Abstract
Stem rust, caused by Puccinia graminis f. sp. tritici (Pgt), is a major disease of wheat. In Kenya, Pgt has caused sporadic but serious losses to farmers since large-scale wheat production began in the early twentieth Century. Breeding for stem rust resistance in Kenya has been conducted since 1910. Mutants of Pgt are common in Kenya as large pathogen populations survive on wheat crops planted throughout the year, with virulence to effective major genes developing shortly after release of resistant cultivars. Gene Sr31 was first deployed in Kenya in ‘Kenya Pa’a’ in 1982 and subsequently in ‘Duma’ in 1993, the latter grown on large acreage. Virulence to Sr31 was first detected in Uganda in 1998, in what became known as Pgt-Ug99 (race TTKSK). Virulence to Sr31 may have occurred earlier in Kenya, but Ug99 was first reported in 2001. Race TTKSK has migrated across East Africa and to Yemen and Iran, spreading to 13 countries with 13 race variants. Other Pgt races (TKTTF, TTRTF, TTTTF) with broad virulence were recently detected in Kenya, likely originating from central Asia. A ‘Sounding the Alarm’ message from Norman Borlaug in 2005 triggered extensive research on worldwide virulence in Pgt, and on finding, characterizing, and developing molecular markers for Sr genes effective to Ug99-lineage pathotypes of Pgt. While fungicides can control Pgt, the best strategy uses host resistance. The best gene stewardship practice to provide enduring resistance combines effective major and minor adult plant resistance (APR) genes prior to release of new wheat cultivars.
Résumé
Partout dans le monde, la rouille noire, causée par Puccinia graminis f. sp. tritici (Pgt), est une grave maladie du blé. Au Kenya, Pgt a occasionné aux producteurs des pertes sporadiques de nature épidémique étant donné que la culture intensive du blé remonte au début du 20e siècle. Au Kenya, la sélection en vue de la résistance à la rouille noire a commencé en 1910. Dans ce pays, le taux de mutation chez Pgt est élevé, puisque de fortes populations d’agents pathogènes survivent sur le blé qui est semé durant toute l’année. La virulence à l’égard des gènes de résistance majeurs s’est développée peu après la commercialization de cultivars résistants. Au Kenya, le gène Sr31 a été utilisé initialement en 1982 dans le cultivar ‘Kenya Pa’a’ et, par la suite, en 1993, dans le cultivar ‘Duma’ semé sur les grandes superficies. La virulence à l’égard de Sr31 a d’abord été détectée en Ouganda en 1998 et l’agent a été décrit comme Pgt-Ug99. La virulence à l’égard de Sr31 est peut-être apparue plus tôt au Kenya, mais Ug99 a été détecté pour la première fois en 2001. Ug99 (race TTKSK) a migré de l’Afrique de l’Est au Yémen et en Iran et s’est répandu dans 13 pays avec 13 variants. D’autres races de Pgt (TKTTF, TTRTF, TTTTF) très virulentes, issues vraisemblablement d’Asie centrale, ont récemment été détectées au Kenya. Un cri d’alarme lancé par Norman Borlaug en 2005 a déclenché de nouvelles études d’envergure sur la virulence de Pgt, et ce, à l’échelle mondiale afin de trouver, de caractériser et de développer des marqueurs moléculaires servant à identifier les gènes Sr pour lutter contre les pathotypes de la lignée Ug99 de Pgt. Bien que Pgt puisse être maîtrisé avec des fongicides, la meilleure stratégie repose sur la résistance de l’hôte. La meilleure pratique d’intendance relative aux gènes visant à établir une résistance durable consiste à combiner les gènes de résistance majeurs et mineurs efficaces des plantes adultes avant de commercialiser de nouveaux cultivars de blé.
Introduction
Wheat (Triticum aestivum L. and Triticum turgidum L.) is the most commonly grown crop worldwide, planted on about 214 million hectares in 2018 and producing about 734 million metric tonnes (FAOSTAT; http://www.fao.org/faostat/en/#data/QC). Wheat production is impacted significantly by many plant diseases, which have been estimated to cause 10.2% actual losses, with a range from 5% to 14% (Oerke Citation2006). Historically, the most dangerous and devastating disease on wheat has been stem rust. Stem rust () is a fungal disease caused by Puccinia graminis Pers.:Pers. f. sp. tritici Eriks. & E. Henn. (Pgt) that has incited epidemics on wheat for thousands of years (Fetch and McCallum Citation2014). Over the past 70 years, stem rust epidemics have occurred in India, North America, Australia, Ethiopia, and Sicily (), and in Kenya it has been an ongoing challenge for wheat production since the early 1900s, with severe devastation from 1906 to 1917 (Pinto and Hurd Citation1970). These epidemics have been attributed mainly to the constant evolution of different races of Pgt following the deployment of stem rust resistance genes (Sr genes). This paper presents the history of rust research in Kenya before and after the discovery of the highly virulent Ug99 race, its impact on wheat breeding worldwide, and discusses putative evolution of Pgt in Kenya that led to the emergence of Ug99.
Table 1. Examples of epidemics and losses due to wheat stem rust infection
Fig. 1 (Colour online) Wheat stem rust infection on stems and leaves (reproduced from Brar et al. Citation2019)

Life cycle and migration
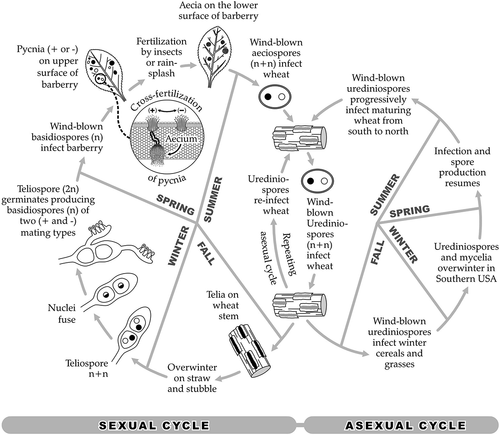
Puccinia graminis f. sp. tritici is an obligate heteroecious and macrocyclic rust pathogen that has five spore stages (). Urediniospores survive year-round in Kenya, as environmental conditions are ideal for infection, and being equatorial with elevation from sea level to over 3000 m the planting of wheat can occur at any time. Volunteer wheat plants also may grow between cropping seasons and provide a ‘green bridge’ that sustains urediniospore production. The maximum occurrence of airborne urediniospore inoculum in Kenya is in September and early October, concurrent with the maturation of the long-rain season planted crop and onset of harvest (Green et al. Citation1970). Planting of the next crop coincides with the short rains occurring in October and November. Thus, the primary life cycle in Kenya consists mainly of repeating asexual propagation of urediniospores. Initially it was thought that the sexual cycle was not operational in Kenya due to the lack of a susceptible alternate host. The government mycologist J. McDonald reported that aeciospores from the local barberry species Berberis holstii did not infect either wheat or oat, but did report that this barberry was mildly susceptible to Pgt (Guthrie Citation1966). However, it was recently found that aecial infections of B. holstii in Ethiopia could generate viable Pgt isolates (Singh et al. Citation2015), and Jin et al. (Citation2020) mentioned that indeed B. holstii functions as an alternate host for P. graminis in East Africa. The extent to which the sexual cycle generates new races of Pgt in Kenya is, however, not clear.
There are two main methods of dispersion of Pgt, by human-mediated travel and by wind. Introduction of exotic rust races via human-mediated travel is rare, but documented cases include the incursion of stripe rust (P. striiformis f. sp. tritici) into Australia from Europe in 1979 (Wellings et al. Citation1987). Since airplane travel has become more commonplace, it is likely that exotic races of rust will be moved by this means more frequently. However, the most common method of dispersal is by wind. Stem rust urediniospores are well-suited for wind dispersal. They are dark-coloured and can tolerate exposure to ultraviolet light, are adapted to a wide humidity and temperature range, and are carried in wind currents at altitudes up to 4.5 km (Visser et al. Citation2019). Urediniospores are rapidly dispersed over short (field to field) and medium (across state/provincial and/or country borders) distances on prevailing winds. In the East African Rift Valley region, airborne dispersal of Pgt has been studied, with northerly movement from June to August, followed by southerly movement from October to December (Meyer et al. Citation2017). The same authors showed that for Kenya, inoculum movement to Ethiopia is frequent in August and September, and is very frequent to Uganda, which predominantly is a spore receptor country.
Currently, there are 13 known variants in the Ug99 lineage (Fetch et al. Citation2016), which have migrated to most countries in east Africa, to Egypt, and to South Africa (Bhavani et al. Citation2019). Additionally, Pgt urediniospores can (rarely) migrate at an intercontinental scale across oceans. There have been at least three documented introductions of novel Pgt races into Australia that have been attributed to long-distance migration of urediniospores from Africa (Watson and de Sousa Citation1982; Park Citation2007; Visser et al. Citation2019). Because races of the Ug99 lineage have now been found in South Africa (Pretorius et al. Citation2010, Citation2012; Visser et al. Citation2011), this poses a direct threat of exotic introduction into Australia. Ug99 also migrated to Yemen in 2006 (Singh et al. Citation2008), and to Iran in 2007 (Nazari et al. Citation2009), attributed to north-easterly wind currents (Singh et al. Citation2011).
History of research in Kenya
The first extensive production of wheat in Kenya dates back to the start of the twentieth century, when Lord Delamere grew about 1200 acres of wheat in the Njoro region (Pinto and Hurd Citation1970). Stem rust and stripe rust infection was severe, with repeated epidemics occurring. Thus, in 1906 G. W. Evans was hired as the first wheat breeder in Kenya. Crosses to improve rust resistance began in 1910, using Italian cultivar ‘Rieti’, ‘Egyptian 3ʹ, Australian cultivars ‘Thew’ and ‘Nut Cut’, and ‘Red Fife’ from Canada (Dixon Citation1960). Evans died in 1914 and was replaced by W. J. Dowson, the government mycologist. The first wheat cultivar released was in 1920, when ‘Equator’ with adaptation to high altitude was selected from a field of Australian wheat at Njoro (Dixon Citation1960). G. J. L. Burton succeeded Dowson in 1921, and using selections from crosses between ‘Red Egyptian’, ‘Marquis’, ‘Equator’, ‘Red Fife’ and ‘White Fife’, released ‘Kenya Droop’ (1924), ‘Kenya Governor’ (1925), and ‘Kenya Standard’ (=B286; 1930).
Significant progress towards breeding for rust resistance was made with the relocation of research at the Scott Laboratories in Nairobi to the new Plant Breeding Station in Njoro in 1927 (Pinto and Hurd Citation1970). At the same time, an assistant wheat breeder (R. J. Lathbury) and mycologist (J. McDonald) were hired. Shortly after the release of ‘Kenya Governor’, which was resistant to stem rust when it was released, it was attacked by stem rust. Thus, in 1927 McDonald began the first stem rust virulence research in Kenya (Guthrie Citation1966). McDonald found two races using the Stakman differentials (Stakman et al. Citation1962), designated as K1 (International [Stakman] race 21, virulent on ‘Kenya Governor’) and K2 (race 17, avirulent on ‘Kenya Governor’). Because the only Stakman line that was useful in differentiating virulence in Kenya at that time was ‘Einkorn’, this line was used along with local Kenyan cultivars to develop the Kenyan differential series (). Cultivar ‘Reliance’ (Sr5), introduced into Kenya in 1930, was found to be susceptible to a new race designated as K3 (race 34) (Lathbury Citation1938). In 1931, a new cultivar (‘Kenya Standard’; = B286) was attacked by yet another new race K4 (race 116). Subsequently, in 1936 race K5 (race 21) that differed from K1 (also race 21) in being virulent on line ‘Kenya 58ʹ (Sr6) was identified.
Table 2. Description of ‘K’ races of Puccinia graminis f. sp. tritici in Kenya from 1928 to 1957
McDonald departed the Njoro station in 1937, and in 1938 H. C. Thorpe joined the station as a plant breeder. In addition to his duties as plant breeder, Thorpe and others also conducted rust race typing as needed. The advent of World War II and the British declaration of war on Germany in 1939 greatly interrupted research at the Njoro station. Fortunately, the wheat cultivar ‘Kenya 117A’ was released to growers in 1938 and provided adequate protection against stem rust during the 1940s. ‘Kenya 117A’ was resistant to new races K6 in 1940 (race 107) and K7 in 1943 (race unknown, appears to be similar to K6) (Thorpe Citation1949). After the war ended, a number of new cultivars derived from ‘Kenya 117A’ were released, and most had the same genetic basis of stem rust resistance, viz. genes Sr7a, Sr9b, and Sr10. This led to a rapid breakdown in resistance, and 12 new races appeared, beginning with K8 (race 24) in 1948 to K19 (race 189) in 1957 () (Thorpe Citation1958; Guthrie Citation1966). G. E. Dixon was hired in 1952 as a plant breeder to join Thorpe, and was responsible for developing cultivars with stem rust resistance from exotic sources (Pinto and Hurd Citation1970).
With the onset of several new virulent races of Pgt, a new plant pathologist (E. J. Guthrie) was hired in 1957. Guthrie replaced the Kenyan differential series with the Stakman lines, plus three Kenyan cultivars as supplemental lines. This was increased to 16 supplemental lines in 1963 (Guthrie Citation1966). Guthrie identified races 295, 296, and 297 in years 1959, 1960, and 1961, respectively (Stakman et al. Citation1962). In the 1950s, attention to wheat quality also became important in Kenya, with several plant breeders joining the wheat breeding effort beginning in 1954 (Pinto and Hurd Citation1970). Efforts to introgress new sources of stem rust resistance began in 1955, using genotypes such as Frontana-Kenya 58-Newthatch (Sr5, Sr6, Sr7a, Sr8a, Sr9b), Mida-McMurachy-Exchange (Sr5, Sr6, Sr23), and CI 12632 (Sr36) (Dixon Citation1960). Because ‘Frontana’ is known to have the Sr57 adult-plant resistance (APR) gene, this gene may have been introduced into Kenyan germplasm from this source. Additionally, in 1961 the International Wheat Rust Testing Centre (IWRTC) was established in Njoro and supported by the Rockefeller Foundation to evaluate stem rust resistance in wheat germplasm, headed by R. Little who was hired as a plant breeder in 1966 (Pinto and Hurd Citation1970). Germplasm from wheat breeders from various countries, CIMMYT, FAO, USDA (International Spring Wheat Rust Nursery), and the World Wheat Collection was evaluated for rust resistance. The IWRTC was suspended in 1970 when Rockefeller support was withdrawn and renamed as the International Nurseries at the Njoro Plant Breeding Station. The International Nurseries were run by various Kenyan scientists, with help from CIMMYT who continued to provide germplasm to select for rust resistance.
Canadian contributions (1956–1984)
One of the most important scientists in early research in identifying stem rust resistance in wheat was Douglas R. Knott from the University of Saskatchewan in Canada. Knott, along with R. Glenn Anderson (who later became a plant breeder with the Canada Department of Agriculture and subsequently went to El Centro Internacional de Mejoramiento de Maiz y Trigo [CIMMYT] to work with Norman Borlaug), identified genes Sr6 and Sr7(a) in ‘Kenya 58ʹ, Sr8(a) and Sr9(a) in ‘Red Egyptian’, Sr9(b) and Sr10 in ‘Kenya 117A’, and Sr11 in ‘Lee’, ‘Gabo’, and ‘Timstein’ (Knott and Anderson Citation1956). Initially the Sr9 alleles were not named, but Knott and Anderson noted that there was no segregation in a Kenya 117A/Red Egyptian cross. Further work by Knott with G. J. Green (Rust Lab in Winnipeg) along with I. A. Watson and A. T. Pugsley in Australia confirmed that there were at least two alleles at the Sr9 locus, with Sr9a in ‘Red Egyptian’ and Sr9b in ‘Kenya 117A’ (Green et al. Citation1960). In a series of studies of rust resistance in Kenyan wheat cultivars from 1956 to 1962, Knott did not identify any additional new Sr genes from those found in 1956 (Knott Citation1962a). However, Knott later identified genes Sr13 and Sr14 in the hexaploid wheat ‘Khapstein’, which were transferred from the tetraploid emmer wheat ‘Khapli’ by W. L. Waterhouse in Australia (Knott Citation1962b). Knott also produced a translocation line using ‘Chinese Spring’ and an Agropyron elongatum line (PW327) using irradiation with gamma rays, X-rays, and thermal neutrons (Knott Citation1961), that ultimately led to the discovery of gene Sr26 (McIntosh et al. Citation1977). Similarly, in 1966 Knott along with D. Sharma produced another wheat-Agropyron line using ‘Agrus’, which was known to contain leaf rust resistance (Sharma and Knott Citation1966). This led to the cultivar ‘Agatha’, which was shown to possess Sr25 linked to Lr19 on chromosome 7D (McIntosh et al. Citation1977). Knott also characterized genes Sr1 and Sr2, renaming Sr1 to Sr9d based on allelism tests to Sr9a from ‘Red Egyptian’, and locating Sr2 to chromosome 3B using Chinese Spring/Hope substitution lines (Knott Citation1971). Finally, Knott and R. A. McIntosh in Australia characterized stem rust resistance in ‘Webster’ and ‘Festiguay’, located on chromosome 5D, and named the gene Sr30 (Knott and McIntosh Citation1978).
By the mid 1960s it became clear to the wheat breeders in Kenya that they required assistance with the ongoing stem rust problems. From 1955 to 1965 a total of 62 cultivars were released, mostly with poor baking quality and susceptibility to stem rust soon after release (Pinto and Hurd Citation1970). Because Canadian scientists had already developed extensive knowledge in stem rust resistance genes and breeding for rust resistance in wheat, they were sought out to help with the stem rust problem. Beginning in 1966, Canada sent plant pathologists, breeders, cereal chemists, and agronomists to work with Kenyan scientists (Kenya gained independence from Britain in 1963) under a 10-year agreement funded by the Canadian International Development Agency (CIDA). The overall objective was to develop and release high-yielding wheat varieties adapted to a wide range of growing conditions, with disease (especially stem rust, stripe rust and leaf rust) resistance and improved milling and baking quality. Facilities (, 4) in Njoro were upgraded, and field and lab equipment and growth cabinets were sent for the scientists to conduct their work. Plant pathologists W. F. Hanna (1966–68), G. J. Green (1968), J. W. Martens (1968–1969), D. E. Harder (1969–73), W. McDonald (1971), and L. Piening (1973–1975) worked to characterize virulence in the Kenyan Pgt population using a new set of differential lines and new nomenclature system (see Virulence section below). Plant breeders R. F. Peterson (1966–68), E. A. Hurd (1967–70), L. E. Evans (1968–69), K. W. Buchannon (1969–1971), R. M. DePauw (1970–72), S. B. Helgason (1971–72), H. H. Muendell (1972–73), T. F. Townley-Smith (1972–74), and K. G. Briggs (1974–75) worked to introgress rust resistance into Kenyan germplasm, and to select for adapted lines with improved yield, milling, and baking quality. Cereal chemists A. O. G. Whiteside (1970) and J. Watson (1971) established milling and gluten strength testing capability. Agronomists A. Martin (1970–71) and A. McMillan (1972–72) conducted macro and micro-nutrient soil fertility trials. W. B. Cormack (1970–73) was team leader.
Fig. 3 (Colour online) Aerial view of bird cages, inoculation building, and greenhouse in 1973. Photo courtesy of Dr. T. F. Townley-Smith

Fig. 4 (Colour online) Plant breeding crosses performed inside bird cages in 1973. Photo courtesy of Dr. T. F. Townley-Smith

The methodology to develop rust-resistant wheat with improved yield and baking quality was first designed by Hurd, Evans, Hanna, and Green. Using a combination of growth cabinets, rust-testing chambers, long-rain and short-rain season field nurseries, and off-season nurseries in irrigated bird-proof cages, it became possible to develop and release new varieties, such as ‘Kenya Nyati’, in as little as four years (Hurd et al. Citation1969 and ). In 1966 the CIMMYT line II.8582–9M-R-2M-1R was selected in the Njoro nursery and released as the variety ‘Romany’. Both ‘Romany’ and ‘Tobari 66ʹ were high-yielding, photoperiod insensitive, semi-dwarf, and adapted to Kenyan growing conditions, thus Canadian breeders used them as recurrent parents in a partial backcross programme. ‘Romany’ also had tolerance to the acid soils of the Eldoret region but had insufficient straw strength. ‘Tobari 66ʹ had good milling and gluten strength characteristics whereas ‘Romany’ had weak gluten strength. When the wheat breeding work began in 1966, the resistance genes present in Kenyan germplasm (Sr5, Sr6, Sr7a, Sr8a, Sr9a, Sr9b, Sr10) and Sr36 from Triticum timopheevii were not effective against race EA4 that was common in Kenya (Green et al. Citation1970), while both ‘Romany’ and ‘Tobari 66ʹ were resistant to EA4 (Evans et al. Citation1969). By 1968, race EA5 was the most common in Kenya and was virulent on ‘Romany’ (Green et al. Citation1970). In subsequent research, ‘Tobari 66ʹ became stem rust susceptible in Kenya due to race EA8 (Evans et al. Citation1969). Thus, an elite panel of 38 lines was evaluated in 1968 at both the seedling stage and in field nurseries to find useful sources of stem rust resistance (Evans et al. Citation1969). Results identified 14 lines with both seedling and field resistance to prevalent Pgt races in Kenya. Most resistant lines had an unknown combination of Sr genes, but two lines had only a single effective gene, Agent (Sr24) and WRT238.5/Pb (Sr27). The lines CI8154/2*Frocor and Wisconsin-245/II-50-17 were two of the 14 that were identified with both seedling and field resistance, and were used by Canadian breeders in their partial backcrossing programme. Interestingly, gene Sr25 (‘Agatha’, T4) was effective at the seedling stage but did not provide protection in the field in 1968, while five lines (‘Conley’, ‘Hope’, ‘Kenya Page’, ‘Africa Mayo’, H44-24) that were susceptible to most races at the seedling stage were resistant in the field. Lines with post-seedling resistance (=adult plant resistance; APR) were found to tolerate the high stem rust pressure in Kenya, while most resistant cultivars only lasted about 4.4 years before succumbing to stem rust (DePauw and Buchannon Citation1975). This was attributed to strong selection pressure on large and variable populations of Pgt when single Sr genes were deployed.
Table 3. Wheat cultivars released in Kenya as part of the Canadian International Development Agency (CIDA) project
Many Kenyan cultivars were released from 1973 to 1989 based on the partial backcross programme using either ‘Romany’ or ‘Tobari 66ʹ as recurrent parents (). The wheat line CI8154/2*Frocor that was found by Evans et al. (Citation1969) to have effective stem rust resistance in Kenya was used in many crosses. With the departure of the Canadian scientists in 1975 when the CIDA project finished, the wheat breeding work was led by Kenyan scientists. A number of Kenyan scientists were trained with the Canadian group during the CIDA project. Additionally, upgrades had been made to the Njoro station and much field equipment was left behind. Wheat cultivars were released from the Kenya Agriculture Research Institute (KARI) National Plant Breeding Research Center (currently Kenya Agriculture and Livestock Research Organization [KALRO]), beginning in 1979 (http://wheatatlas.org/country/varieties/KEN/0?AspxAutoDetectCookieSupport=1). Some of the first resistant cultivars, such as ‘Kenya Tembo’, ‘Kenya Nungu’, and ‘Kenya Fahari’ were cycled back into the breeding programme as parents that resulted in another set of cultivars such as ‘Kenya Kulungo’, ‘Kenya Popo’, ‘Kenya Nyumbu’, ‘Kenya Kima’, and ‘Kenya Tumbili’. Cultivars and germplasm from CIMMYT also were used in crosses to improve rust resistance, or sometimes released directly. In 1980, CIDA provided funding for a second group of Canadian scientists to assist the Kenya wheat breeding programme. Led by E. A. Hurd (1980–84), assistance was provided by breeder K. G. Briggs (1984–84), pathologists W. McDonald (1982–84) and W. P. Skoropad (1983–84), agronomists E. Stobbe (1981–82) and W. Toews (1982–84), physiologist R. Hill (1983–84), and soil scientist A. Riddley (1980–82).
Deployment of gene Sr31
The CIMMYT cultivar ‘Kavko’, which contains the gene Sr31 derived from ‘Kavkaz’, was released as cultivar ‘Kenya Pa’a’ in 1982. This appears to be the first Kenyan cultivar with Sr31, but it was not grown widely. The next cultivar with Sr31 also derived from ‘Kavkaz’ was ‘Kenya Kwale’ (=’Kinglet’) in 1987. Subsequently, cultivar ‘Duma’ with Sr31 derived from ‘Aurora’ was released in 1993 and was grown on significant acreage. In 1999, ‘Duma’ was grown on 35 600 hectares (http:wheatatlas.org/varieties/detail/21888), but rapidly declined due to the evolution of Ug99 with virulence to Sr31.
Discovery of Ug99 isolate in 1998 and research from 1999 to 2004
Following higher than expected stem rust scores on the wheat rust nursery planted at Kalengyere, Uganda in 1998, R. P. Singh from CIMMYT requested on 29 January 1999 (email message to W. Wagoire, T. Payne and J. Huerta-Espino) for field samples to be sent to South Africa for virulence characterization. Because several wheat lines in the nursery were suspected to contain the Sr31 resistance gene, there was concern that the gene may have succumbed to a new Pgt race. The Kalengyere Research Station near Kabale in the south-western corner of Uganda is located at an altitude of 2450 m above sea level and characterized by two rainy seasons per year. According to Kankwatsa et al. (Citation2002), the mean minimum and maximum temperatures at Kalengyere from August to December 1998 were 11°C and 22°C. The average monthly rainfall and relative humidity during these months were 82.9 mm and 89.1%, respectively (Kankwatsa et al. Citation2002).
In liaison with the CIMMYT office in Addis Ababa, Ethiopia, Wagoire dispatched stem rust-infected stems from seven entries () to the University of the Free State in Bloemfontein on 5 February 1999. Rusted entries containing’ Kauz’ in their parentage were of special interest as they most likely carried Sr31 (www.wheatatlas.org/). In general, the stem collections were dry and in poor condition when they arrived in Bloemfontein. Urediniospores were collected in bulk from infected stems using a cyclone collector and sprayed onto seedlings of the Sr31 wheat cultivar ‘Gamtoos’ (= Veery #3). The South African Pgt race BPGSC, known to be avirulent for Sr31, was used as a side-by-side control. Urediniospores from a single large sporulating pustule () originating from the Ugandan sample were collected and bulked in a second round of inoculation before infecting a panel of Sr31 carriers. Infection types on these wheats ranged from ‘3–3’ to ‘4’ (Pretorius et al. Citation2000), and full susceptibility of ‘Kavkaz’, Federation4*/Kavkaz, Bobwhite S, and Alondra S (), all known to have Sr31 (McIntosh et al. Citation1995), confirmed virulence for Sr31. The control race BPGSC produced a ‘1’ infection type in all experiments.
Table 4. Stem rust-infected wheat lines from the PC YR/LR nursery at Kalengyere, Uganda sent to South Africa for race analysis in 1999
Fig. 5 (Colour online) The actual single pustule (right) sampled from a Veery #3 wheat seedling for phenotypic description of original Pgt-Ug99 isolate in 1999. The leaf on the left represent a control isolate avirulent to Sr31.
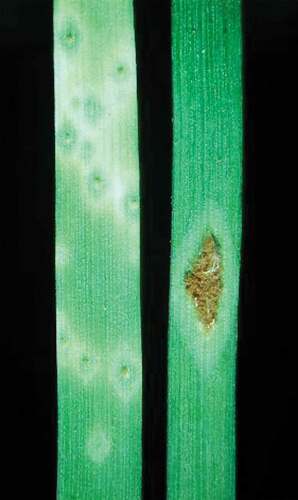
Fig. 6 (Colour online) Confirmation of virulence in isolate Pgt-Ug99 for Sr31 on the wheat genotypes Kavkaz, Federation*4/Kavkaz, Bobwhite ‘S’ and Alondra S

The original single pustule-derived isolate was named Pgt-Ug99 (Pretorius et al. Citation2000), but was soon abbreviated to ‘Ug99’ in communications about East African stem rust. It should be emphasized that this notation does not reflect any formal race nomenclature system as, for example, its TTKSK North American 5-character code (Jin et al. Citation2008). However, ‘Ug99’ has become entrenched in the scientific and popular literature and will probably continue to remain as such in the future. In addition, the isolate was named according to the pathogen, country of origin, and actual year of field sample collection. Virulence for Sr31 was suspected based on field scores taken in 1998, but the race was not officially characterized until 1999.
Further tests of available differential and tester lines indicated avirulence for Sr21, Sr22, Sr24-27, Sr29, Sr32-36, Sr39, Sr40, Sr42-43, Agi and Em, and virulence for Sr5-6, Sr7b, Sr8a, Sr8b, Sr9b, Sr9e, Sr9g, Sr11, Sr15, Sr17, Sr30-31, and Sr38 (Pretorius et al. Citation2000). The classification of avirulence for Sr21 should also be put into perspective. In the first description of Ug99 in South Africa, the Triticum monococcum accession ‘Einkorn’ was the only source of Sr21 available. In follow-up tests using the differential line CS_T.mono-deriv. line containing Sr21 in the hexaploid ‘Chinese Spring’ background, the gene was ineffective (Jin et al. Citation2007).
Little is known about Ug99 in the first years after its description. Singh et al. (Citation2008) stated that the race may have been present in Kenya since 1993. The CIMMYT expert panel report (CIMMYT Citation2005) mentioned the regular occurrence of stem rust in Kenya in 1996, 1999, 2000, and 2002–2004, and Singh et al. (Citation2008) commented that Ug99 was present at several locations in Ethiopia in 2003. However, the identity of the race(s) responsible for these outbreaks is not known as no formal race analysis was done during this period.
‘Sounding the Alarm’
On May 29, 2005, a report was released from CIMMYT that assessed the impact of Ug99 in Kenya and Ethiopia (from which it had been found) and its potential to spread (CIMMYT Citation2005). As a result of this report, the Global Rust Initiative (GRI) was formed and spearheaded by Norman Borlaug. Participants included people from CIMMYT, KARI, Ethiopia, International Center for Agriculture Research in Dryland Areas (ICARDA), Cornell University, Agriculture & Agri-Food Canada, USDA-ARS Cereal Disease Laboratory, Plant Breeding Institute (PBI) Cobbitty in Australia, Sensako in South Africa, Research Institute for Plant Protection in the Czech Republic, and the Instituto Nacional de Investigación Agropecuaria in Uruguay. A meeting was held in Australia later in 2005 that brought together international experts to review the global landscape of cereal rust control (Park et al. Citation2007). The timing of this meeting was a fortuitous coincidence, it had been planned for several years as a response to concerns of a world-wide decline in expertise in rust pathology and genetics amidst the ongoing global threat of rust to cereal production.
The GRI was renamed to the Borlaug Global Rust Initiative (BGRI) in 2008, and the Durable Rust Resistance in Wheat (DRRW) project was started. The DRRW had many objectives, but the focus was to develop new wheat cultivars with resistance to the rust fungi (primarily Ug99) and to monitor the movement and evolution of new races of Pgt. Phase I of the DRRW was conducted from 2008 to 2011 and was funded ($26.8 million USD; https://news.cornell.edu/stories/2008/04/gates-foundation-awards-268m-cu-fight-deadly-wheat-plague) by the Bill and Melinda Gates Foundation (BMGF). Phase II of the DRRW ran from 2011 to 2016 and was funded from both BMGF ($25 million USD) and the United Kingdom Department for International Development (DFID; $15 million USD) (https://news.cornell.edu/stories/2011/02/40m-grant-fight-wheat-rust-threatens-food-security). An additional four years of funding (2016–2019) to build on the DRRW and focus on wheat breeding was again funded by the BMGF ($23 million USD) and DFID ($12 million USD) (http://www.globalrust.org/page/delivering-genetic-gain-wheat). Thus, from 2008 to 2019 over $100 million USD was provided to address the Ug99 threat, with additional in-kind support from advanced research institutions across the world. Pathogen phenotyping and genotyping, discovery and assessment of effective Sr genes, and chemical control efficacy were among the objectives of the DRRW and will be discussed below.
Following the ‘Sounding the Alarm’ announcement and formation of the GRI, numerous articles in popular magazines such as Cosmos, Science, Nature, The Economist, Manitoba Cooperator, New Scientist, Top Crop Manager, Wired, and Science News were published on the Ug99 threat to wheat production (). This, along with the funding provided from the DRRW to its many collaborators, spurred a vast amount of research on stem rust that had not been seen for many years. A search using the Scopus website found 298 scientific publications from 2006 to 2020 (https://www.scopus.com/search/form.uri?display=basic; accessed 24 September 2020) on Ug99 research. In Canada, a $13 million CAD project was approved from 2008 to 2013 to find and characterize new sources of resistance to Ug99 and begin incorporating new Sr genes into wheat breeding programmes. From these funds, a $2.5 million upgrade to a level 3 plant pathogen biocontainment lab was done, which was subsequently used for both the Canadian Ug99 rust resistance project and activities in the DRRW programme on surveillance of virulence in the global population of Pgt. This lab predominantly phenotyped isolates from Kenya and Eritrea for the DRRW project.
Virulence phenotyping of Pgt
Phenotyping of virulence into physiologic races (= biologic forms; biotypes) began in 1916 using 12 ‘standard differential’ cultivars, and by 1962 some 297 distinct races had been characterized (Stakman et al. Citation1962). It was stated that there is an infinite number of races, based on the selection and number of differential hosts that are used. While the basic premise of naming races based on the seedling infection type or reaction (incompatible or compatible) on host differential lines has not changed, several additional nomenclature systems for Pgt have been described. This has led to some confusion and difficulty when trying to compare stem rust virulence over time and across countries. The Stakman and Kenyan (K races) systems used sequential numbers to describe races, but these simply named races in order of discovery and did not provide a systematic means of race comparison. This method was used in Kenya from 1927, when the first stem rust race pathotyping work was started by J. McDonald, to the arrival of the Canadian scientists in 1966. The East Africa (EA) nomenclature was developed by Green in 1968 and used nine differential cultivars, which included ‘Reliance’, ‘Kota’, ‘Einkorn’, and ‘Vernal’ from the original Stakman set (Green et al. Citation1970). Subsequently, the EA system was modified by Harder, using seven of the Green lines and adding three new ones to describe the Pgt races. Harder described nine new races and speculated that somatic recombination could be responsible for the variation in virulence in East Africa (Harder et al. Citation1972). The EA nomenclature also names races in order of discovery, and thus is not a systematic method of race nomenclature.
Following the departure of the Canadians in 1975, no publications can be found on Pgt virulence in Kenya until the discovery of Ug99. Several more nomenclature systems had been developed, including the ‘C-race’ formula system in Canada, the SA system in South Africa, the combined Stakman and additional lines system in Australia, and a ‘letter-code’ International system (Roelfs and Martens Citation1988). Of these, only the letter-code system was mathematically structured, using a hexadecimal system. Additionally, with the development of differential lines that contained single Sr genes, this system described virulence on a gene-for-gene basis. Currently, virulence in Pgt is described in most countries using the letter-code system as was suggested by Fetch et al. (Citation2009), using 20 single-gene differential lines (Jin et al. Citation2008). One important point to keep in mind is that all currently described nomenclature systems use a limited number of differential lines, and thus do not fully describe the virulence of an isolate of Pgt. For example, the race TTKSK isolate (04KEN156/04) used by Nirmala et al. (Citation2017) is virulent on gene Sr8155B1, while the original Ug99 isolate that also phenotypes to race TTKSK is avirulent on Sr8155B1 (8 October 2020 email from W. H. P. Boshoff; Fetch unpublished data). Thus, there is a need to add more Sr differential lines that are useful in describing the virulence spectrum of Pgt. Genes that have been found to be useful in discriminating virulence in Pgt and are not currently used are: Sr7a, Sr8b, Sr13a, Sr13b, Sr15, Sr22, Sr25, Sr27, Sr35, Sr37, Sr42, Sr44, Sr45, Sr46, Sr48, Sr49 and Sr50 (in numerical order). At least one letter, if not two or three, could be added to the current 5-letter nomenclature to provide better characterization of virulence of isolates of Pgt. Another challenge is that many differential genetic seed stocks become mixed over time and are therefore either not pure or wrong. Thus, continual assessment of each differential line using several well-characterized races and DNA markers where available is needed to check for purity and correct phenotype. Finally, the genetic background of the differential line and temperature at the time of incubation also is critical, as both greatly influence the seedling reaction of many Sr genes (McIntosh et al. Citation1995). For example, in the original description (Pretorius et al. Citation2000) of Ug99, gene Sr15 was described as susceptible. Gao et al. (Citation2019) found that if you reduce the incubation temperature to 15–18°C, Sr15 displayed a resistant seedling IT to race TTKSK.
Another gene that is useful in virulence analyses in the east Africa region is Sr28, which is in the Stakman differential line ‘Kota’, along with Sr7b and Sr16 (Park et al. Citation2011). Huerto-Espino (Citation1992) noted a major difference in virulence to Sr28 in African countries (mean of near 50%) compared to other countries, where virulence to Sr28 mostly ranged from 90% to 100%. Races in the Ug99 lineage key out to either standard race 218 or to standard race 143 (Park et al. Citation2011), and both are avirulent to Sr28. Race 143 was found in 1953 in Kenya by Thorpe and originally designated as K14 (Thorpe Citation1958) and later in Tanzania in 1970 by Harder et al. (Citation1972), thus could be a precursor to Ug99. Unfortunately, none of the Kenyan isolates that were pathotyped by Green or by Harder were stored. Nevertheless, because virulence to Sr28 is common worldwide and several races of the Ug99 lineage are avirulent on Sr28 (Singh et al. Citation2015), gene Sr28 could be useful as a ‘thumbprint’ to detect new Ug99 variants along with Sr31, which is effective to most Pgt races worldwide but susceptible to most races within the Ug99 lineage.
Research efforts to characterize the virulence dynamics in the Pgt population in Kenya (and surrounding countries) re-emerged with the ‘Sounding the Alarm’ announcement in 2005 and subsequent funding provided by the DRRW project. Initially, only two institutions (AAFC in Canada, Cereal Disease Laboratory in USA) were conducting pathotyping and race determination of samples collected in Kenya, which expanded to field collections from surrounding countries. While facilities at PBI in Australia and UFS in South Africa perform race-typing, they were not used due to quarantine and biocontainment issues that could be addressed in Canada and the United States. Phase I of the DRRW project began in 2008, and Objective 3 was to track the wheat rust pathogens, which included pathogen race distribution of Ug99, with the information to be integrated into the Global Cereal Rust Monitoring System (GCRMS) (Park et al. Citation2011). This work was continued in Phase II of the DRRW, with Objective 23 that continued GCRMS activities. Both objectives were led by R. F. Park from PBI Cobbitty in Australia. In 2011, the Global Rust Reference Center in Denmark was expanded to include pathotyping of Pgt samples as part of Phase II of the DRRW project. Phase III research began in 2016 under the new DGGW programme, and disease surveillance activities were expanded to include additional diseases such as stripe rust, leaf rust, spot blotch, and Septoria blotch, and was led by D. Hodson of CIMMYT. While stem rust virulence dynamics in Kenya were part of Phase III, the disease research shifted to a more global aspect due to the detection of non-Ug99 races that had become important.
Field nurseries to evaluate stem rust resistance in wheat and barley germplasm, along with differential lines, began in 2005 in Njoro, Kenya. In 2005, scientists Y. Jin from USDA-ARS, R. Singh from CIMMYT, and T. Fetch from AAFC evaluated lines in the Njoro nursery. It was noted that the Sr24 differential line ‘Agent’, which was resistant to the original described strain of Ug99 (Pretorius et al. Citation2000), had large susceptible pustules. A new variant of Ug99, identified as TTKST, was subsequently identified using an additional set of differential lines (expanded from 16 to 20) that included Sr24, Sr31, Sr38, and SrMcN (Jin et al. Citation2008). In 2006 the Njoro nursery was expanded, and virulence on differential lines containing Sr36 were found by T. Fetch and Y. Jin, resulting in the third variant (TTTSK) in the Ug99 lineage (Jin et al. Citation2009). Races PTKST and PTKSK were subsequently found in 2008 and 2009, respectively, and are similar to TTKST and TTKSK but are avirulent to Sr21 in seedling tests (T. Fetch, unpublished data). These five races were dominant in the Kenyan Pgt population until 2014.
The virulence structure in the Kenya Pgt population dramatically shifted from 2013 to 2014. Five new variants were described (TTHSK, TTKTK, TTKTT, PTKTK, TTHST), three with virulence on gene SrTmp (Bhavani et al. Citation2019). New Kenyan wheat cultivars containing SrTmp to provide resistance against Ug99-type races were released, beginning in 2009 with ‘Robin’. As was previously found by Canadian researchers, virulence to new cultivars was detected within a few years of deployment, especially when only a single effective gene was used. As of 2014, there were 13 variants in the Ug99 lineage (Fetch et al. Citation2016), 10 of which were in Kenya, and ranged in distribution from Egypt to South Africa, Yemen, and Iran. In addition to races in the Ug99 lineage, two new races were detected in Kenya in 2016 (TTTTF) and 2019 (TTRTF) (T. Fetch, unpublished data). Several isolates from the 2019 Kenyan survey were virulent to gene Sr25, which is effective against Ug99 but is ineffective to TTRTF (2 March 2020 email from Y. Jin). Race TTRTF was reported in Ethiopia in 2019 (Tesfaye et al. Citation2020), apparently having migrated from Georgia where it was first detected in 2014 (Olivera et al. Citation2019).
Migration of stem rust is typically by wind, but also can rarely occur by human-mediated transport. This was summarized by Singh et al. (Citation2006), who postulated that Ug99 would likely migrate from East Africa to South Asia in a step-wise fashion (Route A), but possibly in a single long-distance event (Route B). At that time, the probability of airborne events was unknown. Recently, the likelihood of airborne dispersal of Ug99 in the Rift Valley zone to the Middle East and South Asia was reported (Meyer et al. Citation2017). Wind currents oscillate in the Rift Valley, from south to north from June to August, then southerly from October to December. Spread of stem rust from Kenya to Ethiopia was frequent, while spread from Ethiopia to Yemen was infrequent. They noted that the probability of spread from Yemen to Iran would be infrequent (Route A), and had little to no chance of continued spread to Pakistan and India unless moderate to large stem rust outbreaks occurred in Iran (Meyer et al. Citation2017). Two other interesting points were made. The first is that airflow from the Middle East towards East Africa is common, and step-wise transmission of stem rust (such as race TKTTF – not part of the Ug99 group) from Iran or Georgia to Ethiopia or Kenya is possible in one year. Race TKTTF that caused epidemic losses on ‘Digalu’ in Ethiopia in 2013–2014 (Olivera et al. Citation2015) subsequently spread to Kenya in 2017 (https://agro.au.dk/forskning/internationale-platforme/wheatrust/). The second point was that spread of stem rust by wind out of Kenya is mostly to Ethiopia and to Uganda. Uganda serves mostly as a spore receptor country (Meyer et al. Citation2017). This is interesting, in that the first report of virulence to Sr31 was from Uganda in 1998. Because Uganda is a receptor country, this would indicate that virulence to Sr31 most likely arose in Kenya prior to 1998. The wheat cultivar ‘Duma’ with stem rust resistance from gene Sr31 was released in Kenya in 1993, and was grown on significant acreage. Given the previous history of evolution of virulence in Kenya, it would be expected that virulence to Sr31 would evolve. Singh et al. (Citation2008) reported that there was evidence that Ug99 may have been present in Kenya since 1993, and thus may be the source country for this virulent race of stem rust.
There also has been discussion on long-distance dispersal of rust, i.e. why has Ug99 not spread farther than Iran? The answer may simply be that there has been very little stem rust in Iran due to dry environmental conditions that are unfavourable for stem rust infection. According to Meyer et al. (Citation2017), even a moderate stem rust epidemic (1000 hectares, >15% severity) would rarely spread rust directly to Pakistan or India. One other possible means of long-distance migration that has been documented previously would be from Africa to Australia across the Indian Ocean. Four exotic incursions of Pgt have occurred in Australia, with three pathotypes likely originating from Africa (Watson and de Sousa Citation1982; Park Citation2007). Visser et al. (Citation2019) studied the long-distance migration of stem rust spores from five regions in Africa to Australia. They found that transmission from Central South Africa (near Bloemfontein) was the most likely (Fest = 0.075, equivalent to once in about 15 years), while transmission from Tanzania was not likely. Because the Ug99-lineage race PTKST was detected in South Africa (near Greytown) in 2009 (Pretorius et al. Citation2010), there is a clear (but rare) threat of Ug99 spread to Australia. Finally, rust spores can spread via clothing (), with one documented case of exotic introduction of stripe rust into Australia from western Europe in 1979 being ascribed to this (Wellings et al. Citation1987). Thus, the spread of rust fungi by human-mediated means does occur, raising the importance of ongoing efforts to prevent transmission using quarantine at points of entry as necessary.
Genomics of Pgt
Despite the technical difficulties of conducting genetic studies with heteroecious rust pathogens like Pgt, a gene-for-gene relationship has been demonstrated, with pathogenicity (virulence/avirulence) usually being governed by single loci and avirulence being dominant (Zambino et al. Citation2000). The complexity of rust genomes (functional diploids that carry two highly heterozygous haploid nuclei) and their recalcitrance to grow on artificial media has made genomic studies difficult. Karyotypic analyses suggested that Pgt carries 18 chromosomes per haploid nucleus (Boehm et al. Citation1992).
The invention and subsequent widespread adoption of the Polymerase Chain Reaction enabled the development and application of DNA-based marker systems to study rust pathogens at the level of the genome. Co-dominant SSR markers have been developed for Pgt and used to study population structure in the USA (Stoxen Citation2012), South Africa (Visser et al. Citation2011), and Australia (Karaoglu et al. Citation2013; Zhang et al. Citation2017), providing evidence of long-term clonality, simple mutation, and somatic hybridization as mechanisms generating genetic variability.
The advent of high throughput whole genome sequencing has led to great advances in understanding the genomic architecture of Pgt. The first genome assembly for Pgt was based on 11x Sanger sequencing of the American strain CDL 75–36-700-3 (Szabo et al. Citation2014). Although this assembly had high gap content and lacked higher-order linkage between scaffolds, it proved valuable in genome-wide comparisons with several other rust pathogens that identified common features including: expansion of genes involved in cell wall modification, transport and antioxidant defence, gene loss events in metabolic pathways including those involved in nitrate and sulphate assimilation, and the presence of large repertoires of genes encoding small secreted (effector) proteins that are lineage specific (Duplessis et al. Citation2011). The most complete genome assembly for Pgt was published recently by Li et al. (Citation2019a), who combined short read sequencing and long read sequencing to generate a haplophased assembly of Australian Pgt pathotype 21–0, which was identified, characterized, and maintained by staff at the University of Sydney who regarded it as being of African origin (Luig Citation1977). This study provided evidence that Ug99 may have arisen via somatic hybridization between pathotype 21-0 and a second unknown isolate of Pgt. While in this case the hybridizational event involved simple nuclear exchange, several studies with P. graminis conducted under controlled conditions have provided evidence for more complex parasexual processes such as mitotic crossing over and vegetative haploidization (Watson Citation1957; see also Park and Wellings Citation2012).
Genome sequence information was also used to isolate the first two genes conferring avirulence for resistance from Pgt, AvrSr35 (Salcedo et al. Citation2017) and AvrSr50 (Chen et al. Citation2017). Both genes encode secreted proteins (AvrSr35, 578 amino acids; AvrSr50, 132 amino acids) that have no homology to known proteins. Salcedo et al. (Citation2017) were able to show that virulence for the resistance gene Sr35 in Pgt has emerged via non-functionalization of the AvrSr35 gene through integration of an inverted transposable element. In contrast, Chen et al. (Citation2017) found that evasion of the Sr50 resistance gene by Pgt was due to either sequence divergence in AvrSr50 or DNA insertion. These discoveries open the possibility of genomic-informed resistance breeding, in which the zygosity of Pgt for avirulence genes within an epidemiological zone is determined and the use of resistance genes for which heterozygosity exists is avoided. The use of such tests, however, needs to take into account the presence of inhibitors of avirulence genes. For example, the flax rust pathogen Melampsora lini carries inhibitors of five avirulence genes (A-M1, A-L1, A-L7, A-L8 and A-L10; Lawrence et al. Citation2007), meaning that an isolate could carry an intact Avr gene and yet be virulent on a host genotype carrying the corresponding R gene. Genome sequencing has also allowed the identification and development of SNP-based diagnostics to supplement race typing in greenhouse seedling tests. These diagnostic tests allow isolates to be placed in a clonal lineage, a group of isolates that are derived from a founding isolates via asexual means. Szabo et al. (Citation2014) reported the development of SNP diagnostics that permit rapid identification of Pgt isolates belonging to the Ug99 lineage, and discrimination between six pathotypes within this lineage.
Control of Pgt
Genetic control and breeding for stem rust resistance
Genetic control is widely accepted as the most efficient strategy to combat rust diseases. However, recurring boom and bust cycles have emphasized the importance of avoiding deployment of new cultivars with only single gene resistance, and creating more durable and complex resistance in a cultivar. Thus, improved knowledge of the genetics of resistance and more efficient selection technologies are considered essential for progressing resistance breeding. At the time of discovery of Ug99 in 1998, there were 45 catalogued stem rust resistance (Sr) loci, plus their alleles and temporary gene designations (https://wheat.pw.usda.gov/ggpages/wgc/98/pathog2.htm#Reaction to Puccinia graminis; accessed 14 October 2020). In the initial evaluation of isolate Pgt-Ug99 using 32 differential lines, virulence was found to 14 genes and avirulence to 18 genes in seedling tests (Pretorius et al. Citation2000). Subsequently, Jin et al. (Citation2007) laid the foundation with information on which resistance genes were effective and ineffective to Ug99 (isolate 04KEN156) in both seedling and field tests using most of the known numbered genes and alleles. They found virulence to 25 Sr genes, and avirulence to 17 Sr genes, with uncertain response on four genes (Sr9e, Sr14, Sr21, Sr29). Only a few of the effective genes (Sr13 in durum, Sr24, Sr36, SrTmp) were used in wheat breeding in the United States (Jin and Singh Citation2006), in Australia (Sr13 in both durum and bread wheat, Sr22, Sr24, Sr26, Sr27 [in triticale], and Sr36; McIntosh et al. Citation1995), and in India (Sr25). Following the detection of variants in the Ug99 group, an updated version of the response of catalogued and temporarily designated genes was provided by Singh et al. (Citation2015). They found virulence to 34 Sr genes but avirulence to 39 Sr genes, most of which originated from wild grass species.
Since the ‘Sounding the Alarm’ declaration in 2005, a dramatic increase of research to identify and characterize new and/or effective Sr genes to Ug99 has occurred. Virulence to Sr24 and Sr36 in Kenya was detected in 2005 (Jin et al. Citation2008) and 2006 (Jin et al. Citation2009), respectively, thus there were few (Sr13a, Sr25, SrTmp) known effective genes left in adapted germplasm. In the 2005 Njoro nursery, the Canadian bread wheat cultivars ‘Peace’ and ‘AC Cadillac’ displayed high field resistance (1R) (Fetch, unpublished data). A subsequent genetic study using DH populations RL6071/’Peace’ and ‘AC Karma’/87E30-S2B1 found that inheritance of resistance was conditioned primarily by two genes (Hiebert et al. Citation2011). One was the APR gene Sr57/Lr34/Yr18, and the other was the major gene SrCad that was located on chromosome 6DS, which can be tracked using SNP markers (Kassa et al. Citation2016). While the SrCad gene alone reduced severity by about 50%, the maximum reduction and expression of high field resistance in Njoro nurseries with Ug99 was achieved by a combination of seedling (SrCad) and APR (Sr57/Lr34/Yr18) genes and accounted for 71% of the variation in stem rust severity. A subsequent QTL study of a DH population of ‘Carberry’/’AC Cadillac’ found that the chromosome 3BS region contributed to stem rust resistance in ‘AC Cadillac’ (Singh et al. Citation2013). It is unclear as to if this is from gene Sr2 or gene Sr12, since both are on chromosome 3B and provide APR. Gene Sr12 maps near the centromere on chromosome 3B and can be detected by SNP marker NB-LRR3, which was detected in ‘AC Cadillac’ (and many other Canadian cultivars) and may be the gene providing APR, which was derived from ‘Thatcher’ wheat (Hiebert et al. Citation2016b).
Other genes in the same region on chromosome 6DS that are effective to TTKSK include Sr42 (Ghazvini et al. Citation2012), SrTmp (Hiebert et al. Citation2016a), and SrTA10187 introgressed from Aegilops tauschii (Olson et al. Citation2013). These four Sr genes can be differentiated using multiple isolates of Pgt, and thus appear to be alleles. Similarly, in the 2006 Njoro nursery the line ‘Webster’ (which has Sr30) used in Canada was resistant, while the one used by the CDL in St. Paul was not. This led to the characterization of SrWeb (Hiebert et al. Citation2010), which was subsequently located on chromosome 2BL at the Sr9 locus in ‘Webster’ and ‘Gabo’ and was renamed as Sr9h (Rouse et al. Citation2014). Babiker et al. (Citation2016) also identified Sr9h in the spring wheat landrace CItr 4311. The value of SrCad, Sr42, SrTmp, and Sr9h is that these genes are present in hexaploid wheat and thus are already in an adapted wheat background. Unfortunately, SrTmp was deployed singly in Kenya in 2009 in the cultivar ‘Kenya Robin’, and virulence to SrTmp due to races TTKTK and TTKTT was detected by 2013 (Bhavani et al. Citation2019). Similarly, Sr9h was overcome by the Ug99-related race TTKSF+, first detected as virulent for the wheat cultivar ‘Matlabas’ in South Africa (Wessels et al. Citation2019).
Since the publication of the Yu et al. (Citation2014) consensus map of Ug99 loci, several new articles aimed at discovering Ug99 resistance in hexaploid wheat have appeared. Bajgain et al. (Citation2016) used genotyping by sequencing and joint mapping to identify 59 QTL for stem rust resistance in 10 cultivars, of which 15 QTL were detected in multiple environments. Prins et al. (Citation2016) evaluated a collection of wheat lines from Africa and used GWAS to identify marker-trait associations (MTA). In two ensuing biparental populations, QTLs for Ug99 resistance were confirmed on chromosomes 3BS, 6AS, and for the Sr57 locus. QTL on chromosomes 2BS, 2DL, 3DL, and 4D were identified in the biparental studies but not in GWAS. Hiebert et al. (Citation2016b) showed that Sr12 or a closely linked gene was responsible for field resistance to stem rust in Kenya, whereas Baraibar et al. (Citation2020) discovered a QTL effective to Ug99 on chromosome 2B in South American wheat that corresponded to Sr28. Babiker et al. (Citation2015) identified a gene, assumed to be closely linked or allelic to Sr15, in the wheat landrace PI 374670.
Alien sources of stem rust resistance
Because of the broad virulence of Ug99 and its related races, many studies mining resistance in wild species also have been conducted. Wulff and Moscou (Citation2014) stated that despite the complexities of identifying and transferring rust resistance genes from wild relatives to bread wheat, several examples exist where such genes have successfully been deployed in commercial cultivars. For example, Sr24 and Sr38 have been deployed in many countries, and Sr26 has been deployed in more than 30 cultivars in Australia since it was first deployed in ‘Eagle’ in 1969 (Park Citation2007). Huang et al. (Citation2018) screened a panel of the diploid grass Aegilops longissima for rust resistance, including Ug99. They found that 81% of the accessions were highly resistant as seedlings, which corroborated previous results by Olivera et al. (Citation2007) who similarly found a high frequency of resistance to Ug99 in an Ae. sharonensis collection. Millet et al. (Citation2017) transferred stem rust resistance from Ae. sharonensis to hexaploid wheat and reported several lines with high levels of seedling and field resistance. Likewise, Yu et al. (Citation2017) identified the resistance gene Sr-1644-1Sh in Ae. sharonensis that was effective to Ug99. Further investigations of Ae. biuncialis, Ae. caudata, Ae. comosa, Ae. cylindrica, Ae. geniculata, Ae. neglecta, Ae. peregrina, Ae. triuncialis, and Ae. umbellulata also revealed a high proportion of entries resistant to Ug99 (Olivera et al. Citation2018). However, genes from wild species can be linked to unwanted characteristics, thus Niu et al. (Citation2011) developed improved lines with Sr39, derived from Ae. speltoides and effective to Ug99. Screening for Ug99 resistance also included an international triticale collection and a panel of tetraploid wheat (Olivera et al. Citation2013). Resistance to Ug99 was common in triticale and appeared to be mostly conferred by single genes. Li et al. (Citation2019b) developed translocation lines with resistance to race PTKST transferred from Thinopyrum ponticum. Using a GWAS approach, (Laidò et al. Citation2015) identified 35 resistance loci to Ug99 in a diverse T. turgidum collection. Of these, 17 QTL were promising with some assumed to be novel. Additionally, transfer of stem rust genes from wild species to hexaploid wheat can be affected by suppressors in the D genome (SuSr-D1), which are frequent in cultivated wheat (Hiebert et al. Citation2020).
Evaluation of the A and D genome ancestors of wheat has also been performed. Rouse and Jin (Citation2011b) evaluated 1061 accessions of T. monococcum and 205 accessions of T. urartu and found nearly 79% of T. monococcum and 93% of T. urartu were resistant to race TTKSK, and 55 T. monococcum accessions were resistant to races TTTTF, TRTTF, QFCSC, and MCCFC as well. A further study on inheritance of resistance of eight T. monococcum lines was conducted, and two accessions (PI 277131–2, PI 306540) were found to have new Sr genes effective to TTKSK that were not Sr21, Sr22, or Sr35 (Rouse and Jin Citation2011a). Subsequently, the two genes in PI 306540 were characterized and mapped, leading to the identification of one gene (Sr60) on chromosome 5AmS that was effective to races QTHJC and SCCSC but not effective to Ug99, and another gene (SrTm5) on chromosome 7AmL that appears to be allelic to Sr22 and is effective to TTKSK (Chen et al. Citation2018). In a study that evaluated 456 accessions of T. tauschii to races TTKSK, TRTTF, TTTTF, TPMKC, RKQQC, and QTHJC, Rouse et al. (Citation2011) found 98 with resistance to Ug99 (TTKSK), but only 12 had resistance to all six races. In addition to using bread wheat ancestors as sources of rust resistance, rye (Secale cereale) also has been used. Recently, gene Sr59, with resistance to several races of Pgt including TTKSK and TTTSK in the Ug99 lineage, was transferred to hexaploid wheat using a 2DS·2DL Robertsonian translocation (Rahmatov et al. Citation2016).
Molecular markers
In addition to discovering new Sr genes, significant progress has been made in molecular marker development and gene cloning. Yu et al. (Citation2014) assembled information from 21 studies on Ug99 resistance and provided a valuable consensus map, covering 141 loci and their linked markers. Additional publications on mapping genes effective to Ug99 since the 2014 consensus map include Sr43 (Niu et al. Citation2014), Sr45 (Periyannan et al. Citation2014), Sr46 (Yu et al. Citation2015a), Sr47 (Yu et al. Citation2015b; Klindworth et al. Citation2017), Sr56 (Bansal et al. Citation2014), SrCad (Kassa et al. Citation2016), SrTmp (Hiebert et al. Citation2016a), Sr8155B1 (Nirmala et al. Citation2017), and SrB (Mago et al. Citation2019). The latter (Sr61, formerly SrB) was recently combined with Sr26 in a single translocated segment (Mago et al. Citation2019). Babu et al. (Citation2020) summarized the genomic tools for improving rust resistance in wheat, pointing out that nine major (=seedling) and two minor (=APR) Sr genes have been cloned. These include Sr13, Sr21, Sr22, Sr33, Sr35, Sr45, Sr46, Sr50, Sr55 (=Lr67/Yr46), Sr57 (=Lr34/Yr18), and Sr60. Two further genes, Sr26 and Sr61, were isolated by Zhang et al. (Citation2020). Cloning was initially done using map-based methods, but is time-consuming as this requires extensive DNA markers, chromosome walking, and determination of the sequence without any reference. New tools to clone genes rapidly include mutant chromosome sequencing (MutChromSeq), resistance gene enrichment sequencing (mutagenesis with resistance gene enrichment sequencing; MutRenSeq), association genetics with resistance gene enrichment (AgRenSeq), and targeted chromosome-based cloning via long range assembly (TACCA). MutChromSeq is based on resistance gene knock-outs that are identified by phenotyping mutant populations with an appropriate rust isolate. The knock-outs and wild type are subjected to chromosome flow sorting to isolate the chromosome on which the target resistance gene resides, and then the specific chromosomes are sequenced and compared to find the target gene. This approach is limited to plants in which chromosomes can be sorted, and relies upon knowing an approximate map location of the gene to be isolated and having access to chromosome flow sorting facilities (Dinh et al. Citation2020).
Resistance gene enrichment sequencing (RenSeq) approaches are restricted to genes that contain NLR domains. They use a customized bait library to capture NLR genes, which are then sequenced. The RenSeq approach has been used in combination with mutagenesis (MutRenSeq) and with association genetics (AgRenSeq) to isolate several stem rust resistance genes (e.g. Sr22, Sr45, Sr46, SrTA662; Zhang et al. Citation2020). While this approach enables rapid pinpointing of resistance genes in the absence of positional mapping, it is restricted to the NLR family of resistance genes and is dependent upon a pan genome to ensure a comprehensive library of baits. Like MutChromSeq, the TACCA approach to gene cloning is based on flow sorted chromosomes of mutant and non-mutant stocks but differs by combining proximity ligation of in-vitro reconstituted chromatin (‘Chicago’) in combination with short read Illumina sequencing to generate a long-range de novo assembly of a specific chromosome (Thind et al. Citation2017).
Several recent reviews on strategies to breed for rust resistance have been published (Ellis et al. Citation2014; Pretorius et al. Citation2017; Bhavani et al. Citation2019). Bhavani et al. (Citation2019) gave a detailed account of progress in breeding for resistance to stem rust, including Ug99, at CIMMYT. As part of their current approach and resources, the establishment of highly efficient stem rust screening and shuttle breeding platforms at Njoro, Kenya, was critical in facilitating accurate phenotyping of Ug99 responses and selection of resistant lines. Over 650,000 wheat and barley entries have been evaluated at Njoro since 2005, contributing to the release of more than 100 moderately resistant or resistant wheat cultivars (Bhavani et al. Citation2019). The primary approach used at CIMMYT emphasizes race-non-specific APR genes in breeding for stem rust resistance. For example, Sr2, in combination with other APR genes, has been the mainstay of many durably resistant wheat cultivars. Huerta-Espino et al. (Citation2020) recently showed that the same association between durability and occurrence of multiple genes for slow rusting exists in a wider collection of Mexican wheats. In addition to Sr2, the pleiotropic genes Sr55, Sr57, and Sr58 have contributed to improved APR in many CIMMYT cultivars. Gene Sr57 (=Lr34/Yr18) has been found to enhance stem rust resistance in many wheat lines, e.g. SrCad alone provided a moderate level of resistance (~30% severity) to Ug99, but when Sr57 was added resistance was high (only 10% severity) (Hiebert et al. Citation2011). However, in addition to using APR genes, CIMMYT also uses genes Sr13, Sr22, Sr23, Sr25, Sr26, Sr32, Sr33, Sr35, Sr38, Sr42, Sr47, Sr50, SrHuw234, SrND643, SrNing, and SrYanac and their linked markers for stacking all-stage resistance genes in wheat (Bhavani et al. Citation2019).
In addition to approximately 90% of the wheat lines exhibiting susceptibility to Ug99 when testing in Njoro began in 2008 (Bhavani et al. Citation2019), most barley germplasm is susceptible. In a study by Steffenson et al. (Citation2017), more that 96% of the 2913 accessions of Hordeum tested to TTKSK were susceptible, and only those carrying rpg4/Rpg5 conditions a high level of all-stage resistance in barley to Ug99 in Kenya. This showed the vulnerability of barley to the Ug99 race group, thus other genes for effective stem rust resistance in barley are needed. Hatta et al. (Citation2020) transferred the cloned Sr22, Sr33, Sr35, and Sr45 wheat genes singly into barley cv. ‘Golden Promise’ using Agrobacterium-mediated transformation. They found that the transgenic barley lines displayed race-specific resistance to Pgt, thus providing a mechanism to introgress new sources of stem rust resistance into barley. The deployment of the same resistance gene in different crop species is, however, undesirable. The deployment of different resistance genes in different crops can differentiate a pathogen population into sub-populations that are specialized to each, reducing the threat of disease development in one to the other (Zwer et al. Citation1992).
Given the significant investments that have been made in resistance gene discovery, their transfer into adapted backgrounds and deployment in agriculture, and the variable nature of the stem rust pathogen, efforts towards responsible gene stewardship are critical. This lesson has unfortunately not been totally learned, as deployment of new wheat cultivars with effective single gene resistance (=boom period) with selection of a mutant with matching virulence from the pathogen population (=bust of R gene) still occurs. In Kenya, deployment of new Sr genes singly has historically resulted in defeat of these genes in about four years on average (DePauw and Buchannon Citation1975). This resulted in the use of a multiline/mixture strategy, where farmers grew several different cultivars in an effort to control stem rust infection. This strategy disappeared upon the release of CIMMYT cultivars that used Sr31, beginning with ‘Kenya Pa’a’ in 1982 and included ‘Duma’ in 1993, which soon was grown on large acreage. Unfortunately, evolution of virulence to Sr31 occurred shortly after the release of ‘Duma’ in Kenya with the discovery of Ug99 in 1998. This underscores the need for responsible deployment of major all-stage resistance genes (R genes). In order to attain enduring resistance, stacks (or pyramids) of effective Sr genes (whether R or APR) are necessary to avoid mutation of virulence to new individual R genes as they are deployed (Zhang et al. Citation2019).
There has been debate on which types of Sr genes are better to use, R genes or APR. While many plant breeders prefer to use major R genes for their ease of introgression and strong resistance response, most are race-specific and are vulnerable to evolution of new virulent Pgt races. In contrast, CIMMYT has chosen to use combinations of 4–5 APR genes to provide ‘near-immunity’ race non-specific (=durable) resistance (Singh et al. Citation2011). Ellis et al. (Citation2014) commented that not all APR genes are race non-specific or durable (see Park et al. Citation1994), but that Lr34 (=Sr57) and Sr2 can be durable and only provide partial resistance. Gene Sr2 has been described as ‘the most important gene for stem rust resistance’ (McIntosh et al. Citation1995) and is instrumental in CIMMYT (Singh et al. Citation2011) and Australian (Park Citation2007) germplasm. Gene Sr2 does not appear to be present in recently released Canadian spring wheat cultivars as assessed by the KASPar wMAS000005 marker (Toth et al. Citation2019), derived from the Mago et al. (Citation2011) csSr2 marker. However, Sr57/Lr34/Yr18 is present in many of the Canadian spring wheat cultivars and is the most important disease gene in wheat in Canada. Gene Sr12 from ‘Thatcher’ wheat is also likely present in many Canadian varieties and providing APR to stem rust (Hiebert et al. Citation2016b). Because expression of APR is usually evaluated in field nurseries, such as in Njoro, Kenya for resistance to the Ug99-lineage, it is important to also test advanced lines at the seedling stage to ascertain that no effective R genes are present and conferring the resistance observed in the field. The best gene stewardship strategy likely is to develop combinations of R and APR genes using marker-assisted selection, or should transgenic approaches to rust control in wheat be accepted, development of gene cassettes that combine multiple Sr genes into a single unit (Ellis et al. Citation2014). Luo et al. (Citation2021) reported the construction of a five-transgene cassette based on the cloned stem rust resistance genes Sr22, Sr35, Sr45, Sr50 and Sr55, its transformation into wheat, and the functionality of at least four of these genes, showing that such transgenic approaches do provide protection from stem rust.
Chemical control
In cases where wheat cultivars are susceptible to stem rust or genetic resistance unexpectedly fails, chemical control is the only short-term strategy to protect yield potential. Several studies have documented the efficacy of fungicides for the control of wheat stem rust (Mayfield Citation1985; Rowell Citation1985; Loughman et al. Citation2005; Wanyera et al. Citation2009, Citation2016; Soko et al. Citation2018). In some instances, the emphasis was on comparing yield and yield components in stem rust-infected and fungicide-protected experimental plots. In other studies, different compounds and timing of applications were investigated. It remains, however, difficult to formulate a chemical control strategy for stem rust that will apply to different production systems. This is understandable as the benefits of fungicide spray treatments on small grain crops depend on several factors including availability of registered products, efficacy and dosage of the active ingredient, crop growth stage at application, number of applications, application equipment, penetration of the canopy cover by spray droplets to reach the target areas, varietal response to rust infection, time of disease onset, environment, and epidemic potential of the pathogen. The decision to apply a fungicide can be complex. Mueller et al. (Citation2013) mentioned economics and risk as key factors in the decision-making process. Cultivar selection, production practices, yield potential, disease severity at regional and farm levels, environmental conditions, and prior experience are central to risk evaluation. Furthermore, the perception that systemic fungicides move without obstruction throughout a plant is not always true. Some compounds are upwardly systemic through xylem tissue, whereas others are only locally systemic (Mueller et al. Citation2013). An additional challenge with fungicidal control is the withholding period; chemicals cannot be applied to a crop within a minimum period of time before harvest. This can be problematic for stem rust, which does most damage towards the end of the season when crops are approaching/entering the ripening phase.
In comparing stem rust-infected wheat alongside triadimefon treatments, Rees and Syme (Citation1981) recorded yield losses of approximately 50% in the spring wheats WW15, ‘Oxley’, and ‘Condor’. Losses in ‘Celebration’ and ‘Banks’, which displayed partial resistance, were insignificant. Using triadimefon and fenapanil, Rowell (Citation1981) concluded that a single, well-timed spray was sufficient during moderate stem rust epidemics but that two applications generally gave better control. Maximum grain yield losses between frequently sprayed and rusted plots ranged from 34% to 56% over three seasons. Loughman et al. (Citation2005) reported yield losses of 10% to 45% due to natural infection of Pgt in Western Australia. Fungicides applied at the first detection of stem rust or before spike emergence provided better protection compared to later applications, indicating that the timing of applications is critical for protection during grain filling. In their experiments, tebuconazole was more consistent in reducing disease than triadimefon or flutriafol.
Wanyera et al. (Citation2009) compared nine commercial fungicides for their ability to reduce Ug99 severity on the wheat cultivar ‘Duma’. In their study conducted over two years at three Kenyan trial sites, which included the application of each fungicide at heading and flowering, azoxystrobin + cyproconazole, tebuconazole, and tebuconazole + triadimenol were the active ingredients that most consistently reduced Ug99 infection. Considering the different year x location data sets, the maximum reduction of AUDPC at Njoro (79.7%) and Eldoret (75.7%) was obtained with the azoxystrobin + cyproconazole treatment, whereas one of the tebuconazole compounds was superior in reducing AUDPC (78.4%) at Mau-Narok. The mean grain yield gain reflecting all fungicides tested exceeded 45% in four of the six trials. Subsequently, Wanyera et al. (Citation2016) investigated the effects of a trifloxystrobin + tebuconazole formulation at different growth stages and concluded that two applications, at tillering and flowering respectively, were optimal to control wheat rust. In experiments to determine the yield response of different wheats to Ug99, Macharia and Wanyera (Citation2012) found grain yield losses of 6% to 66%. In this study four applications of an azoxystrobin – cyproconazole combination, applied from stem elongation to flowering, prevented any stem rust development in the sprayed treatments. Soko et al. (Citation2018) studied yield loss in wheat cultivars with different levels of resistance to Ug99-related race PTKST. Although the objective was to determine the level of protection provided by different resistance phenotypes, their study showed that stem rust could be controlled by a product containing prothioconazole + tebuconazole. A yield loss of 47.9% was recorded in the susceptible control as opposed to 6.4% for a cultivar expressing all-stage resistance. Under the high levels of stem rust which prevailed in the trial, grain yield losses in APR entries ranged from 10.1% to 19.5%.
Several modern-day fungicides contain protective and curative properties necessary to control wheat stem rust. Success will however depend on the timely and effective application of a suitable product, most probably at disease onset followed by at least one more application at heading or flowering. Although commercial farmers generally have access to resources required for chemical control, the availability of products and application equipment, together with a knowledge of chemical crop protection and cost-benefit details, influence management actions in many parts of the world. Finally, fungicide stewardship should remain an important consideration in all chemical control programmes (Mueller et al. Citation2013).
Conclusions
According to historic reports and more recent evidence of Pgt variability and occurrence, stem rust has been and continues to be the most dangerous pathogen on wheat in Kenya. Moreover, the new races that evolve in East Africa threaten wheat production worldwide. Mutation and somatic hybridization appear to be the mechanisms that create new virulence in Pgt in Kenya, which is continual due to the presence of susceptible hosts year-round. Continued global surveillance of Pgt is needed for the early detection of new races with new virulence, but may be difficult to achieve due to lack of sustained funding. Control of Pgt can be accomplished using fungicides, but host resistance is the most economical, efficient, and environmentally conscious method. While both major and minor resistance genes have been deployed, responsible gene stewardship is needed to avoid selection pressure towards virulence to newly deployed genes. Thus, a combination of all-stage and adult-plant resistance genes is recommended to provide enduring resistance to Pgt worldwide. The next generation of rust resistance breeding may involve the use of gene cassettes, which can combine several cloned major and APR genes into a ‘cassette’, which is then introgressed into the desired wheat background using Agrobacterium-mediated transformation (Wulff and Moscou Citation2014). Currently, the use of GM cassettes would be unlikely due to the fear of genetically modified wheat. Given the increasing number of available cloned resistance genes, the use of gene cassettes would be an alternative in countries where rust infection is endemic. In any event, it is critical to deploy a combination of rust-resistance genes to avoid evolution of virulence when only single genes have been deployed, as has been done recently and in the past in Kenya.
Acknowledgements
We gratefully acknowledge the information on cultivars provided by Godwin Macharia and Joseph Nyachiro, information provided by Luis Barnola from the Canadian International Development Agency (now Global Affairs Canada), Brad Fraleigh in AAFC International Engagement, and historical background and photographs provided by Don Harder, Fred Townley-Smith, and Bill Toews. Thanks to Les Szabo and Pablo Olivera Firpo as well for providing information on worldwide Pgt virulence.
References
- Babiker EM, Gordon TC, Chao S, Newcomb M, Rouse MN, Jin Y, Wanyera R, Acevedo M, Brown-Guedira G, Williamson S, et al. 2015. Mapping resistance to the Ug99 race group of the stem rust pathogen in a spring wheat landrace. Theor Appl Genet. 128:605–297. doi:https://doi.org/10.1007/s00122-015-2456-6.
- Babiker EM, Gordon TC, Chao S, Rouse MN, Wanyera R, Newcomb M, Brown-Guedira G, Pretorius ZA, Bonman JM. 2016. Genetic mapping of resistance to the Ug99 race group of Puccinia graminis f. sp. tritici in a spring wheat landrace CItr 4311. Theor Appl Genet. 129:2161–2170. doi:https://doi.org/10.1007/s00122-016-2764-5.
- Babu P, Baranwal DK, Harikrishna PD, Bharti H, Joshi P, Thiyagarajan B, Gaikwad KB, Bhardwaj SC, Singh GP, Singh A. 2020. Application of genomics tools in wheat breeding to attain durable rust resistance. Front Plant Sci. 11:567147. doi:https://doi.org/10.3389/fpls.2020.567147.
- Bajgain P, Rouse MN, Tsilo TJ, Macharia GK, Bhavani S, Jin Y, Anderson JA. 2016. Nested association mapping of stem rust resistance in wheat using genotyping by sequencing. PLoS One. 11(5):e0155760. doi:https://doi.org/10.1371/journal.pone.0155760.
- Bansal U, Bariana H, Wong D, Randhawa M, Wicker T, Hayden M, Keller B. 2014. Molecular mapping of an adult plant stem rust resistance gene Sr56 in winter wheat cultivar Arina. Theor Appl Genet. 127:1441–1448. doi:https://doi.org/10.1007/s00122-014-2311-1.
- Baraibar S, García R, Silva P, Lado B, Castro A, Gutiérrez L, Kavanová M, Quincke M, Bhavani S, Randhawa MS, et al. 2020. QTL mapping of resistance to Ug99 and other stem rust pathogen races in bread wheat. Mol Breed. 40:82. doi:https://doi.org/10.1007/s11032-020-01153-5.
- Bhattacharya S. 2017. Deadly new wheat disease threatens Europe’s crops. Nature. 542:145–146. doi:https://doi.org/10.1038/nature.2017.21424.
- Bhavani S, Hodson DP, Huerta-Espino J, Randhawa M, Singh RP. 2019. Progress in breeding for resistance to Ug99 and other races of the stem rust fungus in CIMMYT wheat germplasm. Front Agric Sci Eng. 6:210–224. doi:https://doi.org/10.15302/J-FASE-2019268.
- Boehm EWA, Wenstrom JC, McLaughlin DJ, Szabo LJ, Roelfs AP, Bushnell WR. 1992. An ultrastructural pachytene karyotype for Puccinia graminis f. sp. tritici. Can J Bot. 70:401–413. doi:https://doi.org/10.1139/b92-054.
- Brar GS, Fetch T, McCallum BD, Hucl PJ, Kutcher HR. 2019. Virulence dynamics and breeding for resistance to stripe, stem, and leaf rust in Canada since 2000. Plant Dis. 103:2981–2995. doi:https://doi.org/10.1094/PDIS-04-19-0866-FE.
- Chen J, Upadhyaya NM, Ortiz D, Sperschneider J, Li F, Bouton C, Breen S, Dong C, Xu B, Zhang X, et al. 2017. Loss of AvrSr50 by somatic exchange in stem rust leads to virulence for Sr50 resistance in wheat. Science. 358:1607–1610.
- Chen S, Guo Y, Briggs J, Dubach F, Chao S, Zhang W, Rouse MN, Dubcovsky J. 2018. Mapping and characterization of wheat stem rust resistance genes SrTm5 and Sr60 from Triticum monococcum. Theor Appl Genet. 131:625–635. doi:https://doi.org/10.1007/s00122-017-3024-z.
- CIMMYT. 2005. Sounding the alarm on global stem rust: an assessment of race Ug99 in Kenya and Ethiopia and the potential for impact in neighboring regions and beyond. p. 26. [accessed 2020 Nov 30]. https://bgri.cornell.edu/wp-content/uploads/2020/09/SoundingAlarmGlobalRust-2005.pdf.
- DePauw RM, Buchannon KW. 1975. Postseedling response of wheat to stem rust. Can J Plant Sci. 55:385–390. doi:https://doi.org/10.4141/cjps75-061.
- Dinh HX, Singh D, Periyannan S, Park RF, Pourkheirandish M. 2020. Molecular genetics of leaf rust resistance in wheat and barley. Theor Appl Genet. 133:2035–2050. doi:https://doi.org/10.1007/s00122-020-03570-8.
- Dixon GE. 1960. A review of wheat breeding in Kenya. Euphytica. 9:209–221. doi:https://doi.org/10.1007/BF00022225.
- Dubin HJ, Brennan JP 2009. Combating stem and leaf rust of wheat. International Food Policy Research Institute Discussion paper 910. p. 33.
- Duplessis S, Cuomo CA, Linc YC, Aerts A, Tisserant E, Veneault-Fourrey C, Joly DL, Hacquard S, Amselem J, Cantarel BL, et al. 2011. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc Nat Acad Sci USA. 108:9166–9171. doi:https://doi.org/10.1073/pnas.1019315108.
- Ellis JG, Lagudah ES, Spielmeyer W, Dodds PN. 2014. The past, present and future of breeding rust resistant wheat. Front Plant Sci. 5:641. doi:https://doi.org/10.3389/fpls.2014.00641.
- Evans LE, Martens JW, Green GJ, Hurd EA. 1969. Sources of resistance to wheat stem rust in East Africa. Can J Plant Sci. 49:649–654. doi:https://doi.org/10.4141/cjps69-116.
- Fetch T, Jin Y, Nazari K, Park RF, Prashar M, Pretorius Z. 2009. Race nomenclature systems: can we speak the same language? In: McIntosh R, editor. Proceedings of the 1st BGRI Technical Workshop; Mar 17–20; Obregon, Mexico. p. 61–64.
- Fetch T, McCallum B. 2014. Increased virulence of wheat rusts and the threat to global crop production. In: Goyal A, Manoharachary C, editors. Future challenges in crop protection against fungal pathogens. New York (NY): Springer; p. 249–266.
- Fetch T, Zegeye T, Park RF, Hodson D, Wanyera R. 2016. Detection of wheat stem rust races TTHSK and PTKTK in the Ug99 race group in Kenya in 2014. Plant Dis. 100:1495. doi:https://doi.org/10.1094/PDIS-11-15-1356-PDN.
- Gao L, Babiker EM, Nava IC, Nirmala J, Bedo Z, Lang L, Chao S, Gale S, Jin Y, Anderson JA, et al. 2019. Temperature-sensitive wheat stem rust resistance gene Sr15 is effective against Puccinia graminis f. sp. tritici race TTKSK. Plant Pathol. 68:143–151. doi:https://doi.org/10.1111/ppa.12928.
- Ghazvini H, Hiebert CW, Zegeye T, Liu S, Dilawari M, Tsilo T, Anderson JA, Rouse MN, Jin Y, Fetch T. 2012. Inheritance of resistance to Ug99 stem rust in wheat cultivar Norin 40 and genetic mapping of Sr42. Theor Appl Genet. 125:817–824. doi:https://doi.org/10.1007/s00122-012-1874-y.
- Green GJ, Knott DR, Watson IA, Pugsley AT. 1960. Seedling reactions to stem rust of lines of Marquis wheat with substituted genes for rust resistance. Can J Plant Sci. 40:524–538. doi:https://doi.org/10.4141/cjps60-069.
- Green GJ, Martens JW, Ribeiro O. 1970. Epidemiology and specialization of wheat and oat stem rusts in Kenya in 1968. Phytopathology. 60:309–314. doi:https://doi.org/10.1094/Phyto-60-309.
- Guthrie EJ. 1966. Investigation into physiologic specialization in wheat stem rust. FAO information bulletin on the Near East wheat and barley improvement and production project. III(1):15–20.
- Harder DE, Mathenge GR, Mwaura LK. 1972. Physiologic specialization and epidemiology of wheat stem rust in Kenya. Phytopathology. 62:166–171. doi:https://doi.org/10.1094/Phyto-62-166.
- Hatta MAM, Arora S, Ghosh S, Matny O, Smedley MA, Yu G, Chakraborty S, Bhatt D, Xia X, Steuernagel B, et al. 2020. The wheat Sr22, Sr33, Sr35 and Sr45 genes confer resistance against stem rust in barley. Plant Biotechnol J. 19(2):273–284. doi:https://doi.org/10.1111/pbi.13460.
- Hiebert CW, Fetch TG, Zegeye T. 2010. Genetics and mapping of stem rust resistance to Ug99 in the wheat cultivar Webster. Theor Appl Genet. 121:65–69. doi:https://doi.org/10.1007/s00122-010-1291-z.
- Hiebert CW, Fetch TG, Zegeye T, Thomas JB, Somers DJ, Humphreys DG, McCallum BD, Cloutier S, Singh D, Knott DR. 2011. Genetics and mapping of seedling resistance to Ug99 stem rust in Canadian wheat cultivars ‘Peace’ and ‘AC Cadillac’. Theor Appl Genet. 122:143–149. doi:https://doi.org/10.1007/s00122-010-1430-6.
- Hiebert CW, Kassa MT, McCartney CA, You FM, Rouse MN, Fobert P, Fetch TG. 2016a. Genetics and mapping of seedling resistance to Ug99 stem rust in winter wheat cultivar Triumph 64 and differentiation of SrTmp, SrCad, and Sr42. Theor Appl Genet. 129:2171–2177. doi:https://doi.org/10.1007/s00122-016-2765-4.
- Hiebert CW, Kolmer JA, McCartney CA, Briggs J, Fetch T, Bariana H, Choulet F, Rouse MN, Spielmeyer W. 2016b. Major gene for field stem rust resistance co-locates with resistance gene Sr12 in ‘Thatcher’ wheat. PLoS One. 11(6):e0157029. doi:https://doi.org/10.1371/journal.pone.0157029.
- Hiebert CW, Moscou MJ, Hewitt T, Steuernagel B, Hernández-Pinzón I, Green P, Pujol V, Zhang P, Rouse MN, Jin Y, et al. 2020. Stem rust resistance in wheat is suppressed by a subunit of the mediator complex. Nat Commun. 11(1):1123. doi:https://doi.org/10.1038/s41467-020-14937-2.
- Huang S, Steffenson BJ, Sela H, Stinebaugh K. 2018. Resistance of Aegilops longissima to the rusts of wheat. Plant Dis. 102:1124–1135. doi:https://doi.org/10.1094/PDIS-06-17-0880-RE.
- Huerta-Espino J, Singh R, Crespo-Herrera LA, Villaseñor-Mir HE, Rodriguez-Garcia MF, Dreisigacker S, Barcenas-Santana D, Lagudah E. 2020. Adult plant slow rusting genes confer high levels of resistance to rusts in bread wheat cultivars from Mexico. Front Plant Sci. 11:824. doi:https://doi.org/10.3389/fpls.2020.00824.
- Huerto-Espino J. 1992. Analyses of wheat leaf and stem rust virulence on a worldwide basis [PhD dissertation]. St. Paul (USA): University of Minnesota.
- Hurd EA, Oggema MW, Evans LE. 1969. New emphasis in wheat breeding in Kenya. East Afr Agric For J. 35(2):213–216. doi:https://doi.org/10.1080/00128325.1969.11662396.
- Jin Y, Kolmer J, Szabo L, Rouse MN, Hovmoller MS, Singh RP, Bhavani S. 2020. Emergence and spread of new races of wheat rust fungi: continued threat to food security and prospects of genetic control. In: Ristaino JB, Records A, editors. Emerging plant diseases and global food security. St. Paul (MN): APS Press; p. 53–79.
- Jin Y, Singh RP. 2006. Resistance in U.S. wheat to recent Eastern African isolates of Puccinia graminis f. sp. tritici with virulence to resistance gene Sr31. Plant Dis. 90:476–480. doi:https://doi.org/10.1094/PD-90-0476.
- Jin Y, Singh RP, Ward RW, Wanyera R, Kinyua M, Njau P, Fetch T, Pretorius ZA, Yahyaoui A. 2007. Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 91:1096–1099. doi:https://doi.org/10.1094/PDIS-91-9-1096.
- Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch T. 2008. Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 92:923–926. doi:https://doi.org/10.1094/PDIS-92-6-0923.
- Jin Y, Szabo LJ, Rouse MN, Fetch T, Pretorius ZA, Wanyera R, Njau P. 2009. Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp. tritici. Plant Dis. 93:367–370. doi:https://doi.org/10.1094/PDIS-93-4-0367.
- Kankwatsa P, Adipala E, Hakiza JJ, Olanya M, Kidanemariam HM. 2002. Effect of integrating planting time, fungicide application and host resistance on potato late blight development in south-western Uganda. J Phytopathol. 150:248–257. doi:https://doi.org/10.1046/j.1439-0434.2002.00750.x.
- Karaoglu H, Lee CMY, Park RF. 2013. Simple sequence repeats in Puccinia graminis: abundance, cross formae speciales and intra-species utility, and development of novel markers. Australas Plant Pathol. 42:271–281. doi:https://doi.org/10.1007/s13313-013-0199-x.
- Kassa MT, You FM, Fetch TG, Fobert P, Sharpe A, Pozniak CJ, Menzies JG, Jordan MC, Humphreys G, Zhu T, et al. 2016. Genetic mapping of SrCad and SNP marker development for marker-assisted selection of Ug99 stem rust resistance in wheat. Theor Appl Genet. 129:1373–1382. doi:https://doi.org/10.1007/s00122-016-2709-z.
- Klindworth DL, Saini J, Long Y, Rouse MN, Faris JD, Jin Y, Xu SS. 2017. Physical mapping of DNA markers linked to stem rust resistance gene Sr47 in durum wheat. Theor Appl Genet. 130:1135–1154. doi:https://doi.org/10.1007/s00122-017-2875-7.
- Knott DR. 1961. The inheritance of rust resistance. VI. The transfer of stem rust resistance from Agropyron elongatum to common wheat. Can J Plant Sci. 41:109–123. doi:https://doi.org/10.4141/cjps61-014.
- Knott DR. 1962a. Inheritance of rust resistance. VIII. Additional studies on Kenya varieties of wheat. Crop Sci. 2:130–132. doi:https://doi.org/10.2135/cropsci1962.0011183X000200020013x.
- Knott DR. 1962b. The inheritance of rust resistance: IX. The inheritance of resistance to races 15B and 56 of stem rust in the wheat variety Khapstein. Can J Plant Sci. 42:415–419. doi:https://doi.org/10.4141/cjps62-068.
- Knott DR. 1971. Genes for stem rust resistance in wheat varieties Hope and H-44. Can J Genet Cytol. 13:186–188. doi:https://doi.org/10.1139/g71-033.
- Knott DR, Anderson RG. 1956. The inheritance of rust resistance.: i. The inheritance of stem rust resistance in ten varieties of common wheat. Can J Agric Sci. 36:174–195.
- Knott DR, McIntosh RA. 1978. Inheritance of stem rust resistance in ‘Webster’ wheat. Crop Sci. 18:365–369. doi:https://doi.org/10.2135/cropsci1978.0011183X001800030003x.
- Laidò G, Panio G, Marone D, Russo MA, Ficco DBM, Giovanniello V, Cattivelli L, Steffenson B, de Vita P, Mastrangelo AM. 2015. Identification of new resistance loci to African stem rust race TTKSK in tetraploid wheats based on linkage and genome-wide association mapping. Front Plant Sci. 6:1033. doi:https://doi.org/10.3389/fpls.2015.01033.
- Lathbury RJ. 1938. The appearance of a new physiologic form of stem rust in Kenya colony. East Afr Agric J. 4:183–185. doi:https://doi.org/10.1080/03670074.1938.11663859.
- Lawrence GJ, Dodds PD, Ellis JG. 2007. Rust of flax and linseed caused by Melampsora lini. Mol Plant Pathol. 8:349–364. doi:https://doi.org/10.1111/j.1364-3703.2007.00405.x.
- Li F, Upadhyaya NM, Sperschneider J, Matny O, Nguyen-Phuc H, Mago R, Raley C, Miller ME, Silverstein KAT, Henningsen E, et al. 2019a. Emergence of the Ug99 lineage of the wheat stem rust pathogen through somatic hybridisation. Nat Commun. 10:5068. doi:https://doi.org/10.1038/s41467-019-12927-7.
- Li H, Boshoff WHP, Pretorius ZA, Zheng Q, Li B, Li Z. 2019b. Establishment of wheat - Thinopyrum ponticum translocation lines with resistance to Puccinia graminis f. sp. tritici race Ug99. J Genet Genomics. 46::405–407. doi:https://doi.org/10.1016/j.jgg.2019.07.005.
- Loughman R, Jayasena K, Majewski J. 2005. Yield loss and fungicide control of stem rust of wheat. Aust J Agric Res. 56:91–96. doi:https://doi.org/10.1071/AR04126.
- Luig NH. 1977. The establishment and success of exotic strains of Puccinia graminis tritici in Australia. Proc Ecol Soc Aust. 10:89–96.
- Luo M, Xie L, Chakraborty S, Wang A, Matny O, Jugovich M, Kolmer J, Richardson T, Bhatt D, Hoque M, et al. 2021. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat Biotechnol. doi:https://doi.org/10.1038/s41587-020-00770-x.
- Macharia JK, Wanyera R. 2012. Effect of stem rust race Ug99 on grain yield and yield components of wheat cultivars in Kenya. J Agric Sci Technol A. 2:423–431.
- Mago R, Brown-Guedira G, Dreisigacker S, Breen J, Jin Y, Singh R, Appels R, Lagudah ES, Ellis J, Spielmeyer W. 2011. An accurate DNA marker assay for stem rust resistance gene Sr2 in wheat. Theor Appl Genet. 122:735–744. doi:https://doi.org/10.1007/s00122-010-1482-7.
- Mago R, Zhang P, Xia X, Zhang J, Hoxha S, Lagudah E, Graner A, Dundas I. 2019. Transfer of stem rust resistance gene SrB from Thinopyrum ponticum into wheat and development of a closely linked PCR-based marker. Theor Appl Genet. 132:371–382. doi:https://doi.org/10.1007/s00122-018-3224-1.
- Mayfield A. 1985. Efficacies of fungicides for control of stem rust of wheat. Aust J Exp Agric. 25:440–443. doi:https://doi.org/10.1071/EA9850440.
- McIntosh R, Dyck P, Green G. 1977. Inheritance of leaf rust and stem rust resistances in wheat cultivars Agent and Agatha. Aust J Agric Res. 28:37–45. doi:https://doi.org/10.1071/AR9770037.
- McIntosh RA, Wellings CR, Park RF. 1995. Wheat rusts: an atlas of resistance genes. Melbourne (Australia): CSIRO Publishing.
- Meyer M, Cox JA, Hitchings MDT, Burgin L, Hort MC, Hodson DP, Gilligan CA. 2017. Quantifying airborne dispersal routes of pathogens over continents to safeguard global wheat supply. Nat Plants. 3:780–786. doi:https://doi.org/10.1038/s41477-017-0017-5.
- Millet E, Steffenson BJ, Prins R, Sela H, Przewieslik-Allen AM, Pretorius ZA. 2017. Genome targeted introgression of resistance to African stem rust from Aegilops sharonensis into bread wheat. Plant Genome. 10:1–11. doi:https://doi.org/10.3835/plantgenome2017.07.0061.
- Mueller DS, Wise KA, Dufault NS, Bradley CA, Chilvers MI. 2013. Fungicides for field crops. St Paul (MN): APS Press.
- Nazari K, Mafi M, Yahyaoui A, Singh RP, Park RF. 2009. Detection of wheat stem rust (Puccinia graminis f. sp. tritici) race TTKSK (Ug99) in Iran. Plant Dis. 93:317. doi:https://doi.org/10.1094/PDIS-93-3-0317B.
- Nirmala J, Saini J, Newcomb M, Olivera P, Gale S, Klindworth D, Elias E, Talbert L, Chao S, Faris J, et al. 2017. Discovery of a novel stem rust resistance allele in durum wheat that exhibits differential reactions to Ug99 isolates. G3: Genes, Genomes, Genet. 7(10):3481–3490. doi:https://doi.org/10.1534/g3.117.300209.
- Niu Z, Klindworth DL, Friesen TL, Chao S, Jin Y, Cai X, Xu SS. 2011. Targeted introgression of a wheat stem rust resistance gene by DNA marker-assisted chromosome engineering. Genetics. 187:1011–1021. doi:https://doi.org/10.1534/genetics.110.123588.
- Niu Z, Klindworth DL, Yu G, Friesen TL, Chao S, Jin Y, Cai X, Ohm JB, Rasmussen JB, Xu SS. 2014. Development and characterization of wheat lines carrying stem rust resistance gene Sr43 derived from Thinopyrum ponticum. Theor Appl Genet. 127:969–980. doi:https://doi.org/10.1007/s00122-014-2272-4.
- Oerke EC. 2006. Crop losses to pests. J Agric Sci. 144:31–43. doi:https://doi.org/10.1017/S0021859605005708.
- Olivera P, Newcomb M, Szabo LJ, Rouse M, Johnson J, Gale S, Luster DG, Hodson D, Cox JA, Burgin L, et al. 2015. Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in southern Ethiopia in 2013–14. Phytopathology. 105:917–928. doi:https://doi.org/10.1094/PHYTO-11-14-0302-FI.
- Olivera PD, Kolmer JA, Anikster Y, Steffenson BJ. 2007. Resistance of sharon goatgrass (Aegilops sharonensis) to fungal diseases of wheat. Plant Dis. 91:942–950. doi:https://doi.org/10.1094/PDIS-91-8-0942.
- Olivera PD, Pretorius ZA, Badebo A, Jin Y. 2013. Identification of resistance to races of Puccinia graminis f. sp. tritici with broad virulence in triticale (×Triticosecale). Plant Dis. 97:479–484. doi:https://doi.org/10.1094/PDIS-05-12-0459-RE.
- Olivera PD, Rouse MN, Jin Y. 2018. Identification of new sources of resistance to wheat stem rust in Aegilops spp. in the tertiary genepool of wheat. Front Plant Sci. 9:1719. doi:https://doi.org/10.3389/fpls.2018.01719.
- Olivera PD, Sikharulidze Z, Dumbadze R, Szabo LJ, Newcomb M, Natsarishvili K, Rouse MN, Luster DG, Jin Y. 2019. Presence of a sexual population of Puccinia graminis f. sp. tritici in Georgia provides a hotspot for genotypic and phenotypic diversity. Phytopathology. 109:2152–2160. doi:https://doi.org/10.1094/PHYTO-06-19-0186-R.
- Olson EL, Rouse MN, Pumphrey MO, Bowden RL, Gill BS, Poland JA. 2013. Introgression of stem rust resistance genes SrTA10187 and SrTA10171 from Aegilops tauschii to wheat. Theor Appl Genet. 126:2477–2484. doi:https://doi.org/10.1007/s00122-013-2148-z.
- Park RF. 2007. Stem rust of wheat in Australia. Aust J Agric Res. 58:558–566. doi:https://doi.org/10.1071/AR07117.
- Park RF, Bariana HS, Wellings CR. 2007. Global landscapes in cereal rust control. Aust J Agric Res Special Issue. 58(6):469. doi:https://doi.org/10.1071/ARv58n6_PR.
- Park RF, Fetch T, Hodson D, Jin Y, Nazari K, Prashar M, Pretorius Z. 2011. International surveillance of wheat rust pathogens: progress and challenges. Euphytica. 179:109–117. doi:https://doi.org/10.1007/s10681-011-0375-4.
- Park RF, McIntosh RA. 1994. Adult plant resistances to Puccinia recondita f. sp. tritici in wheat. New Zealand J Crop Hort Science. 22:151–158.
- Park RF, Wellings CR. 2012. Somatic hybridisation in the uredinales. Annu Rev Phytopathol. 50(1):219–239. doi:https://doi.org/10.1146/annurev-phyto-072910-095405.
- Periyannan S, Bansal U, Bariana H, Deal K, Luo M-C, Dvorak J, Lagudah E. 2014. Identification of a robust molecular marker for the detection of the stem rust resistance gene Sr45 in common wheat. Theor Appl Genet. 127:947–955. doi:https://doi.org/10.1007/s00122-014-2270-6.
- Peturson B. 1958. Wheat rust epidemics in western Canada in 1953, 1954 and 1955. Can J Plant Sci. 38:16–28. doi:https://doi.org/10.4141/cjps58-004.
- Pinto FF, Hurd EA. 1970. Seventy years with wheat in Kenya. East Afr Agric For J. 36(sup1):1–24.
- Pretorius ZA, Ayliffe M, Bowden RL, Boyd LA, DePauw RM, Jin Y, Knox RE, McIntosh RA, Park RF, Prins R, et al. 2017. Advances in control of wheat rusts. In: Langridge P, editor. Achieving sustainable cultivation of wheat: Vol. I: breeding, quality traits, pests, and diseases. Cambridge (UK): Burleigh Dodds Science Publishing; p. 295–343.
- Pretorius ZA, Bender CM, Visser B, Terefe T. 2010. First report of a Puccinia graminis f. sp. tritici race virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. Plant Dis. 94:784. doi:https://doi.org/10.1094/PDIS-94-6-0784C.
- Pretorius ZA, Singh RP, Wagoire WW, Payne TS. 2000. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis. f. sp. tritici in Uganda. Plant Dis. 84:203. doi:https://doi.org/10.1094/PDIS.2000.84.2.203B.
- Pretorius ZA, Szabo LJ, Boshoff WHP, Herselman L, Visser B. 2012. First report of a new TTKSF race of wheat stem rust (Puccinia graminis f. sp. tritici) in South Africa and Zimbabwe. Plant Dis. 96:590. doi:https://doi.org/10.1094/PDIS-12-11-1027-PDN.
- Prins R, Dreisigacker S, Pretorius Z, van Schalkwyk H, Wessels E, Smit C, Bender C, Singh D, Boyd LA. 2016. Stem rust resistance in a geographically diverse collection of spring wheat lines collected from across Africa. Front Plant Sci. 7:973. doi:https://doi.org/10.3389/fpls.2016.00973.
- Rahmatov M, Rouse MN, Nirmala J, Danilova T, Friebe B, Steffenson BJ, Johansson E. 2016. A new 2DS·2RL Robertsonian translocation transfers stem rust resistance gene Sr59 into wheat. Theor Appl Genet. 129:1383–1392. doi:https://doi.org/10.1007/s00122-016-2710-6.
- Rees R, Syme J. 1981. Epidemics of stem rust and their effects on grain yield in the wheat WW15 and some of its derivatives. Aust J Agric Res. 32:725–730. doi:https://doi.org/10.1071/AR9810725.
- Roelfs AP, Martens JW. 1988. An international system of nomenclature for Puccinia graminis f. sp. tritici. Phytopathology. 78:526–533. doi:https://doi.org/10.1094/Phyto-78-526.
- Rouse MN, Jin Y. 2011a. Genetics of resistance to race TTKSK of Puccinia graminis f. sp. tritici in Triticum monococcum. Phytopathology. 101:1418–1423. doi:https://doi.org/10.1094/PHYTO-05-11-0133.
- Rouse MN, Jin Y. 2011b. Stem rust resistance in A-genome diploid relatives of wheat. Plant Dis. 95:941–944. doi:https://doi.org/10.1094/PDIS-04-10-0260.
- Rouse MN, Nirmala J, Jin Y, Chao S, Fetch TG, Pretorius ZA, Hiebert CW. 2014. Characterization of Sr9h, a wheat stem rust resistance allele effective to Ug99. Theor Appl Genet. 127:1681–1688. doi:https://doi.org/10.1007/s00122-014-2330-y.
- Rouse MN, Olson EL, Gill BS, Pumphrey MO, Jin Y. 2011. Stem rust resistance in Aegilops tauschii germplasm. Crop Sci. 51:2074–2078. doi:https://doi.org/10.2135/cropsci2010.12.0719.
- Rowell JB. 1981. Control of stem rust on spring wheat by triadimefon and fenapanil. Plant Dis. 65:235–236. doi:https://doi.org/10.1094/PD-65-235.
- Rowell JB. 1985. Evaluation of chemicals for rust control. In: Roelfs AP, Bushnell WR, editors. The cereal rusts, vol II distribution, epidemiology, and control. Orlando (FL): Academic Press; p. 561–569.
- Salcedo A, Rutter W, Wang S, Akhunova A, Bolus S, Chao S, Anderson N, De Soto MF, Rouse M, Szabo L, et al. 2017. Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science. 358:1604–1606.
- Sharma D, Knott DR. 1966. The transfer of leaf-rust resistance from Agropyron to Triticum by irradiation. Can J Genet Cytol. 8:137–143. doi:https://doi.org/10.1139/g66-018.
- Singh A, Knox RE, DePauw RM, Singh AK, Cuthbert RD, Campbell HK, Singh D, Bhavani S, Fetch T, Clarke F. 2013. Identification and mapping in spring wheat of genetic factors controlling stem rust resistance and the study of their epistatic interactions across multiple environments. Theor Appl Genet. 126:1951–1964. doi:https://doi.org/10.1007/s00122-013-2109-6.
- Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Bhavani S, Njau P, Herrera-Foessel S, Singh PK, Singh S, Govindan V. 2011. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu Rev Phytopathol. 49:465–481. doi:https://doi.org/10.1146/annurev-phyto-072910-095423.
- Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, Herrera-Foessel SA, Ward RW. 2008. Will stem rust destroy the world’s wheat crop? Adv Agron. 98:271–309.
- Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, Njau P, Ward RW. 2006. Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Rev: Perspect Agric Vet Sci Nutr Nat Resour 1(54):1–13. doi:https://doi.org/10.1079/PAVSNNR20061054.
- Singh RP, Hodson DP, Jin Y, Lagudah ES, Ayliffe MA, Bhavani S, Rouse MN, Pretorius ZA, Szabo LJ, Huerta-Espino J, et al. 2015. Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology. 105:872–884. doi:https://doi.org/10.1094/PHYTO-01-15-0030-FI.
- Singh RP, Huerta-Espino J, Bhavani S, Herrera-Foessel SA, Singh D, Singh PK, Velu G, Mason RE, Jin Y, Njau P, et al. 2011b. Race non-specific resistance to rust diseases in CIMMYT spring wheats. Euphytica. 179:175–186. doi:https://doi.org/10.1007/s10681-010-0322-9.
- Soko T, Bender CM, Prins R, Pretorius ZA. 2018. Yield loss associated with different levels of stem rust resistance in bread wheat. Plant Dis. 102:2531–2538. doi:https://doi.org/10.1094/PDIS-02-18-0307-RE.
- Stakman EC, Steward DM, Loegering WQ. 1962. Identification of physiologic races of Puccinia graminis var. tritici. USDA-ARS publ. E617. (Revised).
- Steffenson BJ, Case AJ, Pretorius ZA, Coetzee V, Kloppers FJ, Zhou H, Chai Y, Wanyera R, Macharia G, Bhavani S, et al. 2017. Vulnerability of barley to African pathotypes of Puccinia graminis f. sp. tritici and sources of resistance. Phytopathology. 107:950–962. doi:https://doi.org/10.1094/PHYTO-11-16-0400-R.
- Stoxen S. 2012. Population structure of Puccinia graminis f. sp. tritici in the United States [M. Sc. thesis]. The University of Minnesota. p. 75.
- Szabo LJ, Cuomo C, Park RF. 2014. The stem rust pathogen of grasses, Puccinia graminis. In: Dean RA, editor. Genomics of plant-associated fungi and oomycetes. Berlin (Heidelberg): Springer; p. 177–196.
- Tesfaye T, Chala A, Shikur E, Hodson D, Szabo LJ. 2020. First report of TTRTF race of wheat stem rust, Puccinia graminis f. sp. tritici, in Ethiopia. Plant Dis. 104:293. doi:https://doi.org/10.1094/PDIS-07-19-1390-PDN.
- Thind AK, Wicker T, Simkova H, Fossati D, Moullet O, Brabat C, Vrana J, Dolezel J, Krattinger SG. 2017. Rapid cloning of genes in hexaploid wheat using cultivar-specific long-range chromosome assembly. Nat Biotech. 8:793–796. doi:https://doi.org/10.1038/nbt.3877.
- Thorpe HC. 1949. A note on the release of some new cereal varieties. East Afr Agric J. 14(4):210–211. doi:https://doi.org/10.1080/03670074.1949.11664693.
- Thorpe HC. 1958. Wheat breeding in Kenya. In: Jenkins BC, editor. Proceedings of the 1st International Wheat Genetics Symposium; Aug 11–15; Winnipeg, MB, Canada: Public Press, Ltd. p. 55–66.
- Toth J, Pandurangan S, Burt A, Mitchell Fetch J, Kumar S. 2019. Marker-assisted breeding of hexaploid spring wheat in the Canadian prairies. Can J Plant Sci. 99:111–127. doi:https://doi.org/10.1139/cjps-2018-0183.
- USDA-ARS. 2020. Small grains losses due to rust. https://www.ars.usda.gov/midwest-area/stpaul/cereal-disease-lab/docs/small-grain-losses-due-to-rust/small-grain-losses-due-to-rust/.
- Visser B, Herselman L, Park RF, Karaoglu H, Bender CM, Pretorius ZA. 2011. Characterization of two new Puccinia graminis f. sp. tritici races within the Ug99 lineage in South Africa. Euphytica. 179:119–127. doi:https://doi.org/10.1007/s10681-010-0269-x.
- Visser B, Meyer M, Park RF, Gilligan CA, Burgin LE, Hort MC, Hodson DP, Pretorius ZA. 2019. Microsatellite analysis and urediniospore dispersal simulations support the movement of Puccinia graminis f. sp. tritici from southern Africa to Australia. Phytopathology. 109:133–144. doi:https://doi.org/10.1094/PHYTO-04-18-0110-R.
- Wanyera R, Macharia JK, Kilonzo SM, Kamundia JW. 2009. Foliar fungicides to control wheat stem rust, race TTKS (Ug99), in Kenya. Plant Dis. 93:929–932. doi:https://doi.org/10.1094/PDIS-93-9-0929.
- Wanyera R, Wamalwa M, Odemba M, Wanga H, Kinyanjui P, Onyango V, Owuoche J. 2016. Management of wheat rusts at different growth stages using Nativo 300 SC (trifloxystrobin 100g/L+tebuconazole 200g/L) fungicide. Aust J Crop Sci. 10:1273–1280. doi:https://doi.org/10.21475/ajcs.2016.10.09.p7676.
- Watson IA. 1957. Further studies on the production of new races from mixtures of races of Puccinia graminis var. tritici on wheat seedlings. Phytopathology. 47:510–512.
- Watson IA, de Sousa CNA. 1982. Long distance transport of spores of Puccinia graminis tritici in the southern hemisphere. Proc Linn Soc New South Wales. 106:311–321.
- Wellings CR, McIntosh RA, Walker J. 1987. Puccinia striiformis f.sp. tritici in Eastern Australia possible means of entry and implications for plant quarantine. Plant Pathol. 36:239–241. doi:https://doi.org/10.1111/j.1365-3059.1987.tb02230.x.
- Wessels E, Prins R, Boshoff WHP, Zurn J, Acevedo M, Pretorius ZA. 2019. Mapping a resistance gene to Puccinia graminis f. sp. tritici in the bread wheat cultivar ‘Matlabas’. Plant Dis. 103:2337–2344. doi:https://doi.org/10.1094/PDIS-10-18-1731-RE.
- Wulff BBH, Moscou MJ. 2014. Strategies for transferring resistance into wheat: from wide crosses to GM cassettes. Front Plant Sci. 5:692. doi:https://doi.org/10.3389/fpls.2014.00692.
- Yu G, Champouret N, Steuernagel B, Olivera PD, Simmons J, Williams C, Johnson R, Moscou MJ, Hernández-Pinzón I, Green P, et al. 2017. Discovery and characterization of two new stem rust resistance genes in Aegilops sharonensis. Theor Appl Genet. 130:1207–1222. doi:https://doi.org/10.1007/s00122-017-2882-8.
- Yu G, Klindworth DL, Friesen TL, Faris JD, Zhong S, Rasmussen JB, Xu SS. 2015a. Development of a diagnostic co-dominant marker for stem rust resistance gene Sr47 introgressed from Aegilops speltoides into durum wheat. Theor Appl Genet. 128:2367–2374. doi:https://doi.org/10.1007/s00122-015-2590-1.
- Yu G, Zhang Q, Friesen TL, Rouse MN, Jin Y, Zhong S, Rasmussen JB, Lagudah ES, Xu SS. 2015b. Identification and mapping of Sr46 from Aegilops tauschii accession CIae 25 conferring resistance to race TTKSK (Ug99) of wheat stem rust pathogen. Theor Appl Genet. 128:431–443. doi:https://doi.org/10.1007/s00122-014-2442-4.
- Yu L-X, Barbier H, Rouse MN, Singh S, Singh RP, Bhavani S, Huerta-Espino J, Sorrells ME. 2014. A consensus map for Ug99 stem rust resistance loci in wheat. Theor Appl Genet. 127:1561–1581. doi:https://doi.org/10.1007/s00122-014-2326-7.
- Zambino PJ, Kubelik AR, Szabo L. 2000. Gene action and linkage of avirulence genes to DNA markers in the rust fungus Puccinia graminis. Phytopathology 90:819–826
- Zhang B, Chi D, Hiebert C, Fetch T, McCallum B, Xue A, Cao W, DePauw R, Fedak G. 2019. Pyramiding stem rust resistance genes to race TTKSK (Ug99) in wheat. Can J Plant Pathol. 41:443–449. doi:https://doi.org/10.1080/07060661.2019.1596983.
- Zhang J, Zhang P, Dodds P, Lagudah E. 2020. How target-sequence enrichment and sequencing (TEnSeq) pipelines have catalyzed resistance gene cloning in the wheat-rust pathosystem. Front Plant Sci. 11:678. doi:https://doi.org/10.3389/fpls.2020.00678.
- Zhang J, Zhang P, Karaoglu H, Park RF. 2017. Molecular characterization of Australian isolates of Puccinia graminis f. sp. tritici supports long-term clonality but also reveals cryptic genetic variation. Phytopathology. 107:1032–1038. doi:https://doi.org/10.1094/PHYTO-09-16-0334-R.
- Zwer PZ, Park RF, McIntosh RA. 1992. Wheat stem rust in Australia 1969-1985. Aust J Agric Res. 43:399–431.