Abstract
Apple replant disease (ARD) occurs frequently in old orchards and is an important disease that limits apple production in China. Fusarium oxysporum is one of the causal agents of ARD and its pathogenic mechanism is still unclear. To explore the potential pathogenesis-related genes of this pathogen, a T-DNA insertion library of F. oxysporum HS2 was constructed via optimized Agrobacterium tumefaciens-mediated transformation (ATMT). The ATMT system yielded 160–200 transformants/106 conidia, indicating a relatively high conversion efficiency. The genetically stable transformants were tested through five generations of successive subculture on hygromycin-free media, and the alien gene was detected via PCR. A total of 3500 transformants were obtained to produce a T-DNA insertion library of F. oxysporum HS2, and 223 of these mutants were randomly selected for sporulation and pathogenicity tests. Most of the tested mutants presented weakened virulence, and one of them displayed obviously attenuated sporulation and reduced pathogenicity. The T-DNA in six pathogenicity-deficient mutants existed as a single copy, as confirmed by Southern blotting. The T-DNA flanking sequence was obtained rapidly by successive chromosome walking via the high-efficiency thermal asymmetric interlaced polymerase chain reaction (hiTAIL-PCR) method. The T-DNA right flanking sequences of five transformants were identified further, and the obtained sequences were aligned to the genomic sequence, revealing novel loci related to pathogenesis. This study provided a large number of various pathogenesis-related mutants and pathogenesis-related genes, which will be valuable resources for characterizing the molecular mechanisms of pathogenesis-related genes.
Résumé
La maladie de la replantation du pommier (MRP) se développe fréquemment dans les vieux vergers, et c’est une grave affection qui limite la production de pommes en Chine. Selon nos recherches précédentes, Fusarium oxysporum est un des agents causaux de la MRP dont le mécanisme de la pathogenèse n’est pas encore bien compris. Afin d’explorer les gènes possibles associés à la pathogenèse de cet agent, une banque d’insertion d’ADN-T de F. oxysporum HS2 a été construite à partir d’une transformation optimale avec Agrobacterium tumefaciens (TOAT). La TOAT a donné de 160 à 200 transformants/106 conidies, ce qui indique une efficacité de conversion relativement élevée. Les transformants génétiquement stables ont été testés au cours de cinq générations successives de repiquage sur un milieu de croissance sans hygromycine, et le gène étranger a été détecté par PCR. Il a fallu 3500 transformants pour produire une banque d’insertion d’ADN-T de F. oxysporum HS2, et 223 de ces mutants ont été sélectionnés aléatoirement pour tester la sporulation et la pathogénicité. La plupart des mutants testés ont affiché une virulence affaiblie, et un a affiché une sporulation visiblement atténuée ainsi qu’une pathogénicité réduite. L’ADN-T de six mutants dont la pathogénicité était réduite existait en une seule copie, comme l’a confirmé la technique de Southern. La séquence adjacente d’ADN-T a été obtenue rapidement par marche chromosomique successive au moyen de la PCR asymétrique thermique entrelacée à haut rendement (hiTAIL-PCR). Les séquences adjacentes d’ADN-T de la bordure droite de cinq transformants ont été plus précisément identifiées et les séquences obtenues ont été alignées sur la séquence génomique, révélant de nouveaux locus associés à la pathogenèse. Cette étude a fourni un grand nombre de mutants et de gènes différents associés à la pathogenèse qui constitueront des ressources précieuses pour caractériser les mécanismes moléculaires des gènes associés à la pathogenèse.
Introduction
Apple replant disease (ARD) is a serious apple disease worldwide and is characterized by stunted tree growth, deformed leaves, and the collapse of young trees (Mazzola Citation1998; Singh et al. Citation2019). ARD occurs when a young apple tree is replanted in a spot where a large tree had just died. Although the potential cause of ARD has been widely speculated, the aetiology of ARD remains unclear. Biotic factors (soil-borne microorganisms and parasitic nematodes) are considered important causal factors of ARD (Manici et al. Citation2013; Spath et al. Citation2015). Specifically, fungal pathogens, oomycetes, nematodes, Actinobacteria and endophytic microorganisms have been reported to be causal pathogens of ARD (Tewoldemedhin et al. Citation2011; Yim et al. Citation2013; Manici et al. Citation2013; Caputo et al. Citation2015; Franke-Whittle et al. Citation2015; Mazzola et al. Citation2015). Moreover, Cylindrocarpon-like species, Pythium ultimum, Pythium spp. and Rhizoctonia spp. have been shown to be directly involved in reductions in plant growth. In our previous survey, 293 isolates were isolated from 10 orchards afflicted with ARD in Hebei Province, China, and 116 isolates were identified as Fusarium spp. According to pathogenicity tests of begonia seedlings, Fusarium oxysporum was confirmed to be the major ARD pathogen (Zou et al. Citation2014). However, the pathogenic mechanism of F. oxysporum is still unclear.
Fusarium oxysporum has a wide range of hosts, including coriander, cotton, banana, zucchini, lavender, watermelon, cucumber, and many other cash and food crop species (Martinez et al. Citation2003; Choi et al. Citation2015; Garibaldi et al. Citation2015; Amaradasa et al. Citation2018; Halpern et al. Citation2018; Damodaran et al. Citation2019; Gilardi et al. Citation2019). Substantial information, including population structure, pathogenicity, secondary metabolite synthesis, hyphal differentiation, sexual and asexual reproduction and Fusarium–host interactions, has been reported for other Fusarium species, such as F. oxysporum, F. graminearum and F. verticillioides (Jurado et al. Citation2010; Islam et al. Citation2012; Geng et al. Citation2014; Palmero et al. Citation2014). Although secondary metabolites released into the rhizosphere by F. oxysporum may be an important pathogenicity factor of ARD (Manici et al. Citation2017), knowledge of the pathogenic mechanisms of ARD caused by F. oxysporum is still very limited, and additional molecular approaches are needed to elucidate its pathogenic mechanisms further.
In recent years, various DNA-mediated transformation systems have been developed and applied to different filamentous fungi for genetic research. Traditional tools, such as polyethylene glycol (PEG)/CaCl2-protoplast transformation, restriction enzyme-mediated integration (Liu et al. Citation2010) and genetic engineering via RNA interference techniques (Akashi et al. Citation2005), have been successfully developed for the construction of random mutant libraries for different fungal species (Lu et al. Citation1994; Jiang et al. Citation2013). Compared with the above methods, A. tumefaciens-mediated mutants are more suitable for studying the pathogenesis-related genes of phytopathogens (Dunn-Coleman and Wang Citation1998; Hu et al. Citation2014; Palmero et al. Citation2014). Agrobacterium tumefaciens-mediated transformation of filamentous fungi was first reported in 1998 (De Groot et al. Citation1998). To date, more than 200 fungal species have been transformed via A. tumefaciens-mediated transformation (ATMT) (Mullins et al. Citation2001; Frandsen Citation2011; Islam et al. Citation2012; Geng et al. Citation2014). However, a mutant library of F. oxysporum based on A. tumefaciens-mediated construction has not been reported.
Given the asexual nature of F. oxysporum, it is important to optimize the conditions of ATMT to maximize the number of transformants carrying a single copy of T-DNA. The objectives of this study were to (1) establish and optimize an ATMT system to construct a mutant library of F. oxysporum, (2) generate an ATMT transformation library that contains more than 2000 mutants, (3) screen pathogenicity-deficient mutants of F. oxysporum, (4) identify pathogenesis-related genes, and (5) determine the relationship between the T-DNA insertion site and secondary metabolism.
Materials and methods
Plasmid, strains and culture
Agrobacterium tumefaciens LBA4404 carrying the plasmid pCamhybgfp was cultured at 28ºC in Luria-Bertani (LB) media supplemented with 50 µg mL−1 kanamycin and 100 µg mL−1 streptomycin. The pCamhybgfp plasmids harbour the gene of interest and selectable markers, such as hygromycin resistance, kanamycin resistance and gfp genes.
The virulent strain F. oxysporum HS2, as a recipient strain for fungal transformation, was isolated from diseased apple roots from Handan city, Hebei Province (Zou et al. Citation2014). Fungal spores were produced by culturing the fungus on potato dextrose agar (PDA; 200 g L−1 potato tissue, 20 g L−1 dextrose, 15 g L−1 agar) media for 7 days at 25ºC.
Strains sensitive to hygromycin B
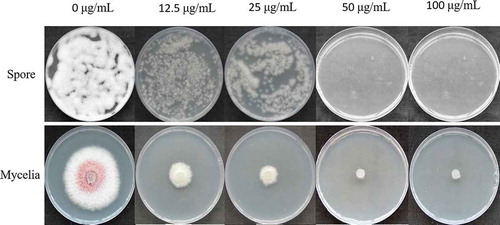
The wild-type (WT) strain F. oxysporum HS2 was reactivated for 5 days on PDA plates at 25°C in the dark. To determine a suitable concentration of hygromycin B, 5 mm hyphal agar discs from the colony edge were transferred to the centre of fresh PDA plates (90 mm in diameter) that contained different concentrations of hygromycin B (0, 12.5, 25, 50 and 100 µg mL−1), after which the plates were incubated at 25ºC. The growth diameters of the strain were measured after 7 days. Each concentration was tested in triplicate to minimize the experimental variations.
Conidia of WT F. oxysporum HS2 were gathered by gently washing the cultures on PDA media with sterile 0.05% Tween 80 solution. The conidial suspension was filtered with four layers of sterile gauze to remove mycelial fragments and then resuspended in sterile deionized water after being washed twice. The conidial concentration was adjusted to 106 conidia mL−1 via a hemocytometer. Two hundred microlitres of the conidial suspension was spread on PDA plates of different concentrations of hygromycin B (0, 12.5, 25, 50 and 100 µg mL−1), which were then incubated at 25ºC. Conidium germination under different concentrations of hygromycin B was observed 18 h later.
Agrobacterium tumefaciens-mediated transformation of F. oxysporum
The transformation procedure was based on a modified protocol (Mullins et al. Citation2001). Agrobacterium tumefaciens strain LBA4404 carrying the pCamhybgfp plasmid was cultivated on LB agar plates that were supplemented with 50 µg mL−1 kanamycin and 100 µg mL−1 streptomycin at 28°C for 2 days. A single colony was transferred to LB broth that contained 50 µg mL−1 kanamycin and 100 µg mL−1 streptomycin and incubated at 28ºC with shaking at 220 rpm for 24 h to an OD600 value of 0.5–0.8. The bacterial suspension was then centrifuged at 5000 rpm for 2 min at room temperature, washed twice with 1 mL of induction medium (IM) (liquid), diluted in 10 mL of IM (Bundock et al. Citation1995; Wang et al. Citation2012) that contained different concentrations of acetosyringone (AS; 0, 100, 200, 300 and 400 μmol mL−1), and subsequently incubated at 28ºC with shaking at 220 rpm for 4–6 h to an OD600 value of 0.2–0.3.
The conidial suspension of strain HS2 was collected and adjusted to 105, 106 and 107 spores mL−1. A. tumefaciens suspensions at different OD600 values, 0.1, 0.2, 0.3, 0.4 and 0.5, and an equal volume of conidial suspension of F. oxysporum HS2 were mixed together. The Agrobacterium-conidia mixture (200 µL per plate) was applied to sterile qualitative filter paper and overlaid on the surface of a co-cultivation medium (CM) plate (the same as that used for IM except that it contained 0.02% glucose and 1.5% agar). The co-cultivation plates were incubated for different periods of time (24, 36, 48 and 60 h) at different temperatures (15, 17, 19, 21, 23 and 25ºC). The filter paper was cut into strips and transferred onto PDA medium that contained 100 µg mL−1 hygromycin B. Individual hygromycin B-resistant transformants appeared after 3 days.
Green fluorescence observation
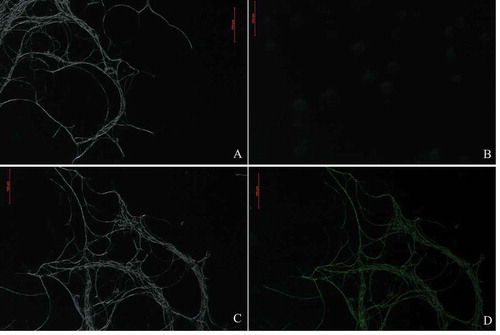
GFP becomes activated when the T-DNA integrates into the genome of strain HS2 randomly. The green fluorescence of the mutant was observed using a fluorescence microscope (Olympus IX71, Japan). The excitation wavelength was 460–550 nm, and the emission wavelength was 590 nm.
DNA extraction and PCR analyses
The transformants were incubated in 50 mL of liquid potato dextrose broth (PDB) that contained 100 µg mL−1 hygromycin B for 5 days at 25ºC with shaking at 160 rpm. Hyphae were harvested via sterile toothpicks and ground into a powder in liquid nitrogen via a sterile mortar and pestle. Fungal genomic DNA was isolated according to the cetyl-trimethylammonium bromide (CTAB) method (Hallen et al. Citation2003). To detect the integration of T-DNA in the putative transformants, PCR amplification was carried out by amplifying the hygromycin phosphotransferase (hph) gene with the specific primers HYG-F and HYG-R. The following procedure was used: 94°C for 5 min; 30 cycles of 94°C for 40 s, 51°C for 40 s and 72°C for 2 min; and then 72°C for 10 min (Wang et al. Citation2012). The genomic DNA of the WT strain HS2 was used as a negative control, and the plasmid pCamhybgfp was used as a positive control. The PCR products were detected via 1.0% agarose gel electrophoresis.
Mitotic stability test
The F. oxysporum transformants with the hph gene and the green fluorescence protein gene were subcultured on PDA that contained 100 µg mL−1 hygromycin B for 5 days. A mycelial plug from the edge of the colony was transferred to a fresh PDA plate that lacked hygromycin B and subsequently incubated at 25ºC for 5 days. After five generations, the mycelia were transferred to PDA plates that contained 100 µg mL−1 hygromycin B and then incubated at 25ºC for 5 days to check the growth of the mycelia. This assay was repeated three times.
Phenotypic analysis of transformants
The WT strain HS2 and the genetically stable transformants were subcultured on PDA at 25ºC for 8 days in darkness, and the colony diameters and colours were recorded to calculate the mycelial growth rate. The spores were collected by washing the cultures on PDA plates with 10 mL of sterile water after 10 days of incubation. The spore suspensions were subsequently filtered through four layers of sterile gauze, and a hemocytometer was used to calculate spore production of the transformants. Each strain was analysed in triplicate.
Screening of pathogenicity-deficient transformants
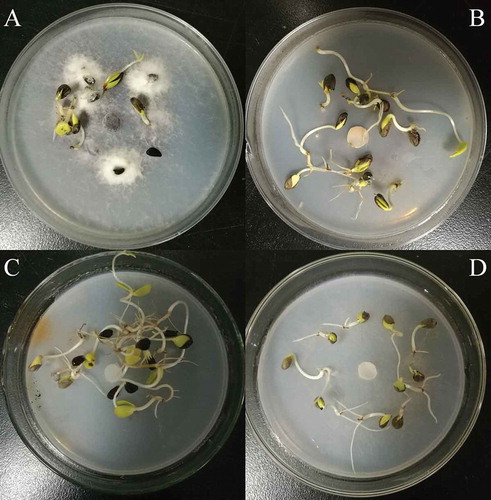
Crabapple seed germination was induced in sterile wet sand at 0–5ºC for 3 months and 37ºC sterile water for 24 h. The WT strain HS2 and its ATMT transformants were incubated on water agar (WA) media supplemented with 0.02% glucose at 25ºC for 3 days to generate actively growing colonies. The germinated seeds with a short radicle (1–2 mm) were placed on the margin of the actively growing colonies. The disease incidence was evaluated on the basis of the number of browning and shortened radicles that were infected by the WT or the transformants at 10 days post-inoculation (dpi). Each strain was inoculated onto 10 germinated seeds (constituting one biological repeat), and the experiment was conducted three times (Zou et al. Citation2014).
Southern blotting
The fragments of the pathogenicity-deficient transformants harbouring the hph gene were amplified with primers HF1 and HR1 and labelled with digoxigenin (DIG)-dUTP using a DIG High Prime DNA Labelling and Detection Starter Kit I (Roche, Mannheim, Germany). Genomic DNA (30 μg) of the transformant and WT strains was digested overnight at 37°C with a HindIII restriction enzyme (Takara Co., Ltd, Dalian, China), and pCamhybgfp linearized with the HindIII restriction enzyme was used as a positive control. The digestion products were separated by electrophoresis. The gels were treated with denaturation buffer containing 1.5 mol L−1 HCl and 0.5 mol L−1 NaOH before being blotted onto a nylon membrane. The prehybridization and hybridization temperatures were 49ºC, and the high-stringency washes of the membrane were performed at 68ºC.
Identification of T-DNA integration sites
Genomic DNA sequences flanking the T-DNA insertions were amplified by high-efficiency thermal asymmetric interlaced polymerase chain reaction (hiTAIL-PCR) as described previously (Schuster et al. Citation2012). Sequencing of the PCR product was also performed as previously described (Phoulivong et al. Citation2010). The right border of the inserted T-DNA and its flanking genomic sequences was analysed by searching the NCBI GenBank database, transmembrane domains within a predicted protein were analysed via TMHMM, and signal peptides within a predicted protein were analysed with SignalP 5.0.
DNA and RNA manipulation
RNA was extracted from the hyphal mixture and incubated for 3 days via a TransZol Up Plus RNA Kit (Trans, China) according to the manufacturer’s protocol. EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (Trans, China) were used in accordance with the manufacturer’s instructions. PCR and reverse-transcriptase polymerase chain reaction (RT-PCR) amplifications were performed by the use of 2x Es Taq Master Mix (CWBIO, China) as previously described (Wang et al. Citation2012). For detecting the transcripts of the five genes, the following primers were used: H520F and H520R (H520), H711F and H711R (H711), H2040F and H2040R (H2040), and H2026F and H2026R (H2026). The F. oxysporum β-actin gene, amplified with primers Act F and Act R, was used as a reference gene ().
Table 1. List of oligonucleotides used in this study
Relationship between insertion sites and the fusaric acid production capability of the different transformants
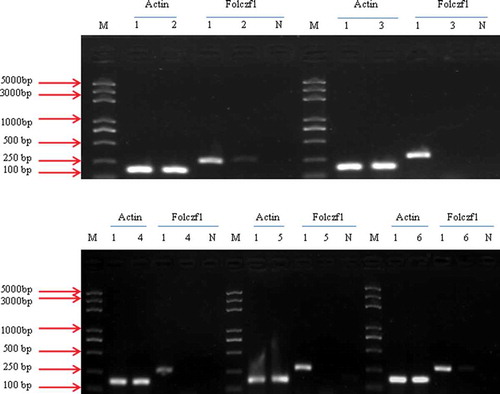
To explain the relationship between the insertion site and the capability of secondary metabolite production by the different mutants, we quantified the transcripts of the folczf1 gene (Yun et al. Citation2019) from five pathogenicity-deficient transformants. The F. oxysporum β-actin gene amplified with primers ActF and ActR was used as the reference gene.
Data analysis
All the data were analysed via SPSS software (version 24.0). One-way ANOVA was used, and the significance between treatments in each experiment was evaluated by Duncan’s multiple range test.
Results
Sensitivity of F. oxysporum to hygromycin B
The mycelial growth and conidial germination of F. oxysporum were dramatically inhibited in the presence of hygromycin B. The mycelial growth of F. oxysporum was partially inhibited by hygromycin B at a final concentration of 50 µg mL−1 and was completely inhibited at a final concentration of 100 µg mL−1 (). Therefore, a final concentration of 100 μg mL−1 hygromycin B in the PDA was selected as the optimal concentration to screen the transformants.
Optimization of ATMT conditions
To identify the optimal concentration of AS, different concentrations of AS were added to the PDA plates. With the increase in the AS concentration, the number of obtained transformants gradually increased, although the number of transformants did not significantly differ between the treatments with 200, 300 and 400 μmol mL−1 AS (). These results indicated that 200 μmol L−1 AS was suitable for F. oxysporum transformation.
Fig. 2 Single-factor comparison of Agrobacterium tumefaciens-mediated transformation of Fusarium oxysporum. The factors include the co-cultivation duration (a), the co-cultivation temperature (b), the OD600 of A. tumefaciens (c), the conidium concentration of F. oxysporum (d), and the concentration of AS (e). The error bars represent the standard deviations of the repeats, and the different letters indicate significant differences between different treatments (P < 0.05)
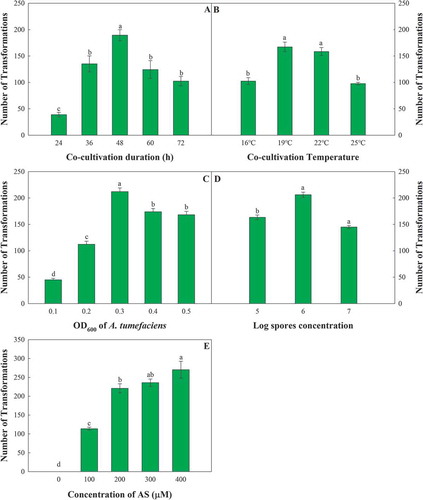
To screen suitable co-cultivation conditions to maintain high efficiency for ATMT, different temperatures and incubation periods were tested for the co-cultivation of F. oxysporum and A. tumefaciens. With the increase in treatment temperature, the number of obtained transformants tended to decrease after first increasing, with a peak occurring at 19°C (). Interestingly, the trend of the incubation period was similar to that of the co-cultivation temperature, with a peak occurring at 48 h (). Thus, the suitable co-cultivation conditions for ATMT in this study consisted of 19°C for 48 h.
To obtain individual transformants and avoid overlapping of different transformants, the concentrations of the A. tumefaciens bacterial suspension and F. oxysporum conidial suspension were optimized. The highest transformation frequency was recorded with an A. tumefaciens OD600 of 0.3 () and an F. oxysporum conidial concentration of 1 × 106 conidia mL−1 ().
After the ATMT conditions were optimized, the transformation efficiency reached 300 transformants/106 conidia. With this optimized ATMT system, a library containing more than 5000 transformants was generated.
Identification of transformants
The positive transformation of T-DNA was confirmed by amplifying the hph gene by the use of the genomic DNA of the transformants, which contained a 1000 bp fragment (Fig. S1). The presence of the hph gene was confirmed in 290 out of the 300 tested hygromycin B-resistant transformants on selective media. Observations of GFP expression by fluorescence microscopy revealed that GFP was expressed constitutively in the hyphae of transformants rather than the hyphae of WT F. oxysporum (). The results indicated that the hph gene could be effectively integrated into the WT strain HS2 by ATMT.
Mitotic stability of transformants
Three hundred randomly selected transformants were subcultured for five successive generations in the absence of hygromycin B and subsequently screened for hygromycin B resistance in the sixth generation. The results showed that all putative transformants could grow on selective media that contained hygromycin B (), implying that the transformants obtained by the ATMT method are mitotically stable.
Morphology, growth rate and sporulation of the different transformants
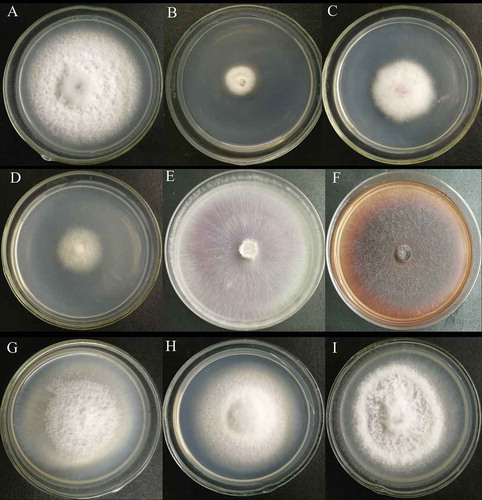
The morphology of 10% of the mutants was different from that of the WT strain. The strains HS2-100, HS2-908 and HS2-1017 grew slowly, and the hyphae turned white and were delicate. The hyphae of HS2-532, HS2-527 and HS2-511 became thinner and more distributed, and those of HS2-711 and HS2-1015 became denser, delicate and slightly darker in colour (). The growth rate of 3% of the mutants were slower than that of the WT, and the spore production of 10% of the mutants was less than that of the WT. Among the transformants with reduced sporulation, HS2-2229 did not sporulate, and the sporulation of strains HS2-2019 and HS2-100 decreased most significantly ().
Table 2. Sporulation number of partial transformants per unit area
Screening of pathogenicity-deficient transformants
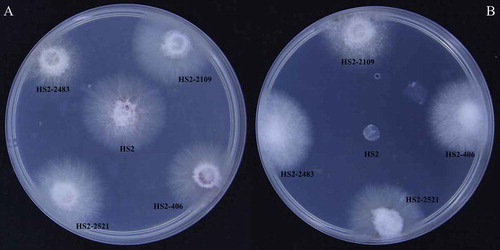
A total of 223 genetically stable transformants whose spore production obviously varied were used to test the pathogenicity. The results showed that the pathogenicity among the detected mutants significantly differed. The pathogenicity of HS2-311, HS2-2319 and HS2-520 was significantly weakened; a total of three transformants completely lost their virulence (). Among them, the pathogenicity of strains HS2-2521 and HS2-2483 was completely lost, but their spore-producing capability remained unchanged. The pathogenicity of strain HS2-2109 was completely lost (), and the spore-producing capability of this strain significantly decreased. Combined with the previous results, the pathogenicity-deficient mutants of strains HS2-2483, HS2-2109 and HS2-2521 were selected as the pathogenicity-deficient F. oxysporum mutants.
Table 3. Pathogenicity test of partial mutants
Southern blot analysis of T-DNA insertion events
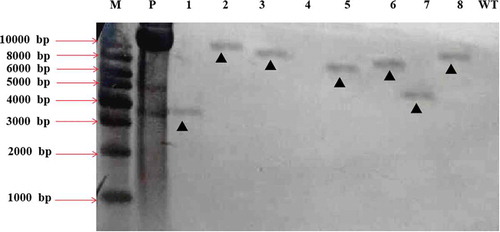
To investigate the T-DNA integration pattern and copy number of the transformants, Southern blotting was performed on eight pathogenicity-deficient mutants. Seven out of the eight tested mutants produced a single band, suggesting a single T-DNA insertion (). Combined with the previous results, the mutants HS2-2483, HS2-2109 and HS2-2521, whose hph genes had integrated at a single site, could be used for further analysis.
Fig. 7 Southern blot analyses of 8 different transformants of Fusarium oxysporum HS2 generated by ATMT. M represents the DNA marker; P represents the positive control of pCamhybgfp; Lanes 1–8 represent the transformant strains HS2-2483, HS2-2521, HS2-520, HS2-1106, HS2-2109, HS2-511, HS2-311 and HS2-2460, respectively; Lane WT represents the WT strain of F. oxysporum, HS2
Identification of T-DNA integration sites
For further characterization of the pathogenicity-deficient mutants, we chose five transformants (HS2-711, HS2-2109, HS2-2040, HS2-780 and HS2-2026). The right T-DNA border sequences and adjacent sequences of these mutants were amplified via hiTAIL-PCR. To identify the T-DNA insertion sites, the PCR products were sequenced and subjected to BLAST searches of the NCBI genomic database. Among these analysed transformants, the T-DNA was integrated within the annotated gene in three transformants, and the T-DNA was integrated within an upstream region of the tagged gene for two of five transformants (). The BLAST results revealed that the five tagged genes were FOIG_07731, FOIG_09359, FOIG_00043, CEK26_004779 and Fso1 ().
Table 4. Analysis of identified genes possibly involved in the deficient pathogenicity of Fusarium oxysporum mutants
Relative expression analysis of mutant genes and the folczf1 gene
The RT-PCR results revealed that the transcripts of four genes (FOIG_09359, FOIG_00043, CEK26_004779 and Fso1) were not detected in the T-DNA insertion mutants; the FOIG_0773 gene was detected, but RT-PCR revealed a strongly decreased expression level of FOIG_0773, and all of these gene transcripts were present in the WT isolate HS2 (). These results suggested that mutations in these five genes were responsible for the variation in pathogenicity.
Fig. 8 RT-PCR analysis of the transcription of FOIG_07731, FOIG_00043, FOIG_09359, Fso1 and CEK26_00479. The β-actin gene was amplified and used as a control. Lane M represents the DNA marker; Lanes 2–6 represent different transformants mediated by ATMT; Lane 1 represents the WT strain of Fusarium oxysporum, HS2; Lane N represents the negative control
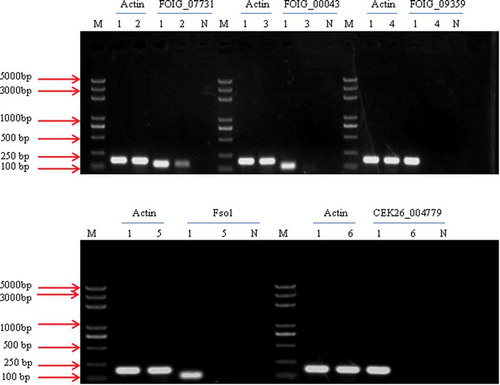
We performed RT-PCR to determine the relative expression level of folczf1 in the different pathogenicity-deficient mutants. Folczf1 expression was not detected in the HS2-711, HS2-2040 or HS2-2026 mutant; the folczf1 gene was detected in HS2-520 and HS2-2109, and all the gene transcripts were present in the WT isolate HS2 (). These results suggested that there is no direct relationship between the T-DNA insertion site and fusaric acid synthesis.
Discussion
One of the aims of this study was to verify the feasibility of ATMT as a tool for insertional mutagenesis of F. oxysporum. In this study, this method for constructing F. oxysporum mutants with high transformation efficiency was optimized. Compared with other methods for generating mutants, ATMT has several advantages. One of the principal advantages is the flexibility in choosing the starting material (protoplasts, hyphae, spores or mycelial tissues) to be transformed (Abuodeh et al. Citation2000; Rogers et al. Citation2004; Van Schoor et al. Citation2009; Liu et al. Citation2010). ATMT usually has high transformation efficiency and a high rate of single-copy insertions (Betts et al. Citation2007), and the insertion sites are easy to identify using T-DNA flanking sequences (Choi et al. Citation2007).
Several factors can affect transformation efficiency. It was previously shown that preculturing A. tumefaciens cells with AS could influence the transformation frequency of fungi via ATMT. Pre-induction delays the F. oxysporum transformation process (Mullins et al. Citation2001). In the case of F. oxysporum, pre-induction was essential for the success of ATMT. Pre-induction for 6 h promoted a yield of more than 100 transformants, which was significantly greater than the number of transformants without pre-induction.
The concentration of A. tumefaciens was one of the most important factors affecting the transformation efficiency, and high concentrations of Agrobacterium are often associated with high conversion efficiency (Meyer et al. Citation2003; Michielse et al. Citation2004). However, in the present study, the highest transformation efficiency was obtained when the OD600 of A. tumefaciens reached 0.3 and when the concentration of F. oxysporum conidia was 1 × 106 conidia mL−1. This is consistent with the results of Harpophora oryzae mutant construction (Liu et al. Citation2010). A higher concentration of A. tumefaciens and the WT strain may lead to overlap of the mycelia of the transformants, and it would be difficult to isolate individual strains effectively (Lu et al. Citation2017).
The co-cultivation period also significantly affected the efficiency of transformation, and this period of time was different with different filamentous fungi. For instance, the co-cultivation time was 108 h for an ectomycorrhizal symbiont (Mullins et al. Citation2001), but 48 h was the optimal co-cultivation time for Fusarium avenaceum (Sørensen et al. Citation2014). Extended co-cultivation periods decreased the transformation efficiency and increased the number of false positives obtained because of the excess growth of the fungus. In this study, the number of colonies increased with the time of co-cultivation. Nevertheless, a thick layer of mycelia developed on nitrocellulose filters after 48 h of co-cultivation, which made the attainment of individual transformants more difficult.
Pre-treating A. tumefaciens cells with AS also increased the number of generations of transformants (Bundock et al. Citation1995; Choi et al. Citation2007). AS has an important role in producing Vir proteins, including VirF, VirH and VirE3, which are essential for transformation (Michielse et al. Citation2004). Transformation efficiency significantly decreases in the absence of AS, and sometimes no transformants are produced (Takahara et al. Citation2004; Kemppainen et al. Citation2005; Islam et al. Citation2012). Similar results occurred in our study; AS seems to be essential for transformation during co-cultivation. With increasing AS concentration, the transformation frequency significantly increased. However, excess AS can also produce false-positive colonies.
The radicle assay (Zou et al. Citation2014) was used to screen for pathogenicity-deficient transformants in this study. Crabapple radicles become brown and short when infected by WT strains of F. oxysporum; in contrast, they remain white and long when the seeds are infected by pathogenicity-deficient F. oxysporum mutants. The radicle assay was used to screen strains to save time and improve the efficiency of selecting antagonistic bacteria for the control of Phytophthora blight of pepper (Sung et al. Citation2001). PCR and GFP fluorescence were used to detect whether the pathogenicity-deficient mutants were positive. To verify whether phenotypic differences of mutants are caused by a single-gene-insertion mutation, Southern blot hybridization is typically used (De Groot et al. Citation1998; Mullins et al. Citation2001). In this study, 80% of the detected mutants were single-gene T-DNA insertion mutations.
Thermal asymmetric staggered PCR is the most widely used technique to obtain flanking sequences. However, studies have shown that when T-DNA is inserted into the genome via ATMT, the obtained sequence may be a vector sequence or target fragment (He et al. Citation2007). In the present study, 5 mutants were cloned by hiTAIL-PCR, and 5 flanking sequences were obtained. Three sequences were related to hypothetical proteins according to NCBI BLAST. The transformant HS2-2026 showed complete loss of pathogenicity on WA media, and the lone T-DNA insertion site was located within the exon of the FOIG_09359 gene, which encodes benzoate 4-monooxygenase, an enzyme that has been identified in the fungal pathogen Cochliobolus lunatus as a crucial player in the detoxification of benzoic acid (BA), which is a key intermediate in the metabolism of aromatic compounds in fungi (Sabina et al. Citation2012). The transformant HS2-2040 also presented complete loss of pathogenicity on WA media, and the lone T-DNA insertion site was located within the exon of the fso1 gene, which encodes the derived www domain protein Fos1, a putative two-component histidine kinase that may play a role in the regulation of cell wall assembly (Clemons et al. Citation2002). In addition, three genes (FOIG_00043, CEK26_004779 and FOIG_07731) were discovered to encode proteins of unknown function; these proteins did not contain conserved domains on the basis of an InterProScan analysis, indicating that these genes may be novel genes involved in the pathogenicity of fungi. Elucidating the functions of these novel genes could facilitate the understanding of F. oxysporum.
Fusaric acid is a secondary metabolite of F. oxysporum and is a non-specific toxin. To analyse the secondary metabolite-producing ability of strains with significantly weakened pathogenicity caused by T-DNA insertion, five mutants with significantly weakened pathogenicity were evaluated. RT-PCR was used to detect the expression of the fusaric acid synthesis gene of F. oxysporum. The results showed that the expression of this gene was significantly reduced in three mutants, HS2-711, HS2-2026 and HS2-2040, and that it did not significantly differ between the WT and the HS2-520 and HS2-2109 mutants. The reason may be related to the T-DNA insertion site because T-DNA inserts randomly, and the gene at the insertion site does not always affect the production of secondary metabolites.
The pathogenicity-deficient mutants screened from the mutant library of F. oxysporum allow the localization of genes associated with pathogenesis to be identified and allow their function to be studied. Therefore, ATMT is a feasible tool for random insertional mutagenesis, can advance the identification of genes in F. oxysporum and provides a foundation for future genetic studies of other filamentous fungi.
supplemental_figure.pptx
Download MS Power Point (483.1 KB)Acknowledgements
We thank Dr Junxiang Zhang from the Chinese Academy of Agricultural Sciences for kindly providing A. tumefaciens strain LBA4404 and the pCamhybgfp vector.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Abuodeh RO, Orbach MJ, Mandel MA, Das A, Galgiani JN. 2000. Genetic transformation of Coccidioides immitis facilitated by Agrobacterium tumefaciens. J Infect Dis. 181(6):2106–2110. doi:10.1086/jid.2000.181.issue-6.
- Akashi H, Matsumoto S, Taira K. 2005. Gene discovery by ribozyme and siRNA libraries. Nat Rev Mol Cell Bio. 6(5):413–422. doi:10.1038/nrm1646.
- Amaradasa BS, Beckham K, Dufault N, Sanchez T, Ertek TS, Iriarte F, Paret M, Ji P. 2018. First report of Fusarium oxysporum f. sp. niveum race 3 causing wilt of watermelon in Florida, USA. Plant Dis. 102(5):1029. doi:10.1094/PDIS-10-17-1649-PDN.
- Betts MF, Tucker SL, Galadima N, Meng Y, Patel G, Li L, Donofrio N, Floyd A, Nolin S, Brown D, et al. 2007. Development of a high throughput transformation system for insertional mutagenesis in Magnaporthe oryzae. Fungal Genet Biol. 44(10):1035–1049. doi:10.1016/j.fgb.2007.05.001.
- Bundock P, Dulk-Ras AD, Beijersbergen A, Hooykaas PJ. 1995. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to saccharomyces cerevisiae. Embo J. 14(13):3206–3214. doi:10.1002/embj.1995.14.issue-13.
- Caputo F, Nicoletti F, Picione FDL, Manici LM. 2015. Rhizospheric changes of fungal and bacterial communities in relation to soil health of multi-generation apple orchards. Biol Control. 88:8–17. doi:10.1016/j.biocontrol.2015.04.019.
- Choi IY, JU-Hee JH, Lee WH, Park JH, Shin HD. 2015. First report on fusarium wilt of zucchini caused by Fusarium oxysporum, in Korea. Mycobiology. 43(2):174–178. doi:10.5941/MYCO.2015.43.2.174.
- Choi J, Park J, Jeon J, Chi MH, Goh J, Yoo SY, Park J, Jung K, Kim H, Park SY, et al. 2007. Genome-wide analysis of T-DNA integration into the chromosomes of Magnaporthe oryzae. Mol Microniol. 66(2):371–382. doi:10.1111/mmi.2007.66.issue-2.
- Clemons KV, Miller TK, Selitrennikoff CP, Stevens DA. 2002. fos-1, a putative histidine kinase as a virulence factor for systemic aspergillosis. Med Mycol. 40(3):259–262. doi:10.1080/mmy.40.3.259.262.
- Damodaran T, Mishra VK, Jha SK, Gopal R, Rajan S, Ahmed I. 2019. First report of fusarium wilt in banana caused by Fusarium oxysporum f. sp. cubense tropical race 4 in India. Plant Dis. 103(5):1022–1023. doi:10.1094/PDIS-07-18-1263-PDN.
- De Groot MJA, Bundock P, Hooykaas PJJ, Beijersbergen AGM. 1998. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat Biotechnol. 16(9):839–842. doi:10.1038/nbt0998-839.
- Dunn-Coleman N, Wang H. 1998. Research news - Agrobacterium T-DNA: A silver bullet for filamentous fungi? Nat Biotechnol. 16(9):817–818. doi:10.1038/nbt0998-817.
- Frandsen RJN. 2011. A guide to binary vectors and strategies for targeted genome modification in fungi using Agrobacterium tumefaciens-mediated transformation. J Microbiol Meth. 87(3):247–262. doi:10.1016/j.mimet.2011.09.004.
- Franke-Whittle IH, Manici LM, Insam H, Stres B. 2015. Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil. 395(1–2):317–333. doi:10.1007/s11104-015-2562-x.
- Garibaldi A, Bertetti D, Pensa P, Ortu G, Gullino ML. 2015. First report of fusarium oxysporum causing Wilt on Allard’s Lavender (Lavandula × allardii) in Italy. Plant Dis. 99(12):1868. doi:10.1094/PDIS-02-15-0234-PDN.
- Geng Z, Zhu W, Su H, Zhao Y, Zhang KQ, Yang J. 2014. Recent advances in genes involved in secondary metabolite synthesis, hyphal development, energy metabolism and pathogenicity in Fusarium graminearum (teleomorph Gibberella zeae). Biotechnol Adv. 32(2):390–402. doi:10.1016/j.biotechadv.2013.12.007.
- Gilardi G, Franco-Ortega S, Gullino ML, Garibaldi A. 2019. First report of fusarium wilt of coriander (Coriandrum sativum) caused by Fusarium oxysporum in Italy. Plant Dis. 103:1021.
- Hallen HE, Watling R, Adams GC. 2003. Taxonomy and toxicity of conocybe lactea and related species. Mycol Res. 107(8):969–979. doi:10.1017/S0953756203008190.
- Halpern HC, Bell AA, Wagner TA, Liu J, Nichols RL, Olvey J, Woodward JE, Sanogo S, Jones CA, Chan CT, et al. 2018. First report of fusarium wilt of cotton caused by Fusarium oxysporum f. sp. vasitzfectum race 4 in Texas, USA. Plant Dis. 102(2):446. doi:10.1094/PDIS-07-17-1084-PDN.
- He CP, Wang KD, Liao QH, Zheng FC. 2007. Spotting the positions of T-DNA on the genome for Magnaporthe grisea transformants and study on the mode of T-DNA ibsertion. Acta Microbiologica Sinica. 4:588–592.
- Hu Y, Dai Q, Liu Y, Yang Z, Song N, Gao X, Voegele RT, Kang Z, Huang L. 2014. Agrobacterium tumefaciens-mediated transformation of the causative agent of valsa canker of apple tree valsa mali var.mali. Curr Microbiol. 68(6):769–776. doi:10.1007/s00284-014-0541-8.
- Islam MN, Nizam S, Verma PK. 2012. A highly efficient Agrobacterium mediated transformation system for chickpea wilt pathogen Fusarium oxysporum f. sp ciceri using DsRed-Express to follow root colonisation. Microbiol Res. 167(6):332–338. doi:10.1016/j.micres.2012.02.001.
- Jiang D, Zhu W, Wang Y, Sun C, Zhang KQ, Yang J. 2013. Molecular tools for functional genomics in filamentous fungi: recent advances and new strategies. Biotechnol Adv. 31(8):1562–1574. doi:10.1016/j.biotechadv.2013.08.005.
- Jurado M, Marin P, Callejas C, Moretti A, Vázquez C, González-Jaén MT. 2010. Genetic variability and fumonisin production by Fusarium proliferatum. Food Microbiol. 27(1):50–57. doi:10.1016/j.fm.2009.08.001.
- Kemppainen M, Circosta A, Tagu D, Martin F, Pardo AG. 2005. Agrobacterium-mediated transformation of the ectomycorrhizal symbiont Laccaria bicolor S238N. Mycorrhiza. 16(1):19–22. doi:10.1007/s00572-005-0008-7.
- Liu T, Liu L, Jiang X, Hou J, Fu K, Zhou F, Chen J. 2010. Agrobacterium-mediated transformation as a useful tool for the molecular genetic study of the phytopathogen Curvularia lunata. Eur J Plant Pathol. 126(3):363–371. doi:10.1007/s10658-009-9541-0.
- Lu S, Lyngholm L, Yang G, Bronson C, Yoder OC, Turgeon BG. 1994. Tagged mutations at the tox1 locus of cochliobolus heterostrophus by restriction enzyme-mediated integration. Proc Natl Acad Sci U S A. 91(26):12649–12653. doi:10.1073/pnas.91.26.12649.
- Lu Y, Xiao S, Wang F, Sun J, Zhao L, Yan L, Xue C. 2017. Agrobacterium tumefaciens-mediated transformation as an efficient tool for insertional mutagenesis of Cercospora zeae-maydis. J Microbiol Met. 133:8–13. doi:10.1016/j.mimet.2016.12.010.
- Manici LM, Caputo F, Saccà ML. 2017. Secondary metabolites released into the rhizosphere by Fusarium oxysporum and Fusarium spp. as underestimated component of non specific replant disease. Plant Soil. 415(1–2):85–98. doi:10.1007/s11104-016-3152-2.
- Manici LM, Kelderer M, Franke-Whittle IH, Rühmer T, Baab G, Nicoletti F, Caputo F, Topp A, Insam H, Naef A. 2013. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl Soil Eco. 72:207–214. doi:10.1016/j.apsoil.2013.07.011.
- Martinez R, Aguilar MI, Guirado ML, Alvarez A, Gomez J. 2003. First report of fusarium wilt of cucumber caused by Fusarium oxysporum in Spain. Plant Pathol. 52(3):410. doi:10.1046/j.1365-3059.2003.00832.x.
- Mazzola M. 1998. Elucidation of the microbial complex having a causal role in the development of apple replant disease in Washington. Phytopathology. 88(9):930–938. doi:10.1094/PHYTO.1998.88.9.930.
- Mazzola M, Hewavitharana SS, Strauss SL. 2015. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen re-infestation. Phytopathology. 105(4):460–469. doi:10.1094/PHYTO-09-14-0247-R.
- Meyer V, Mueller D, Strowig T, Stahl U. 2003. Comparison of different transformation methods for Aspergillus giganteus. Curr Genet. 43(5):371–377. doi:10.1007/s00294-003-0406-3.
- Michielse CB, Ram AFG, Hooykaas PJJ, Hondel CAMJJVD. 2004. Role of bacterial virulence proteins in Agrobacterium-mediated transformation of Aspergillus awamori. Fungal Genet Biol. 41(5):571–578. doi:10.1016/j.fgb.2004.01.004.
- Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S. 2001. Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology. 91(2):173–180. doi:10.1094/PHYTO.2001.91.2.173.
- Palmero D, Rubio-Moraga A, Galvez-Patón L, Nogueras J, Abato C, Gómez-Gómez L, Ahrazem O. 2014. Pathogenicity and genetic diversity of Fusarium oxysporum isolates from corms of Crocus sativus. Ind Corp Prod. 61:186–192. doi:10.1016/j.indcrop.2014.06.051.
- Phoulivong S, Cai L, Chen H, McKenzie EHC, Abdelsalam K, Chukeatirote E, Hyde KD. 2010. Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers. 44(1):33–43. doi:10.1007/s13225-010-0046-0.
- Rogers CW, Challen MP, Green JR, Whipps JM. 2004. Use of REMI and Agrobacterium -mediated transformation to identify pathogenicity mutants of the biocontrol fungus, coniothyrium minitans. FEMS Microbiol Lett. 241(2):207–214. doi:10.1016/j.femsle.2004.10.022.
- Sabina B, Barbara P, Neja Z, Metka N, Nada K, Samo T, Branka K, Ljerka L, Erika S, Jure S, et al. 2012. Virtual screening yields inhibitors of novel antifungal drug target, benzoate 4 monooxygenase. J Chem Inf Model. 52(11):3053–3063. doi:10.1021/ci3004418.
- Schuster A, Tisch D, Seidl-Seiboth V, Kubicek CP, Schmoll M. 2012. Roles of protein kinase A and adenylate cyclase in light modulated cellulase regulation in Trichoderma reesei. Appl Environ Microbiol. 78(7):2168–2178. doi:10.1128/AEM.06959-11.
- Singh J, Silva KJP, Fuchs M, Khan A. 2019. Potential role of weather, soil and plant microbial communities in rapid decline of apple trees. PLoS One. 14(3):e0213293. doi:10.1371/journal.pone.0213293.
- Sørensen LQ, Lysøe E, Larsen JE, Khorsand-Jamal P, Nielsen KF, Frandsen RJN. 2014. Genetic transformation of Fusarium avenaceum by Agrobacterium tumefaciens mediated transformation and the development of a USER-brick vector construction system. BMC Mol Biol. 6(1):15. doi:10.1186/1471-2199-15-15.
- Spath M, Insam H, Peintner U, Kelderer M, Kuhnert R, Franke-Whittle IH. 2015. Linking soil biotic and abiotic factors to apple replant disease: a greenhouse approach. J Phytopathol. 163(4):287–299. doi:10.1111/jph.2015.163.issue-4.
- Sung HC, Min SK, Yun SK, Jung YL. 2001. A rapid radicle assay for prescreening antagonistic bacteria against Phytophthora capsici on pepper. Mycobiology. 29(4):218–223. doi:10.1080/12298093.2001.12015791.
- Takahara H, Tsuji G, Kubo Y, Yamamoto M, Toyoda K, Inagaki Y, Ichinose Y, Shiraishi T. 2004. Agrobacterium tumefaciens-mediated transformation as a tool for random mutagenesis of Colletotrichum trifolii. J Gen Plant Pathol. 70(2):93–96. doi:10.1007/s10327-003-0099-y.
- Tewoldemedhin YT, Mazzola M, Labuschagne I, Mcleod A. 2011. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biol Biochem. 43(9):1917–1927. doi:10.1016/j.soilbio.2011.05.014.
- Van Schoor L, Denman S, Cook NC. 2009. Characterisation of apple replant disease under South African conditions and potential biological management strategies. Sci Hortic-amsterdam. 119(2):153–162. doi:10.1016/j.scienta.2008.07.032.
- Wang M-J, Li P, Wu M, Fan YS, Gu SQ, Dong JG. 2012. Constuction and evaluation of ATMT mutant library of Setosphaeria turcica. Scientia Agricultura Sinica. 45:2384–2392.
- Yim B, Smalla K, Winkelmann T. 2013. Evaluation of apple replant problems based on different soil disinfection treatments-links to soil microbial community structure? Plant Soil. 366(1–2):617–631. doi:10.1007/s11104-012-1454-6.
- Yun YZ, Zhou X, Yang S, Wen Y, You HX, Zheng YR, Norvienyeku J, Shim WB, Wang ZH. 2019. Fusarium oxysporum f. sp. lycopersici C2H2 transcription factor FolCzf1 is required for conidiation, fusaric acid production, and early host infection. Curr Gent. 65(3):773–783. doi:10.1007/s00294-019-00931-9.
- Zou QJ, Wang ST, Liang KJ, Wang YN, Hu TL, Han ZQ, Cao KQ. 2014. Suspected pathogenic Fusarium spp. isolated from apple orchard soils in Hebei Province. Mycosystema. 5:976–983.