant stand was significantly higher when seeds were treated with either mefenoxam or metalaxyl than with the sodium salt of phosphorous acid or the control in soil from field 3 ().
Table 2. Effect of seed treatments on per cent germination (G) and per cent stand (S) of ‘Dark Green’ zucchini in greenhouse tests using soil sampled from three commercial fields near AAFC-GPCRC in 2005 where Phytophthora capsici was previously reported in 2004.
Evaluation of fungicides for control of phytophthora blight under greenhouse conditions
Crown rot severity was consistently greater than overall plant disease symptoms for the majority of treatments regardless of vegetable crop examined (). Water-treated control plants (A) had the highest disease ratings for crown, root or plant in all trials except tomato trials 3 and 5 (data 3 not shown). Phytophthora capsici was recovered from diseased tissue of water-treated control plants and occasionally from metalaxyl-treated plants, but never from any other fungicide-treated plants. Metalaxyl (Ridomil) at 0.125 mL L−1 water was generally less effective than other fungicides in control of P. capsici regardless of the type of vegetable treated (, ).
Fig. 3 (Colour online) Efficacy of fungicides for control of Phytophthora capsici in greenhouse tomato (T-1, 2), cucumber (C-1, 2), and pepper (P-1, 2) trials. Drench treatments consisted of: A-control (water), B-fluazinam (Allegro), C-mandipropamid (Revus), D-cyazofamid (Ranman), E-metalaxyl (Ridomil), F-control (No Treatment applied). Plant, roots and crown tissues of tomato, cucumber and pepper were rated separately using 0 (healthy) to 5 (dead) scale 21 days after inoculation (dai) for T-1, 2, 15 and 17 dai for C-1, 2 respectively, and 20 and 13 dai for P-1, 2 respectively. The vertical lines denote standard error.
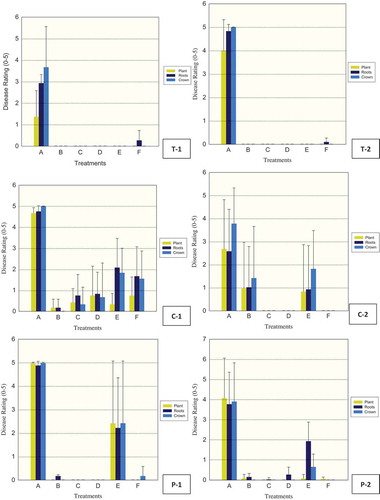
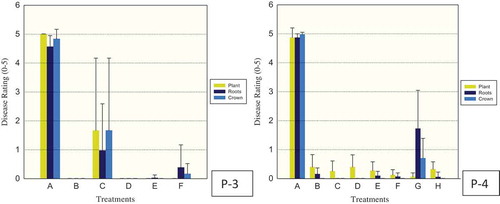
Fig. 4 (Colour online) Efficacy of fungicides for control of Phytophthora capsici in greenhouse pepper (P-3, 4) trials. Drench treatments for P-3 consisted of: A – control (water), B – fluopicolide (Presidio), C, D, E – cyazofamid (Ranman) at 0.15, 0.25 and 0.35 ml L−1, respectively, F – control (No Treatment applied). Drench treatments for P-4 consisted of: A – control (water), B – fluazinam (Allegro), C – mandipropamid (Revus), D, E, F – cyazofamide (Ranman) at 0.15, 0.25 and 0.35 ml L−1, respectively, G – metalaxyl (Ridomil), H – control (No Treatment applied). Plant, roots and crown tissues of pepper were rated separately using 0 (healthy) to 5 (dead) scale 18 and 19 days after inoculation for P-3, 4, respectively. The vertical lines denote standard error.
Fluazinam (B, Allegro), mandipropamid (C, Revus), and cyazofamid (D, Ranman) were effective in providing control against P. capsici in greenhouse tomato in trials 1, 2, 3 and 4 (, trials 1 and 2 corresponding to T-1 and 2 shown) when plants were evaluated 21, 21, 14 and 16 days after inoculation, respectively. In trial 5 (not shown), however, these materials were no longer effective 63 days after inoculation with P. capsici. Metalaxyl (E, Ridomil) was effective in control in trials 1 and 2 (, T-1, 2) but not in trials 3, 4 or 5. In all five trials, the inoculated control (A, fungus inoculation only) showed extensive development of plant, root and crown symptoms.
In greenhouse cucumber, fluazinam (B, Allegro) was effective in trial 1, 15 days after fungus inoculation, but less effective in trial 2, 17 days after inoculation with P. capsici, while cyazofamid (D, Ranman) and mandipropamid (C, Revus) were effective in both trials and metalaxyl (E, Ridomil) was less effective than the preceding materials in either trial (, trials 1, 2 corresponding to C-1, 2 shown).
In greenhouse pepper, fluazinam (B, Allegro), mandipropamid (C, Revus) and cyazofamid (D, Ranman) were effective in trials 1 and 2 (, trials 1, 2 corresponding to P-1, 2 shown), when plants were evaluated 20 and 13 days after inoculation, respectively. In trial 3, 18 days after fungus inoculation, cyazofamid (D, Ranman) at the lowest rate (0.15 ml L−1 water) was not effective in comparison to the control water while the higher rates of 0.25 ml L−1 and 0.35 ml L−1 were effective as well as fluopicolide (Presidio) in control of P. capsici (, P-3). In trial 4, 19 days after inoculation with P. capsici, all rates of cyazofamid (D, Ranman) were effective as well as fluazinam (B, Allegro), mandipropamid (C, Revus) and metalaxyl (E, Ridomil) (, P-4).
Evaluation of disinfectants for control of the pathogen in the greenhouse
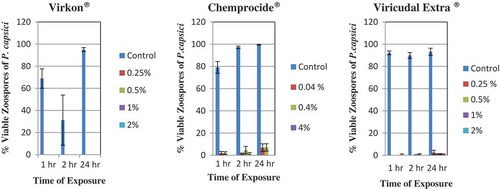
All rates of Virkon and exposure times completely inhibited zoospore germination () in the disinfection trials. Virkon® was the only disinfectant with 0% zoospore germination. Chemprocide® disinfectant did not completely inhibit zoospore germination of P. capsici (). Germinated zoospores were observed at every concentration of Chemprocide® (0.04%, 0.4% and 4.0%), although per cent germination was consistently low (0–7.1%) compared with the per cent germination in the water controls (79.1–99.6%). At 1 and 2 h, the germinated zoospores on the discs treated with Chemprocide® appeared just like the germinated zoospores found on the control slides. At 24 h, however, the germinated zoospores on the discs treated with Chemprocide® had much shorter germination tubes than those of the germinated zoospores on the control slides – only 1–1.5 times the diameter of the zoospore instead of massive branched hyphal growth in the control. There was only one germinated zoospore found at 4% Chemprocide®. Although germinated zoospores were found on discs treated with Virucidal Extra®, mean per cent germination values were very low, varying from 0% to 2.5% (). In contrast, the mean per cent germination values for the control were high (≥ 90%). The few germ tubes that did develop on treated discs were much shorter (0.6–1.3 times the zoospore diameter) than the germ tubes on the control slides (25 times the zoospore diameter).
Fig. 5 (Colour online) Effect of hours of contact with various concentrations of disinfectants, Virkon®, Chemprocide® and Virucidal Extra® on per cent germination of zoospores of Phytophthora capsici. Control is sterile distilled water. The vertical lines denote standard error.
After 5 days, there was no mycelial growth of P. capsici from cellophane discs that were transferred onto culture media, following exposure to Virkon® and Virucidal Extra® for 1 or 2 h, for any concentration evaluated, indicating fungistatic activity. This was also evident at 4% and 0.4% Chemprocide® but not at 0.04% where some mycelial growth was evident after 2 h exposure to the disinfectant. The corresponding controls for all disinfectants had mycelial growth.
Discussion
This study represents the first report of P. capsici affecting greenhouse vegetable production in Canada. The disease was also reported on greenhouse cucumbers in Mexico (Fernández-Pavía et al. Citation2006) and in open hydroponic systems under shade cloth and plastic in South Africa (Labuschagne et al. Citation2003). Identification of Ontario isolates was based on cultural, morphological and molecular aspects (Erwin & Ribeiro Citation1996; Zhang et al. Citation2006). Mean length (46.3 µm) and width (31.7 µm) of sporangia was within the range reported (32.8–65.8 µm by 17.4–38.7 µm) as well as the length to width ratio of 1.47 versus 1.3–2.1 reported elsewhere (Ristaino Citation1990; Erwin & Ribeiro Citation1996; Islam et al. Citation2005; Granke et al. Citation2011). The mean length and width of sporangia of two BC field isolates of P. capsici was greater at 55.2 and 58.3 µm, and 35.0 and 33.3 µm, respectively, than the Ontario isolates while mean pedicel length was considerably less at 47.8 µm for BC isolates (Sholberg et al. Citation2007), and 67.9 µm noted by Granke et al. (Citation2011) compared with 83.3 µm for Ontario isolates. Similarly, mean range of oospore diameter of Ontario isolates, 29.3–32.0 µm, was within the range of 23.7–34.9 µm, 22.2–38.4 µm and 21.7–36.9 µm reported by Erwin and Ribeiro (Citation1996), Islam et al. (Citation2005) and Granke et al. (Citation2011), respectively, although the mean wall thickness from 1.1 to 2.2 µm was low compared with 2–6 µm cited by Erwin and Ribeiro (Citation1996). Mean oospore diameter for the BC isolates was greater at 38.5 µm (Sholberg et al. Citation2007). Colony growth pattern of the six isolates was cottony or petaloid in comparison to Islam et al. (Citation2005) where cottony, petaloid, rosaceous and stellate growth patterns were reported with 57 isolates, and three and eight of the isolates did not grow at 36°C and 38°C, respectively. Additionally, the molecular results confirm the morphological observations since the PCR involving the primer set PC-1 and PC-2 was able to amplify the unique DNA fragment of about 560 bp from the P. capsici isolates and DNA sequences were found to be identical to the NCBI sequences reported for the fungus (Zhang et al. Citation2006).
Symptom development on greenhouse tomato in rock wool substrate following P. capsici zoospore suspension application occurred after several weeks in contrast to a report by Quesada-Ocampo and Hausbeck (Citation2010) who used a potting soilless media and reported plant death as early as 7–8 days following inoculation in some tomato genotypes. They noted that the medium, tomato genotypes, and the isolates used in their study may have influenced the development of symptoms and that responses in the greenhouse may not be consistent with that in the field. Greenhouse pepper and cucumber plants inoculated with P. capsici exhibited typical root, crown and stem symptoms observed in the field (Ristaino & Johnston Citation1999; Hausbeck & Lamour Citation2004; Granke et al. Citation2012). The very hot and dry conditions during most of the growing season in southwestern Ontario in 2005 precluded the occurrence and development of phytophthora blight (P. capsici) on any susceptible hosts such as pepper, cucumber, zucchini or tomato in the field trials near Harrow, ON or in commercial grower fields in south-western Ontario. The average air temperatures at Harrow were: June 22.7°C, July 23.4°C, August 22.9°C and September 19.2°C while the 30-year (1961–1990) average temperatures were: June 19.7°C, July 22.1°C, August 21.1°C and September 17.4°C (http://climate.weather.gc.ca/climate_normals/results_1961_1990). During the June to September period, a total of 215.4 mm rain fell in contrast to the 30-year average of 340 mm for the same period. Consequently, disease development and subsequent field efficacy trials were insufficient for determination of control of P. capsici. Nevertheless, Pristine provided best yield, per cent stand and per cent germination under such conditions in our trial. Phytophthora blight was also not observed in 2006 at the same experiment location although environmental conditions were more conducive to disease development, with average air temperatures of 19.6°C, 23.1°C, 21.5°C and 16.3°C for the months June to September, respectively, and a total rainfall of 376.6 mm from planting to harvest. Similarly, in 2007, no cucumber, pepper or tomato plants were observed with any phytophthora blight in another field trial located at a vegetable farm near St Williams, ON where the disease was reported the previous year. Again, in 2007, hot, dry conditions prevailed during the trial, with average air temperatures of 22.1°C, 22.7°C., 23.3°C and 19.7°C for the months June to September, respectively, and with only 39 mm of rain during a 40-day period from 28 June until 6 August. Schlub (Citation1983) demonstrated that neither sporangia nor mycelia of P. capsici survived a week in leaf tissue at 15, 23 or 31°C if the atmospheric relative humidity was 47% or less or at 31°C if the relative humidity was 75% or less. Similarly, sporangial development was significantly reduced at 87% relative humidity versus higher levels of relative humidity (Schlub Citation1983). In another study, complete oospore mortality was achieved when moistened soil was exposed to 40°C for 199 h or to 45°C for 22 h (Etxeberria et al. Citation2011). These observations suggest that environmental conditions were important in limiting disease development in fields with a previous history of phytophthora blight in Ontario. Additionally, infected greenhouse transplants with low levels of inoculum and poor survival of this inoculum under adverse environmental conditions that occurred may have contributed to the field observations.
Thick-walled oospores of P. capsici can persist in field soil for years under harsh environmental conditions (Bowers et al. Citation1990; Lamour & Hausbeck Citation2003; French-Monar et al. Citation2007; Babadoost & Pavon Citation2013) and provide the main source of initial inoculum for subsequent crops (Ploetz et al. Citation2002). Survival of mycelium in buried pepper tissue is less than 120 days, and that of sporangia and zoospores in soil is less than 75 days (Ansani & Matsuoka Citation1983) regardless of depth of burial in soil (Bowers et al. Citation1990). Islam et al. (Citation2005) noted that chlamydospores of P. capsici from pumpkin isolates in Illinois may also overwinter in fields and serve as a primary inoculum source; however, chlamydospores were not observed with our Ontario isolates. The fungus survived for 65 days as encysted zoospores and was disseminated in recycled surface run-off water from diseased plants in fields in Florida (Roberts et al. Citation2005) to adjacent fields or to nearby water sources used for irrigation (Hausbeck & Lamour Citation2004). Such dissemination from a diseased field near GPCRC in 2004, for example, to a nearby field adjacent to the trial conducted in 2005 was not observed; however, such survival is likely possible in recirculated water in greenhouse vegetable production in Ontario due to optimum temperatures during plant growth (Nielsen et al. Citation2006a, Citation2006b; Gevens et al. Citation2007).
In the field, a major means of dissemination of P. capsici inoculum, such as caducous sporangia or oospores, involves splashing rain and run-off water and not wind dissemination (Schlub Citation1983; Granke et al. Citation2009). In Michigan, crop rotation and use of mefenoxam are not considered adequate to provide economic control of the disease in the field and long-distance dispersal of inoculum via wind is not considered to be a significant concern (Lamour & Hausbeck Citation2003). In greenhouses in Ontario, the primary means of dissemination is likely through P. capsici-infested re-circulated water or dissemination of inoculum between plants within the same substrate medium such as the rock wool slab (Stanghellini et al. Citation1996, Citation2000; Nielsen et al. Citation2006a, Citation2006b). Consequently, in the Ontario greenhouse ebb-and-flow and re-circulating hydroponic production, rapid detection of P. capsici using molecular methods (Zhang et al. Citation2006) is crucial to minimize losses in successful disease management and samples need to be taken about 4–6 h after sunset from surface water for optimal recovery of zoospores (Nielsen et al. Citation2006b). In contrast, in Ontario fields, another means of P. capsici inoculum survival at low levels and dissemination is by latent colonization of roots of some weed species (Alex & Switzer Citation1988), such as American black nightshade (Solanum americanum Mill.) and common purslane (Portulaca oleracea L.) (Ploetz et al. Citation2002), while on black nightshade (Solanum nigrum L.) infection by P. capsici causes plant mortality (French-Monar et al. Citation2006).
Phytophthora blight remains as a major disease problem in neighbouring US states, and the disease is very likely to increase in nearby southwestern Ontario. When inoculum levels are low to moderate in commercial fields, disease control strategies can involve limiting movement of free water by planting on well-drained sites, planting on raised beds, use of trickle irrigation, plastic mulching, and use of trellises for tomato. When warm temperatures and rain occur, providing environmental conditions that are ideal for disease development, chemical or other control strategies are less effective. In the USA and Canada, there are few resistant commercial field pepper, tomato or cucumber cultivars available (Gevens et al. Citation2006; Sy et al. Citation2008; Candole et al. Citation2010; Quesada-Ocampo & Hausbeck Citation2010) and none are available for greenhouse vegetable production in Ontario (Growing greenhouse vegetables Citation2005); however, age-related (i.e. ontogenetic) resistance in fruit of various cucurbits, especially field cucumber (Ando et al. Citation2009; Meyer & Hausbeck Citation2013b; Colle et al. Citation2014; Krasnow et al. Citation2014) and in root and stem tissue of pepper (Kim et al. Citation1989) and tomato (Roberts et al. Citation2000) plants has been reported. The field pepper cultivars ‘Emerald Isle’ and ‘Reinger’ possess resistance to the crown rot phase of phytophthora blight, but do not possess sufficient horticultural characteristics to be commercially acceptable, while ‘Paladin’ has resistance to the crown rot phase of phytophthora blight but does not provide sufficient resistance toward the aerial phase. ‘Aristotle’ field pepper provides tolerance to the crown rot phase but it has insufficient resistance to the aerial phase. Both ‘Paladin’ and ‘Aristotle’, which are available in Ontario, have good horticultural characteristics similar to ‘Camelot’ field pepper, but a “silvering’ pattern may develop on the fruit and ‘Paladin’ may also develop small shoulder cracks when allowed to mature to the red stage. Additional phytophthora tolerant field pepper hybrids include ‘Conquest’ and ‘Revolution’ (Ristaino & Johnston Citation1999; Candole et al. Citation2010). However, Yin et al. (Citation2012) demonstrated that resistance of some field pepper cultivars to P. capsici is not always stable in different locations in Georgia due to presence of different P. capsici populations. Consequently, in Ontario, disease management strategies need to be based on knowledge of the nature of local pathogen populations. Enzenbacher and Hausbeck (Citation2012) observed that the most virulent isolates on cucurbits were collected from solanaceous hosts, suggesting that tomato, pepper and eggplant should not be part of crop rotations with cucurbits in management of phytophthora blight in the field.
Grafting has been utilized with members of the Solanaceae and Cucurbitaceae for control of soilborne pathogens (Louws et al. Citation2010) primarily in Asia (King et al. Citation2010; Lee et al. Citation2010), and increasingly in southern Europe (Gilardi et al. Citation2013). In Ontario, grafting is used primarily in greenhouse tomato production (Growing greenhouse… Citation2005), where about 40% of commercial production involves grafted plants (S. Khosla, pers. comm.), and elsewhere in North America (Kubota et al. Citation2008). In the greenhouse, grafting susceptible scions to P. capsici-resistant eggplant or pepper rootstocks limited phytophthora root rot and increased yield and fruit quality (Gisbert et al. Citation2010; Foster et al. Citation2013; Gilardi et al. Citation2013). Eggplant lines EG195 and EG203 were resistant to most P. capsici isolates that were tested and these lines could be potential rootstock sources for tomatoes, peppers and eggplants (Foster et al. Citation2013), while ‘Charlot’ and ‘Foc’ pepper hybrids showed a high level of tolerance to P. capsici when used as rootstocks for commercial peppers ‘Coyote’ and ‘Almuden’ (Gisbert et al. Citation2010). ‘Tecnico F1’, ‘Terrano’, ‘Graffito’ and ‘Gc 1002’ pepper rootstocks provided complete control of the disease with various pepper scions in greenhouse trials conducted in Italy (Morra & Bilotto Citation2006; Gilardi et al. Citation2013). In Spain, grafted pepper plants in soils that had bio-fumigation consisting of amendment with sheep and chicken manure, and soil solarization, were not affected by P. capsici (Ros et al. Citation2005); however, in Italy, the addition of compost showed little added value for control of P. capsici (Gilardi et al. Citation2013). These approaches are not used in Ontario greenhouses since crop production involves soilless substrates (Growing greenhouse… Citation2005). Additionally, splash dispersal of the pathogen onto above-ground foliage and fruit where genetic resistance may be limited (Ristaino Citation1990; Tian & Babadoost Citation2004; Sy et al. Citation2005; Quesada-Ocampo & Hausbeck Citation2010; Foster & Hausbeck Citation2010a), in comparison to P. capsici-resistant rootstock, may limit use of grafting to where splash dispersal does not occur. However, over-reliance on resistant rootstocks without an integrated disease management approach may lead to selection for more virulent strains of the pathogen. In Ontario greenhouses, limited propagation experience with grafting, intensive labour input requirement, and resulting high costs of grafted seedling production, limit adoption of this strategy (Kubota et al. Citation2008).
In this study, fluazinam (Allegro), mandipropamid (Revus) and cyazofamid (Ranman) were effective in controlling P. capsici infection on greenhouse tomato, cucumber and pepper for at least a 14-day period (), and for at least 18–19 days with fluopicolide (Presidio) in greenhouse pepper (). Mandipropamid and cyazofamid are foliar penetrants with curative activity (Mitani et al. Citation2001; Blum et al. Citation2010) while fluopicolide is a penetrant with some systemic activity (Toquin et al. Citation2008) that is active in suppression of sporangium formation, zoospore germination and mycelial growth of P. capsici (Jackson et al. Citation2010). In our trials, application of these materials was made as a drench to the base of the plant and to the rock wool block to provide better contact with the root system and therefore better disease control than with foliar applications. Matheron and Porchas (Citation2014) reported that fluazinam and cyazofamid were less effective for control of P. capsici than mandipropamid or fluopicolide when applied to either stem and foliage or to roots. Foster and Hausbeck (Citation2010b) and Meyer and Hausbeck (Citation2013a) also obtained better control of P. capsici with drench applications than foliar applications of fluopicolide, mandipropamid, cyazofamid and dimethomorph on both field and greenhouse pepper and summer squash plants, respectively. Matheron and Porchas (Citation2000) obtained significantly better control of P. capsici on chili pepper plants and fruit with mefenoxam than with fluazinam, both at 1200 µg mL−1. However, a single application of fluazinam in addition to mefenoxam significantly prolonged the life of chili pepper plants growing in a field soil infested with the pathogen compared with plants not receiving a fungicide treatment (Matheron & Porchas Citation2000). Further disease control may be possible by an additional spray of fungicides onto the stem and foliage of the plant, thus providing increased protection against infection by P. capsici. In our greenhouse experiments, the stem was not as well protected and the assumption was that the majority of inoculum in a commercial greenhouse setting would most likely enter via the roots of young plants. Additionally, the foliar phase of the disease is easily identified and affected plants may be rouged out to reduce aerial and nutrient flow dispersal of inoculum. Using a new phosphonate formulation (AG3) as a drench or as a seed treatment, Abbasi et al. (Citation2011) demonstrated efficacy in control of phytophthora blight on cucumber and bell pepper plants grown in a peat-based mix or sandy-loam soil under growth room conditions. They also observed that growth of P. capsici culture plugs submerged for 24 h in AG phosphonate was not affected, suggesting a fungistatic rather than a fungicidal effect. In addition, under greenhouse conditions, the treatment of water sources may be necessary using products such as non-ionic synthetic surfactants (Stanghellini et al. Citation1996) or rhamnolipid and saponin biosurfactants (Nielsen et al. Citation2006a) previously shown to be effective in controlling phytophthora blight of greenhouse pepper in recirculating hydroponic systems. The ebb-and-flow system used in the greenhouse was less conducive to spread of P. capsici than a top-irrigated cultural system, because incoming water in the latter system drains through the entire volume of the substrate, and synthetic surfactants provided excellent control in both systems (Stanghellini et al. Citation2000). Disease control was also demonstrated with rhamnolipid and saponic biosurfactants (Kim et al. Citation2000; Nielsen et al. Citation2006a). Such approaches may be useful in greenhouse vegetable production for control of this disease.
In our greenhouse trials, metalaxyl was less effective for control of phytophthora blight on greenhouse cucumber and pepper than the other fungicides that were evaluated. The decreased sensitivity of P. capsici to metalaxyl in our subsequent trials may be due to the development of resistance to this single site mode of action fungicide, since isolations from plants in trials 1 and 2 were conducted to obtain new isolates of the pathogen. Resistance to metalaxyl in P. capsici has also been reported from North Carolina (Parra & Ristaino Citation1998, Citation2001), Michigan (Lamour & Hausbeck Citation2000, Citation2001), Georgia (Jackson et al. Citation2010) and Florida (Ploetz et al. Citation2002). Additionally, insensitivity to cyazofamid, a fungicide that was effective in our greenhouse trials, has been observed in isolates of P. capsici from various locations in southeastern USA (Kousik & Keinath Citation2008; Jackson et al. Citation2012) while baseline sensitivity to fluopicolide varied in isolates from different regions of the USA (Keinath & Kousik Citation2011). The use of any single site mode of action fungicide will require a limited number of applications in the greenhouse, preferably only one during the course of the growing season, followed by rotation with fungicides with different modes of action to reduce the likelihood of development of resistance in populations of the pathogen (Matheron & Porchas Citation2014). Such fungicides may suppress certain stages of P. capsici, such as mycelial growth and sporangial production, but not zoospore germination (Jackson et al. Citation2012). Essential oils from aromatic plants such as red thyme (Thymus vulgaris L.), oregano (Origanum syriacum L.) and palmarosa (Cymbopogon martini (Roxb.)Wats.) that inhibit growth of P. capsici isolates that are resistant to fluopicolide may be an alternative to such fungicides and other single site mode of action fungicides in resistance management strategies in the greenhouse and field (Bi et al. Citation2012). Additionally, the efficacy of acibenzolar-S-methyl (Actigard®), an activator of plant disease resistance, in combination or in alternation with mefenoxam, can be used to reduce development of resistance to the latter fungicide (Matheron & Porchas Citation2002, Citation2014). Suppressing P. capsici progress on pepper roots by supplying silicon in a hydroponic system (Lee et al. Citation2004) or other growth medium (French-Monar et al. Citation2010) was demonstrated and may also be useful in conjunction with a fungicide program in greenhouse vegetable production. Additional control measures, such as use of sanitation procedures, early identification and removal of affected plants, disinfection of tools, equipment, clothing, and worker education will help to reduce inoculum levels and the necessity for further applications of reduced-risk materials in the greenhouse.
Re-circulation of the nutrient solution is used to save water and fertilizers in greenhouse vegetable production in Ontario (Growing greenhouse… Citation2005). This recycled nutrient solution acts as both a primary inoculum source and an effective mechanism for dispersal of inoculum for species of Phytophthora and Pythium (Bush et al. Citation2003). Zoospores of P. capsici are the primary propagules and the only motile propagule found in recirculating nutrient solution (Stanghellini et al. Citation1996) and cannot survive free available chlorine, a disinfectant, at 2 mg L−1 (2 ppm) (Hong et al. Citation2003). Decontamination of recycled water prior to use, and other preventive sanitation methods such as treatment of greenhouse structures, troughs, foot mats, pruning tools, nutrient feed lines and emitters with disinfectants (Russell et al. Citation1999) such as Virkon®, Virucidal Extra® and Chemprocide® are necessary to reduce losses in the greenhouse due to P. capsici and other pathogens in Ontario (Growing greenhouse… Citation2005). Current methods for disinfesting nutrient solutions that may be useful for management of P. capsici in greenhouses include: slow sand filtration, ultraviolet irradiation, heating, ozonation, non-ionic surfactants, chlorination, other methods such as acidic electrolysed water, and use of hydrogen peroxide or iodine (Russell et al. Citation1999; Wohanka Citation2002; Hong & Moorman Citation2005; van Os Citation2009; Stewart-Wade Citation2011). In our trials, Virkon® was 100% effective at 0.25%, 0.5%, 1% and 2% while Chemprocide® at 0.04%, 0.4% and 4% was not fully effective, and Virucidal Extra® was intermediate in efficacy in preventing P. capsici zoospore germination. In contrast, James et al. (Citation2012) found Chemprocide® at 1.35% to be fully effective in inhibiting mycelial growth from plugs of Phytophthora ramorum (S. Werres, A.W.A.M. de Cock & W.A. Man in’t Veld) while Virkon® at 1% was less effective and Virucidal Extra® at 1% had intermediate efficacy. This was also evident with respect to mycelial fragments, chlamydospores and sporangia of P. ramorum.
Numerous detailed descriptions of integrated disease management approaches for P. capsici are described for field vegetables (Ristaino & Johnston Citation1999; Hausbeck & Lamour Citation2004; Granke et al. Citation2012; Sanogo & Ji Citation2012) but no such approaches are available for greenhouse vegetables affected by P. capsici in Ontario. In soilless greenhouse culture, the substrate, transplant, plant treatment and sanitation are key elements that must be integrated in management of P. capsici. The substrate, whether rock wool, coconut coir or sawdust, must be free of the pathogen and may be treated by additional means such as steam pasteurization (Growing greenhouse… Citation2005) that are not available in a field production system. Similarly, sanitation involving disinfection of greenhouse structures and related equipment/tools, and recycled nutrient feed, is an important means of disease management in the greenhouse. The role of the condition of the transplant, the degree of host plant resistance, and plant treatment with fungicides, bio-controls, other materials, and procedures such as use of grafting, do not differ between the field and soilless environment. It may be possible to manage phytophthora blight on greenhouse vegetables in Ontario by application of relevant disease management principles from field vegetable production with integration of various aspects of disease control in the greenhouse.
Acknowledgements
Craig MacNair was funded by the Federal Public Sector Youth Internship Program. The assistance of Janice LeBoeuf (OMAFRA, Ridgetown, ON) in locating pepper fields for survey is gratefully acknowledged. The technical assistance of Michelle Miller, Sarah Rota, Michael Pest, Jeffery Rudd, Adam Hesch and Jacqueline Gilmet is also gratefully acknowledged. M. Miller, J. Gilmet and J. Rudd were funded by the YMCA-Federal Public Sector Youth Internship Program. A. Hesch is a University of Waterloo Co-op student, and M. Pest and S. Rota are students at the University of Western Ontario. AG3 was kindly provided by Pervaiz Abbasi (AAFC-London). Funding provided by the Ontario Greenhouse Vegetable Growers, Ontario Processing Vegetable Growers and the Federal Minor Use Program is very gratefully acknowledged.
References
- Abbasi PA, Lazarovits G, Weselowski B. 2011. Effectiveness of AG3 phosphonate formulation in suppressing phytophthora blight in cucumber and bell pepper plants under growth room conditions. Can J Plant Pathol. 33:150–158.
- Alex JF, Switzer CM. 1988. Ontario weeds: descriptions, illustrations and keys to their identification. Publication 505, Ontario Ministry of Agriculture and Food. Toronto (ON): Queen’s Printer for Ontario.
- Anderson TR, Garton R. 2000. First report of blight of field peppers caused by Phytophthora capsici in Ontario. Plant Dis. 84:705.
- Ando K, Hammar S, Grumet R. 2009. Age-related resistance of diverse cucurbit fruit to infection by Phytophthora capsici. J Amer Soc Hort Sci. 134:176–182.
- Ansani CV, Matsuoka K. 1983. Sobre-vivencia de Phytophthora capsici Leonian no solo. Fitopatol Bras. 8:269–276.
- Babadoost M. 2000. Outbreak of Phytophthora foliar blight and fruit rot in processing pumpkin fields in Illinois. Plant Dis. 84:1345.
- Babadoost M, Pavon C. 2013. Survival of oospores of Phytophthora capsici in soil. Plant Dis. 97:1478–1483.
- Bi Y, Jiang H, Hausbeck MK, Hao JJ. 2012. Inhibitory effects of essential oils for controlling Phytophthora capsici. Plant Dis. 96:797–803.
- Blum M, Boehler M, Randall E, Young V, Csukai M, Kraus S, Moulin F, Scalliet G, Avrova AO, Whisson SC, Fonne-Pfister R. 2010. Mandipropamid targets the cellulose synthase-like PiCesA3 to inhibit cell wall biosynthesis in the oomycete plant pathogen, Phytophthora infestans. Mol Plant Pathol. 11:227–243.
- Bowers JH, Papavizas GC, Johnston SA. 1990. Effect of soil temperature and soil-water matric potential on the survival of Phytophthora capsici in natural soil. Plant Dis. 74:771–777.
- Bush EA, Hong CX, Stromberg EL. 2003. Fluctuations of Phytophthora and Pythium spp. in components of a recycling irrigation system. Plant Dis. 87:1500–1506.
- Candole BL, Conner PJ, Ji P. 2010. Screening Capsicum annuum accessions for resistance to six isolates of Phytophthora capsici. HortSci. 45:254–259.
- Cerkauskas RF. 2004a. Pepper diseases - phytophthora blight factsheet [internet]. AVRDC Publication 04-579. Available from: http://www.avrdc.org/?page_id=417
- Cerkauskas RF. 2004b. Tomato diseases – buckeye rot factsheet [internet]. AVRDC Publication 04- 612. Available from: http://www.avrdc.org/?page_id=417
- Colle M, Straley EN, Makela SB, Hammar SA, Grumet R. 2014. Screening the cucumber plant introduction collection for young fruit resistance to Phytophthora capsici. HortSci. 49:244–249.
- Dhingra OD, Sinclair JB. 1995. Basic plant pathology methods. Boca Raton (FL): CRC Press, Inc.
- Enzenbacher TB, Hausbeck MK. 2012. An evaluation of cucurbits for susceptibility to cucurbitaceous and solanaceous Phytophthora capsici isolates. Plant Dis. 96:1404–1414.
- Erwin DC, Ribeiro OK. 1996. Phytophthora diseases worldwide. St. Paul (MN): APS Press.
- Etxeberria A, Mendarte S, Larregla S. 2011. Thermal inactivation of Phytophthora capsici oospores. Revista Iberoamer de Micologia. 28:83–90.
- Fernández-Pavía SP, Rodríguez-Alvarado G, López-Ordaz A, Fernández-Pavía YL. 2006. First report of Phytophthora capsici causing wilt on hydropronically grown cucumber in Mexico. Plant Dis. 90:1552.
- Foster JM, Hausbeck MK. 2010a. Resistance of pepper to phytophthora crown, root, and fruit rot is affected by isolate virulence. Plant Dis. 94:24–30.
- Foster JM, Hausbeck MK. 2010b. Managing phytophthora crown and root rot in bell pepper using fungicides and host resistance. Plant Dis. 94:697–702.
- Foster JM, Naegele RP, Hausbeck MK. 2013. Evaluation of eggplant rootstocks and pepper varieties for potential resistance to isolates of Phytophthora capsici from Michigan and New York. Plant Dis. 97:1037–1041.
- French-Monar RD, Jones JB, Ozores-Hampton M, Roberts PD. 2007. Survival of inoculum of Phytophthora capsici in soil through time under different soil treatments. Plant Dis. 91:593–598.
- French-Monar RD, Jones JB, Roberts PD. 2006. Characterization of Phytophthora capsici associated with roots of weeds on Florida vegetable farms. Plant Dis. 90:345–350.
- French-Monar RD, Rodrigues FA, Korndörfer GH, Datnoff LE. 2010. Silicon suppresses phytophthora blight development on bell pepper. J Phytopathol. 158:554–560.
- Gevens AJ, Ando K, Lamour K, Grumet R, Hausbeck MK. 2006. Development of a detached cucumber fruit assay to screen for resistance and effect of fruit age on susceptibility to infection by Phytophthora capsici. Plant Dis. 90:1276–1282.
- Gevens AJ, Donahoo RS, Lamour KH, Hausbeck MK. 2007. Characterization of Phytophthora capsici from Michigan surface irrigation water. Phytopathology. 97:421–428.
- Gilardi G, Baudino M, Moizio M, Pugliese M, Garibaldi A, Gullino ML. 2013. Integrated management of Phytophthora capsici on bell pepper by combining grafting and compost treatment. Crop Prot. 53:13–19.
- Gilbert G, Lacroix M, Hamel D. 2001. Diseases diagnosed on commercial crops submitted to the MAPAQ diagnostic laboratory in 2000. Can Plant Dis Surv. 81:40–58.
- Gisbert C, Sánchez-Torres P, Raigón MD, Nuez F. 2010. Phytophthora capsici resistance evaluation in pepper hybrids: agronomic performance and fruit quality of pepper grafted plants. J Food Agric Environ. 8:116–121.
- Granke LL, Quesada-Ocampo L, Hausbeck MK. 2011. Variation in phenotypic characteristics of Phytophthora capsici isolates from a worldwide collection. Plant Dis. 95:1080–1088.
- Granke LL, Quesada-Ocampo L, Lamour K, Hausbeck MK. 2012. Advances in research on Phytophthora capsici on vegetable crops in the United States. Plant Dis. 96:1588–1600.
- Granke LL, Windstam ST, Hoch HC, Smart CD, Hausbeck MK. 2009. Dispersal and movement mechanisms of Phytophthora capsici sporangia. Phytopathology. 99:1258–1264.
- Growing greenhouse vegetables. 2005. Publication 371, Ontario Ministry of Agriculture & Food. Toronto (ON): Queen’s Printer for Ontario.
- Hausbeck MK, Lamour KH. 2004. Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 88:1292–1303.
- Hong CX, Moorman GW. 2005. Plant pathogens in irrigation water: challenges and opportunities. Crit Rev Plant Sci. 24:189–208.
- Hong CX, Richardson PA, Kong P, Bush EA. 2003. Efficacy of chlorine on multiple species of phytophthora in recycled nursery irrigation water. Plant Dis. 87:1183–1189.
- Islam SZ, Babadoost M, Lambert KN, Ndeme A. 2005. Characterization of Phytophthora capsici isolates from processing pumpkin in Illinois. Plant Dis. 89:191–197.
- Jackson KL, Yin J, Csinos AS, Ji P. 2010. Fungicidal activity of fluopicolide for suppression of Phytophthora capsici on squash. Crop Prot. 29:1421–1427.
- Jackson KL, Yin J, Ji P. 2012. Sensitivity of Phytophthora capsici on vegetable crops in Georgia to mandipropamid, dimethomorph, and cyazofamid. Plant Dis. 96:1337–1342.
- James D, Varga A, Becker E, Sumampong G, Bailey K, Elliott M, Masri S, Shamoun SF. 2012. Screening of several disinfectants to assess their efficacy in controlling mycelia growth, sporangia germination, and recovery of viable Phytophthora ramorum. Crop Prot. 42:186–192.
- Keinath AP, Kousik CS. 2011. Sensitivity of isolates of Phytophthora capsici from the eastern United States to fluopicolide. Plant Dis. 95:1414–1419.
- Kim BS, Lee JY, Hwang BK. 2000. In vivo control and in vitro antifungal activity of rhamnolipid B, a glycolipid antibiotic, against Phytophthora capsici and Colletotrichum orbiculare. Pest Manag Sci. 56:1029–1035.
- Kim YJ, Hwang BK, Park KW. 1989. Expression of age-related resistance in pepper plants infected with Phytophthora capsici. Plant Dis. 73:745–747.
- King SR, Davis AR, Zhang X, Crosby K. 2010. Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Sci Horticul. 127:106–111.
- Kousik CS, Keinath AP. 2008. First report of insensitivity to cyazofamid among isolates of Phytophthora capsici from the southeastern United States. Plant Dis. 92:979.
- Krasnow CS, Naegele RP, Hausbeck MK. 2014. Evaluation of fruit resistance in Cucurbita germplasm resistant to Phytophthora capsici crown rot. HortSci. 49:285–288.
- Kubota C, McClure MA, Kokalis-Burelle N, Bausher MG, Rosskopf EN. 2008. Vegetable grafting: history, use, and current technology status in North America. HortSci. 43:1664–1669.
- Labuschagne N, Thompson AH, Botha WJ. 2003. First report of stem and root rot of tomato caused by Phytophthora capsici in South Africa. Plant Dis. 87:1540.
- Lamour KH, Hausbeck MK. 2000. Mefenoxam insensitivity and the sexual stage of Phytophthora capsici in Michigan cucurbit fields. Phytopathology. 90:396–400.
- Lamour KH, Hausbeck MK. 2001. The dynamics of mefenoxam insensitivity in a recombining population of Phytophthora capsici characterized with amplified fragment length polymorphism markers. Phytopathology. 91:553–557.
- Lamour KH, Hausbeck MK. 2003. Effect of crop rotation on the survival of Phytophthora capsici in Michigan. Plant Dis. 87:841–845.
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics. 23:2947–2948.
- Lee J-M, Kubota C, Tsao SJ, Bie Z, Hoyos Echevarria P, Morra L, Oda M. 2010. Current status of vegetable grafting: diffusion, grafting techniques, automation. Sci Horticul. 127:93–105.
- Lee JS, Seo ST, Wang TC, Jang HI, Pae DH, Engle LM. 2004. Effect of potassium silicate amendments in hydroponic nutrient solutions to suppress phytophthora blight (Phytophthora capsic) in pepper (Capsicum annuum). Plant Pathol J. 20:277–282.
- Louws FJ, Rivard CL, Kubota C. 2010. Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci Horticul. 127:127–146.
- Madden T. 2002. Chapter 16, The BLAST sequence analysis tool. In: McEntyre J, Ostell J, editors. The NCBI handbook. Bethesda (MD): National Center for Biotechnology Information (US).
- Matheron ME, Porchas M. 2000. Comparison of five fungicides on development of root, crown, and fruit rot of chile pepper and recovery of Phytophthora capsici from soil. Plant Dis. 84:1038–1043.
- Matheron ME, Porchas M. 2002. Suppression of phytophthora root and crown rot on pepper plants treated with acibenzolar-S-methyl. Plant Dis. 86:292–297.
- Matheron ME, Porchas M. 2014. Effectiveness of 14 fungicides for suppressing lesions caused by Phytophthora capsici on inoculated stems of chile pepper seedlings. Plant Health Prog. 15:166–171.
- Meyer MD, Hausbeck MK. 2013a. Using soil-applied fungicides to manage phytophthora crown and root rot on summer squash. Plant Dis. 97:107–112.
- Meyer MD, Hausbeck MK. 2013b. Age-related resistance to phytophthora fruit rot in ‘Dickenson Field’ processing pumpkin and ‘Golden Delicious’ winter squash fruit. Plant Dis. 97:446–552.
- Mitani S, Araki S, Yamaguchi T, Takii Y, Ohshima T, Matsuo N. 2001. Antifungal activity of the novel fungicide cyazofamid against Phytophthora infestans and other plant pathogenic fungi in vitro. Pestic Biochem Physiol. 70:92–99.
- Morra L, Bilotto M. 2006. Evaluation of new rootstocks for resistance to soil-borne pathogens and productive behavior of pepper (Capsicum annuum L.). J Hort Sci Biotech. 81:518–524.
- Neely D, Himelick EB. 1966. Simultaneous determination of fungistatic and fungicidal properties of chemicals. Phytopathology. 56:203–209.
- Neter J, Wasserman W, Kutner MH. 1985. Applied linear statistical models. 2nd ed. Homewood (IL): Richard D. Irwin, Inc.
- Nielsen CJ, Ferrin DM, Stanghellini ME. 2006a. Efficacy of biosurfactants in the management of Phytophthora capsici on pepper in recirculating hydroponic systems. Can J Plant Pathol. 28:450–460.
- Nielsen CJ, Ferrin DM, Stanghellini ME. 2006b. Cyclic production of sporangia and zoospores by Phytophthora capsici on pepper roots in hydroponic culture. Can J Plant Pathol. 28:461–466.
- OMAFRA. 2012. Area, production, value and sales of specified commercial vegetable crops, Ontario, 2011. [ cited 2012 Oct 3]. Available from: http://www.omafra.gov.on.ca/english/stats/hort/veg_all10-11English.pdf
- Parra G, Ristaino JB. 1998. Insensitivity to Ridomil Gold (mefenoxam) found among field isolates of Phytophthora capsici causing phytophthora blight on bell pepper in North Carolina and New Jersey. Plant Dis. 82:711.
- Parra G, Ristaino JB. 2001. Resistance to mefenoxam and metalaxyl among field isolates of Phytophthora capsici causing phytophthora blight of bell pepper. Plant Dis. 85:1069–1075.
- Ploetz R, Heine G, Haynes J, Watson M. 2002. An investigation of biological attributes that may contribute to the importance of Phytophthora capsici as a vegetable pathogen in Florida. Ann Appl Biol. 140:61–67.
- Quesada-Ocampo LM, Hausbeck MK. 2010. Resistance in tomato and wild relatives to crown and root rot caused by Phytophthora capsici. Phytopathology. 100:619–627.
- Ristaino JB. 1990. Intraspecific variation among isolates of Phytophthora capsici from pepper and cucurbit fields in North Carolina. Phytopathology. 80:1253–1259.
- Ristaino JB, Johnston SA. 1999. Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Dis. 83:1080–1089.
- Roberts PD, Urs RR, French-Monar RD, Hoffine MS, Seijo TE, McGovern RJ. 2005. Survival and recovery of Phytophthora capsici and oomycetes in tailwater and soil from vegetable fields in Florida. Ann Appl Biol. 146:351–359.
- Roberts PD, Urs RR, McGovern RJ. 2000. Age and varietal response of tomato to infection by Phytophthora capsici. Phytopathology. 90:S65.
- Ros C, Guerrero MM, Martínez MA, Barcelló N, Martínez MC, Rodríguez I, Lacasa A, Guirao P, Bello A. 2005. Resistant sweet pepper rootstocks integrated into the management of soilborne pathogens in greenhouse. Acta Hortic. 698:305–310.
- Russell AD, Hugo WB, Ayliffe GAJ. 1999. Disinfection, preservation and sterilization. London (England): Blackwell Science Ltd.
- Sambrook J, Russell DW. 2001. Molecular cloning – a laboratory manual. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press.
- Sanogo S, Ji P. 2012. Integrated management of Phytophthora capsici on solanaceous and cucurbitaceous crops: Current status, gaps in knowledge and research needs. Can J Plant Pathol. 34:479–492.
- SAS Institute Inc. 2000. SAS/STAT© user’s guide. Version 8.1 [computer program]. Cary (NC): SAS Institute
- Schlub RL. 1983. Epidemiology of Phytophthora capsici on bell pepper. J Agric Sci, Camb. 100:7–11.
- Sholberg PL, Walker MC, O’Gorman DT, Jesperson GD. 2007. First report of Phytophthora capsici on cucurbits and peppers in British Columbia. Can J Plant Pathol. 29:153–158.
- Stanghellini ME, Kim DH, Rasmussen SL, Rorabaugh PA. 1996. Control of root rot of peppers caused by Phytophthora capsici with a nonionic surfactant. Plant Dis. 80:1113–1116.
- Stanghellini ME, Nielsen CJ, Kim DH, Rasmussen SL, Rorbaugh PA. 2000. Influence of sub-versus top-irrigation and surfactants in a recirculating system on disease incidence caused by Phytophthora spp. in potted pepper plants. Plant Dis. 84:1147–1150.
- Steel RGD, Torrie JH. 1960. Principles and procedures of statistics. New York (NY): McGraw- Hill.
- Stewart-Wade SM. 2011. Plant pathogens in recycled irrigation water in commercial plant nurseries and greenhouses: their detection and management. Irrig Sci. 29:267–297.
- Sy O, Bosland PW, Steiner R. 2005. Inheritance of Phytophthora stem blight resistance as compared to phytophthora root rot and phytophthora foliar blight resistance in Capsicum annuum L. J Amer Soc Hortic Sci. 130:75–78.
- Sy O, Steiner R, Bosland PW. 2008. Recombinant inbred line differential identifies race-specific resistance to phytophthora root rot in Capsicum annuum. Phytopathology. 98:867–870.
- Tian D, Babadoost M. 2004. Host range of Phytophthora capsici from pumpkin and pathogenicity of isolates. Plant Dis. 88:485–489.
- Toquin V, Barja F, Sirven C, Bffa R. 2008. Fluopicolide, a new anti-oomycete fungicide with a new mode of action inducing perturbation of a spectrin-like protein. In: Krämer W, Schirmer U, editors. Modern crop protection compounds. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; p. 875–682.
- van Os EA. 2009. Comparison of some chemical and non-chemical treatments to disinfect a recirculating nutrient solution. Acta Hort. 843:229–234.
- Wohanka K. 2002. Nutrient solution disinfection. In: Savvas D, Passam H, editors. Hydroponic production of vegetables and ornamentals. Athens (Greece): Embryo Publications; p. 345–372.
- Yin J, Jackson KL, Candole BL, Csinos AS, Langston DB, Ji P. 2012. Aggressiveness and diversity of Phytophthora capsici on vegetable crops in Georgia. Ann Appl Biol. 160:191–200.
- Zhang ZG, Li YQ, Fan H, Wang YC, Zheng XB. 2006. Molecular detection of Phytophthora capsici in infected plant tissues, soil and water. Plant Pathol. 55:770–775.
