ABSTRACT
Corneal endothelium is the innermost layer of the cornea which has both barrier and pump function and very important to maintain cornea clarity. Unlike epithelium, endothelium does not have regenerative potential; hence, endothelial damage or dysfunction could lead to corneal edema and visual impairment. Advanced corneal transplantation which involves selective replacement of dysfunctional endothelium has led to improved and faster visual rehabilitation. But in recent times, alternative therapies in the management of corneal edema and endothelial diseases have been reported. In this review, we aim to give a comprehensive review of various strategies for the management of corneal endothelial dysfunction in order to give treatment which is precisely tailored for each individual patient. A review of all peer-reviewed publications on novel strategies for the management of endothelial dysfunction was performed. The various approaches to the management of endothelial dysfunction are compared and discussed. Shortage of human donor corneas globally is fuelling the search for keratoplasty alternatives. Corneal endothelial dysfunction can be caused following surgery, laser or corneal endothelial dystrophies which could be amenable to treatment with pharmacological, biological intervention and reverse the endothelial dysfunction in the early stages of endothelial failure. Pharmacological and surgical intervention are helpful in cases of good peripheral endothelial cell reserve, and advanced cases of endothelial cell dysfunction can be targeted with cell culture therapies, gene therapy and artificial implant. Treatment strategies which target endothelial dysfunction, especially FECD in its early stages, and gene therapy are rapidly evolving. Therapies which delay endothelial keratoplasty also are evolving like DSO and need more studies of long-term follow-up and patient selection criteria.
INTRODUCTION
Corneal endothelium is the innermost layer of the cornea, which has both barrier and pump function and very important to maintain cornea clarity.Citation1,Citation3 Corneal endothelial cells are incapable of replicating in vitro because their further replication is arrested at the G1 phase of the cell cycle. There is gradual debilitation of the endothelial cells because of the arrested cell cycle leading to decrease in both density and the number of endothelial cells with advancing age. The density of endothelial cells at birth is 3500–4000 cells/mmCitation2, gradually declining to 2000 cells/mmCitation2 by the age of 85.Citation4,Citation5 When endothelial cells are damaged, a cascade of events are initiated which leads the cells in the periphery to migrate towards the centre and form the new tight junction and finally regain the endothelial pump function. This migration is by an irregular endothelial cell which transforms into a hexagonal shape but with decrease in the density of the cells.Citation6 This process continues throughout life, but the minimum density needed to maintain a transparent and functional cornea is 500 cells/mm.Citation2,Citation7
Corneal oedema post cataract surgery and endothelial dystrophies are two most common endothelial pathologies which are treated by corneal transplantation. Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK), which comprise of selective replacement of corneal layers, are the current standard of care and preferred over penetrating keratoplasty (PK).Citation8 In pursuit of minimally invasive therapies to treat endothelial diseases, several alternative strategies have gained interest. These strategies are broadly classified into three main groups, namely, surgical techniques, pharmacological modalities and cell-based therapies.Citation9
This review focuses on the various therapies that have been explored as an alternative to the conventional management with endothelial keratoplasty.
MATERIALS AND METHODS
A search in PubMed was performed using the following keywords: endothelial dysfunction, newer development for endothelial replacement, Rho-kinase inhibitors, Descemet stripping only, and Endothelial cell culture. All the selected articles were reviewed for relevance by going through the abstract. Articles on gene therapies and artificial implant clinical trials were additionally reviewed and added.
DESCEMET STRIPPING ONLY (DSO)/DWEK
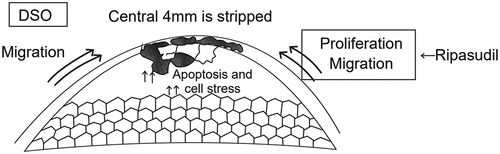
Paufique in 1955 described that Descemet stripping could be a treatment option for Fuchs endothelial corneal dystrophy.Citation10 Later many authors reported spontaneous clearance of the cornea after iatrogenic trauma. Initial reports on the outcomesCitation11–13 of this procedure were mixed. The reports suggested that a larger area of Descemet stripping of about 8 mm was most commonly associated with failure.Citation14 Price described clearing of cornea in two out of three cases with Descemet stripping of 6–6.5 mm, and one of them had residual oedema.Citation15 Later on, Borkar et al. described favourable outcomes in a DSO with a smaller area of Descemet stripping of about 4 mm ().Citation16 The outcomes of DSO are governed by several inherent characteristics and surgery-related factors. Young age,and intact Descemet membrane promote cell migration and a roughened stromal surface, or bare stroma hinders endothelial movement suggesting the impact of surgical factors on this procedure.Citation17,Citation18 Davis et al. found that when descemetorhexis woundsCitation19 were created with a peeling technique, the cells at the margin of the wound retained their morphology.Citation20 Hence, peelingCitation21–24 technique over scoring has been strongly recommended to maximize both cell preservation and migration.Citation25
Good peripheral endothelial cell reserve is cardinal for success of the DSO because it is believed that endothelial cell migration is the driving force for corneal clearance where DSO is performed. DSO cannot be performed in cases of diffuse endothelial cell damage like aphakic or pseudophakic bullous keratopathy.Citation26,Citation27 Dysfunctional endothelial cells act like a barrier to the migration of the healthy cells; hence, Descemet removal in a particular area removes the mechanical barrier and resolves the corneal edema. DSO has been performed in cases of FECD and post surgical corneal oedema.Citation26 Most of the reports suggested that endothelial cell density of 1000/mm2 is a crucial parameter for DSO because the actual reduction of peripheral cell counts in literature is reported to be 10%.
Surgical techniqueCitation25
Initially surgical preparation begins from the pre-operative area which comprises of marking the cornea at the centre of the pupil just before the peribulbar block and especially in mesopic condition to help with centration. A dilated pupil guides better for descemetorhexis. With the help of calipers, a 4-mm-diameter marking is made around the centre of the pupil to delineate the descemetorhexis. Then, a 2-mm clear corneal incision is made and the anterior chamber is filled with cohesive viscoelastic. A Reverse Sinskey hook is placed at the margin of the 4-mm circle and then with small side-to-side movements a descemet membrane tag is created and during this procedure a gentle pressure is maintained. The tag is then held with a grasping forceps and the tag is then further propagated in the form of a circle. After the descemetorhexis, the cohesive viscoelastic is thoroughly washed and the main wound is hydrated.
Clinical suggestives of healing response after surgery are clearance of oedema, honeycomb oedema (transient in nature), pseudoguttata and endothelial pigmentation.Citation25
Refractive Outcomes after DSO
According to the literature, visual acuity after DMEK and DSO has been comparable but postoperatively complicated and was noted to be higher in the DMEK group but none was long-lasting.Citation25 Visual acuity according to most of the published cohorts on DSO was between −0.12 and 0.001log Mar.Citation16 But ghosting and irregular astigmatism are known to decrease the quality of the vision despite the better-achieved visual acuity and clear cornea. This is known to be caused by the central corneal thinning and posterior stromal scarring.Citation25,Citation28
Role of ROCK Inhibitors in DSO
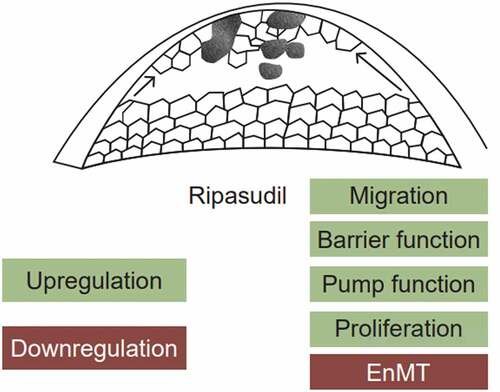
ROCK inhibitors as adjuvant in DSO help to modify the basic concept of the procedure from endothelial cell migration to endothelial cell proliferation. In DSO the central area is stripped of the dysfunctional DM and endothelium for the healthy peripheral cells to migrate, resulting in clearance of the corneal oedema, resulting in decrease in the peripheral cell count by 10% which can be addressed by the use of ROCK inhibitors ().Citation29 Endothelial wound healing with ROCK inhibitors leading to cell proliferation is maximum at the wound edge and stops immediately after the healing is complete; hence, ROCK inhibitors should be added immediately after DSO.Citation28,Citation29
Although the literature is minimum with studies of smaller size, this approach eliminates rejection, decreases corticosteroid use, has less side effects and decreases the need for long-term medical care. Another added benefit of adding ROCK inhibitors to DSO is that it extends the benefits of including candidates who were previously considered as borderline candidates for DSO only.Citation20
Current Status and Its Applications
The literature is sparse with studies of smaller sample sizes (Table 1). This approach is of potential interest in eyes with FECD with good peripheral endothelial count as it eliminates the risk of rejection, decreases corticosteroid use, has less side effects and decreases the need for long-term medical care.
RHO-KINASE INHIBITORS
Ripasudil
Rho-associated coiled-coil containing protein kinase (ROCK) inhibitors are serine/threonine kinases that act on small Rho GTPases and are involved in cytoskeleton organization, cell migration and cell-cell adhesion.Citation30 Earlier the primary application of ROCK inhibitors was to promote the efficiency of culturing endothelial cells for cell therapy purposes because they have the ability to enhance cell adhesion, curb apoptosis and promote proliferation ().Citation31–36In vivo experiments conducted on rabbits and monkey showed that after cryogenic damage to endothelium, application of topical ROCK inhibitor resulted in higher CEC density and hastened wound healing when compared with the placebo group.Citation34 ROCK inhibitors are also known to help reconstruct endothelial layer in cell-based therapies in bullous keratopathy patients.Citation37 The Rho kinase (ROCK1 and ROCK2) inhibitor ripasudil hydrochloride hydrate (Glanatec ophthalmic solution 0.4%, Kowa Co Ltd., Nagoya, Japan) was ratified in Japan in 2014 for the treatment of glaucoma and ocular hypertension. ROCK inhibitors are also used as a salvage agent for failing case of Fuchs dystrophy where DSO was done as a primary intervention. Citation38
Central endothelial cells are maximum affected in late stages of FECD, and Ripasudil can actuate cell cycle progression, hasten cell cycle progression and surge up pump and barrier function and without causing adverse phenotypic changes on the endothelial cells. ROCK inhibitors in a single dose of 30 µM are sufficient to incite and succor expression changes up to 72 hours and it independent of the age of the patient. Ripasudil is also known to cause beneficial changes in the gene and protein expression which happen in the presence of a strong inhibitory environment formed by the cell-cell contact inhibition and pathologically changed Descemet membrane and guttae. Ripasudil helps to preserve the regenerative potential and also improve the functionality of endothelial cells.Citation38–40
Netarsudil
Another popular ROCK inhibitor being used is Netarsudil which inhibits the Rho family of small protein G kinase. Netarsudil is known to decrease the oedema and improve the scotopic distance visual acuity in FECD patients. Netarsudil reportedly clears both focal and diffuse cases of corneal oedema within one month of starting the treatment. Viable endothelial cells available for proliferation and hurdles to cytoskeleton development along the endothelium are two important factors governing the clearance of corneal oedema by Netarsudil.Citation41,Citation42
Current Status and Its Applications
ROCK inhibitors help to delay the need for endothelial keratoplasty, decrease in oedema improves the surface symmetry resulting in better biometry, and this ultimately bolsters refractive outcomes of cataract surgery in patients with FECD. ROCK inhibitors reinforce the barrier function of endothelium by altering the tension of endothelial cell junction, which decreases ingress of aqueous and thus improving the corneal hydration.Citation41
CULTURED CORNEAL ENDOTHELIAL CELLS
Corneal endothelial cells have the capacity to expand in vitro, but ability to do so in vivo is limited. The first reported protocol for in vitro expansion of endothelial cells was in 1965, and ever since there have been a plethora of protocols explaining isolation and in vitro expansion of endothelial cells because it is extremely challenging to isolate and expand endothelial cells in vitro. Culture and expansion of endothelial cells are achieved by collectively tweaking the following steps: selection of suitable donor tissue, peeling of the corneal endothelium and DM from donor corneas, enzymatic digestion to isolate the hCEC, seeding of the resulting cell suspension using a combination of culture media and growth factors, and expansion and proliferation on appropriate substrates that mimic the in vivo conditions.Citation9 Cell growth is also boosted by the loss of cell contact, supplemented culture medium and growth factors.Citation43 Endothelial to mesenchymal transition is induced by the activation of intra-cellular pathways which causes the CEC to acquire a fibroblast-like phenotype, thereby losing their morphological features and function.Citation43–45Hence, a protocol to achieve isolation, expansion and propagation of CEC along with maintaining cell morphology and function needs to be developed.
3.a Cell Sources for Culture
Sources for primary endothelial cells for culture are cadaveric donor cornea, stem cells and hCEC-like cells with stem cell potential which could be obtained from adipose tissue, umbilical cord blood or bone marrow, directed differentiation competent embryonic stem cells, induced pleuripotent stem cells and hCEC precursors.Citation46–51
3.b Isolation of Corneal Endothelial Cells
Peel and digest technique is used for the isolation and expansion of endothelial cells obtained from the cadaver tissue.Citation52 Descemet membrane endothelial donor is peeled from the stroma and then cell junctions are split in order to achieve the separation of hCEC from the descemet membrane. Enzymatic and non-enzymatic are two tissue digestion strategies.Citation53 Collagenase, trypsin and dispase are used for enzymatic digestion procedures, and ethylenediaminetetraacetic acid (EDTA) helps to dissociate cell-cell junctions as a part of non-enzymatic digestion.Citation52
3.c Culture media and growth factors
Many culture media have been tried for the growth of the endothelial cells; two of the them are single and dual media approach. Peh et al. explained that dual media could be used to cultivate isolated corneal endothelial cells using a culture system, which comprises of a proliferative medium rich in growth factors and a serum supplemented culture medium without growth factors which helps to avoid epithelial-mesenchymal transition (EMT). Later a slightly modified dual media was developed to increase the yield of successful cultures from elderly donors.Citation54,Citation55
3.d Preventing transition of EMT transition of corneal endothelial cells during culture
Loss of endothelial cell phenotype as a result of EMT of hCEC during culture is a major limitation in the use of cultured hCEC in tissue engineering, which is applied for corneal regeneration. Increasing cell seeding density helps to achieve cell confluency and polarity as soon as possible during culture so that dissociation of cell junction could be avoided.Citation56
3.e Coatings
Descemet membrane is the natural coating for endothelial cell which is a basement membrane secreted by the corneal endothelium and includes proteins, such as fibronectin, laminin, collagen type IV and VIII and proteoglycans. Endothelial cells can attach and grow on bare tissue culture plates; however, there are reports that coating used in culture plates has resulted in superior results.Citation53,Citation57,Citation58 Collagen, fibronectin admixed with collagen and most popular and widely accepted coating is albumin.Citation57,Citation59–61
Basement membrane has another major component, laminin, which governs cell migration, proliferation and differentiation. A recombinant form of laminin E8 was developed which offers the option of xeno-free compliant substrate.Citation62 Following this another xeno-free substrate of pericellular matrix of decidua-derived mesenchymal cells (PCM-DM) was tested and results showed promising results as a substrate for human ES cells.Citation63
Endothelial Cell Sheet Transplantation
Corneal endothelial cells when cultured and expanded in vitro need a carrier to be placed inside the eye as they are too fragile. Ideal carrier is transparent, permeable, easily reproducible and thick enough to provide sufficient mechanical strength, flexible to adjust to the corneal curvature, biocompatible, promoting hCEC-carrier interactions and finally allowing interaction between the cultured hCEC layer and the recipient stroma in terms of exchange of nutrients and small molecules.Citation46
Potential carriers can be natural tissue materials, such as amniotic membrane, human anterior lens capsules (HALC), amniotic membrane and decellularized DM or stroma or polymeric materials (natural and synthetic), and have been investigated as carriers.Citation46,Citation64–66
Bioengineered Carriers
Natural tissue material carries the risk of biotoxicity and incompatibility, and makes it more difficult for practical uses, and hence increasing interest to avoid biological carrier has led to the development of bioengineered carrier.Citation67 There are natural polymers like collagen, gelatin and silk which possess the properties of basement membrane and are biocompatible, but synthetic polymers lead to fine customization of all the desirable properties. Thermo-responsive polymers such as poly(N-isopropylacrylamide) (PNIPAAm) and copolymers possess the ability to modulate hydrophobic and hydrophilic properties facilitating separation of the cell sheets without the need for enzymatic digestion. Temperature modulatory property of the thermo-responsive polymer needs a detailed analysis of its effect on the functionality of the hCEC is inadequate.Citation9
Cell Injection Therapy
Cultured endothelial cells can be directly injected into the anterior chamber without the need for carrier, and this concept was for the first time elaborated in rabbits. The landmark paper regarding the cell injection therapy was published in Japan by Kinoshita et al. in patient with bullous keratopathy.Citation68–70
This study comprised of 11 patients of bullous keratopathy receiving 1 × 106 cultured human CECs suspended in 300 μl of modified Opti-MEM I medium containing the ROCK inhibitor into the anterior chamber. It is important to maintain 3 hours of prone position to enhance adherence of the cultured cells to the posterior cornea. This study comprised of patients with chronic corneal oedema due to various endothelial dysfunctions (s/p Argon laser iridotomy bullous keratopathy, FECD, pseudoexfoliative bullous keratopathy, pseudophakic bullous keratopathy) and 82% of patients reported increase in the visual acuity 6 months after surgery. Cultured hCEC retained both morphology and functionality of endothelial cells. Duration required for the cornea to clear varied and factors influencing them need a more detailed analysis. At 5-year follow-up, normal corneal endothelial function was restored in 10 of 11 eyes and the mean endothelial cell density was 1257 cells/mm2 (range, 601 to 2067 cells/mm2).Citation37 Central density at 5-year follow-up was >2067 cells/mmCitation2 and increase in the proportion of hexagonal cells was also noted, indicating that cells became more biophysically stable with time than in the early post-operative period. Outcomes of cell injection therapy are determined by micro-environment, indication for endothelial dysfunction and the seeds.Citation71 Supermagnetic microspheres when added to cultured hCEC before injection into the anterior chamber and application of magnetic field to direct cells in ex vivo model proved to enhance the number of cells, which adhered to the posterior cornea.Citation72
Ong at al. reported that outcomes improved when direct cell injection and dual media approach were combined. It was noted that DM-EC tissue when stored in a stabilization medium for 48 hours then hCEC can be isolated by mild enzymatic digestion. At 3 weeks it was noted that corneal thickness had significantly decreased in the rabbit’s bullous keratopathy.Citation73
Parikumar et al. reported that human corneal endothelial precursors (hCEP) obtained from donors which are not suitable for transplantation are suitable for in vitro expansion and direct injection along with nanocomposited gel sheets which improved the adherence of the cells. This approach is safe and also alleviates the symptoms of patients with bullous keratopathy, although it still needs a larger population study.Citation74
Current Status and Its Applications
Most of the literature reported regarding cell injection therapy that involved small sample groups but none of the studies reported adverse events. However, safety issues still need to be better defined before applying this technique on a large scale. The fate of unattached cells and their effect upon entering the trabecular meshwork or the systemic circulation is not yet fully understood.Citation37
PLURIPOTENT/MULTIPOTENT STEM CELLS
Human embryonic stem cells and pluripotent cells already being thoroughly investigated in Cardiology and Neurology, but their application for the treatment of Corneal endothelial dysfunction is still an expanding horizon. Hurdle for their usage lies in the optimization of a clearly defined cell differentiation media towards a corneal endothelial cell phenotype.Citation75,Citation76 This can be addressed by developing pluripotent cell into periocular mesenchyme, which is vital for development of various cell types in the cornea. SMAD inhibition helps for further differentiation of neural crest cells into corneal endothelial cells.Citation77,Citation78 Although this appears to be a promising option, the risk of tumorogenesis does still exist and needs to be further evaluated.
ENDOTHELIAL STEM CELL
Schwalbe line cells were initially believed to be a unique sub-population of cells which lines the anterior trabecular meshwork with stem cells like characteristics.Citation79 Yam et al. described the presence of stem cells in the transition zone at the posterior limbus. The transition zone is divided into two zones: outer and inner transition zones. The inner zone is rich with progenitor marker for peripheral endothelium and project as multicellular clusters,Citation80 although this horizon needs to be investigated further.
GENETIC AND RNA APPROACHES
In the past couple of decades, there have been rapid advances in understanding trinucleotide repeat expansion disorders, especially in the field of neuromuscular degenerative condition like spinocerebellar ataxia, myotonic dystrophy and Huntington's disease.Citation81 Recently, it has been known that a significant proportion of FECD has trinucleotide repeat disease (TCF4 gene), thus making gene therapies theoretically an option for FECD. Human genome project has led to significant knowledge and techniques for the treatment of both systemic and ophthalmologic diseases. In the recent times, the development of molecular scissors specifically cleaved the diseased targets so that defective genes can be deleted, replaced or repaired in situ. Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated 9 (Cas9) nucleases are the most interesting innovations in the field of human biomedicine by removing the abnormal DNA segments directly.Citation70 mRNA transcription can be inhibited by designing ssRNA oligonucleotides, which directly attach to the promoter sequences.Citation82
ARTIFICIAL DEVICE (ENDOART)
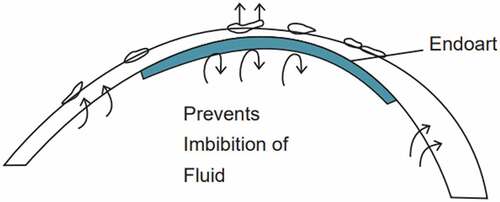
Endoart, a recently developed artificial endothelial device, was awarded Breakthrough therapy designation by the US Food and Drug Administration. It has been proven to be a suitable alternative for the conventional DMEK, thus reducing the need for donor corneas. Endoart is composed of hydrophilic acrylic material identical to the material of intraocular lens; hence, biocompatibility is already established. Endoart is 6-mm-diameter contact lens implanted with 50-μ thickness that acts like fluid barrier in the posterior cornea (). Patients with chronic corneal oedema like FECD, pseudophakic/aphakic bullous keratopathy or failed endothelial grafts are ideal candidates for this artificial implant. Auffarth et al. reported a small case series of three patients with chronic corneal edema who had endoart implantation and reported to have favourable outcomes, although a large case series and long-term outcomes need to be reported.Citation83
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996;37:645–655.
- Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002;43:2152–2159.
- Joyce NC. Proliferative capacity of the corneal endothelium overall clinical outcomes of descemet membrane endothelial keratoplasty in 600 consecutive eyes: a large retrospective case series. Indian J Ophthalmol. 2003;68(6):1044–1053. doi:10.4103/ijo.IJO_1563_19. 2020.
- Murphy C, Alvarado J, Juster R, Maglio M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci. 1984;25:312–322.
- Gambato C, Longhin E, Catania AG, Lazzarini D, Parrozzani R, Midena E. Aging and corneal layers: an in vivo corneal confocal microscopy study. Graefes Arch Clin Exp Ophthalmol. 2015;253(2):267–275. doi:10.1007/s00417-014-2812-2.
- DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598.
- Engelmann K, Bednarz J, Valtink M. Prospects for endothelial transplantation. Exp Eye Res. 2004;78:573–578.
- Das AV, Mohamed A, Chaurasia S. Recent indications of endothelial keratoplasty at a tertiary eye care center in South India. Int Ophthalmol. May 2021;22:1–9.
- Spinozzi D, Miron A, Bruinsma M, et al. New developments in corneal endothelial cell replacement. Acta Ophthalmol. 2021;99(7):712–729. doi:10.1111/aos.14722.
- Paufique. Lamellar keratoplasty. In: Rycroft BW, ed. Corneal Grafts. England: Butterworth and Co., Ltd; 1955:132–133.
- Braunstein RE, Airiani S, Chang MA, Odrich MG. Corneal edema resolution after ‘descemetorhexis’. J Cataract Refract Surg. 2003;29(7):1436–1439. doi:10.1016/S0886-3350(02)01984-3.
- Patel DV, Phang KL, Grupcheva CN, et al. Surgical detachment of Descemet’s membrane and endothelium imaged over time by in vivo confocal microscopy. Clin Exp Ophthalmol. 2004;32(5):539–542. doi:10.1111/j.1442-9071.2004.00875.x.
- Zvi T, Nadav B, Itamar K, Tova L. Inadvertent descemetorhexis. J Cataract Refract Surg. 2005;31(1):234–235. doi:10.1016/j.jcrs.2004.11.001.
- Bleyen I, Saelens IEY, van Dooren Bt, van Rij G, van Dooren BTH. Spontaneous corneal clearing after Descemet’s stripping. Ophthalmology. 2013;120(1):215. doi:10.1016/j.ophtha.2012.08.037.
- Price FW FW, Price MO. Spontaneous corneal clearance despite graft detachment after descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2010;149(1):173–174. author reply 174–175. doi:10.1016/j.ajo.2009.09.003.
- Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by descemet stripping without endothelial keratoplasty. Cornea. 2016;35(10):1267–1273. doi:10.1097/ICO.0000000000000915.
- Soh YQ, Peh G, George BL, et al. Predicative factors for corneal endothelial cell migration. Invest Ophthalmol Vis Sci. 2016;57(2):338–348. doi:10.1167/iovs.15-18300.
- Okumura N, Matsumoto D, Fukui Y, et al. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PLoS One. 2018;13(1):e0191306. doi:10.1371/journal.pone.0191306.
- Shah RD, Randleman JB, Grossniklaus HE. Spontaneous corneal clearing after Descemet’s stripping without endothelial replacement. Ophthalmology. 2012;119(2):256–260. doi:10.1016/j.ophtha.2011.07.032.
- Davies E, Jurkunas U, Pineda R. Predictive factors for corneal clearance after descemetorhexis without endothelial keratoplasty. Cornea. 2018;37(2):137–140. 2nd. doi:10.1097/ICO.0000000000001427.
- Macsai MS, Shiloach M. Use of topical Rho kinase inhibitors in the treatment of Fuchs dystrophy after descemet stripping only. Cornea. 2019;38(5):529–534. doi:10.1097/ICO.0000000000001883.
- Artieda JA, Wells M, Devasahayam RN, Moloney G. 5-year outcomes of Descemet stripping only in Fuchs dystrophy.Cornea.2020 Aug 1;39(8):1048–1051.
- Moloney G, Congote DG, Hirnschall N, et al. Descemet stripping only supplemented with topical ripasudil for fuchs endothelial dystrophy 12-month outcomes of the sydney eye hospital study Cornea 2021 Mar 1 43 320–326 10.1097/ICO.0000000000002437
- Hirabayashi KE, Mark D, Lau J, Lin CC. Descemet stripping only for a chronic descemet detachment after cataract surgery. Cornea. 2020 Mar;39(3):379. doi:10.1097/ICO.0000000000002195.
- Garcerant D, Hirnschall N, Toalster N, et al. Descemet’s stripping without endothelial keratoplasty. Curr Opin Ophthalmol. 2019;30(4):275–285. doi:10.1097/ICU.0000000000000579.
- Artieda J, Wells, Wells M, et al. 5-Year outcomes of descemet stripping only in fuchs dystrophy. Cornea. 2020;39(8):1048–1051.
- Hirabayashi KE, Mark D, Lau J, et al. Descemet stripping only for a chronic descemet detachment after cataract surgery. Cornea. 2020;39(3):379–381.
- Huang MJ, Kane S, Dhaliwal DK. Descemetorhexis without endothelial keratoplasty versus DMEK for treatment of fuchs endothelial corneal dystrophy. Cornea. 2018;37(12):1479–1483. doi:10.1097/ICO.0000000000001742.
- Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis Without Grafting for Fuchs Endothelial Dystrophy—Supplementation With Topical Ripasudil. Cornea. 2017;36(6):642–648. doi:10.1097/ICO.0000000000001209.
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nature Reviews Molecular Cell Biology. 2003;4(6):539–542. doi:10.1038/nrm1128.
- Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. investigative Opthalmology & Visual Science. 2009;50(8):3680–3687. doi:10.1167/iovs.08-2634.
- Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. the American Journal of Pathology. 2012;181(1):268–277. doi:10.1016/j.ajpath.2012.03.033.
- Okumura N, Kay EP, Nakahara M, Hamuro J, Kinoshita S, Koizumi N. Inhibition of TGF-β signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PloS One. 2013;8(2):e58000. doi:10.1371/journal.pone.0058000.
- Okumura N, Nakano S, Kay EP, et al. Involvement of cyclin D and p27 in cell proliferation mediated by ROCK inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2014;55(1):318–329. doi:10.1167/iovs.13-12225.
- Okumura N, Sakamoto Y, Fujii K, et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci Rep. 2016a;6(1):26113. doi:10.1038/srep26113.
- Okumura N, Fujii K, Kagami T, et al. Activation of the Rho/Rho kinase signaling pathway is involved in cell death of corneal endothelium. Invest Ophthalmol Vis Sci. 2016b;57(15):6843–6851. doi:10.1167/iovs.16-20123.
- Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995–1003. doi:10.1056/NEJMoa1712770.
- Haydari MN, Perron MC, Laprise S, et al. A short-term in vivo experimental model for fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2012;53(10):63436354. doi:10.1167/iovs.12-9708.
- Zaniolo K, Bostan C, Rochette Drouin O, et al. Culture of human corneal endothelial cells isolated from corneas with Fuchs endothelial corneal dystrophy. Exp Eye Res. 2012;94(1):22–31. doi:10.1016/j.exer.2011.10.018.
- Schlötzer-Schrehardt U, Zenkel M, Strunz M, et al. Potential functional restoration of corneal endothelial cells in fuchs endothelial corneal dystrophy by ROCK Inhibitor (Ripasudil). Am J Ophthalmol. 2021;224:185–199. doi:10.1016/j.ajo.2020.12.006.
- Price MO, Price FW Jr. Randomized, double-masked, pilot study of netarsudil 0.02% ophthalmic solution for treatment of corneal edema in fuchs dystrophy. Am J Ophthalmol. 2021;227:100–105. doi:10.1016/j.ajo.2021.03.006.
- Davies E. Case series: novel utilization of rho-kinase inhibitor for the treatment of corneal edema. Cornea. 2021;40(1):116–120. doi:10.1097/ICO.0000000000002421.
- Roy O, Leclerc VB, Bourget JM, Theriault M, Proulx S. Understanding the process of corneal endothelial morphological change invitro. InvestOphthalmolVisSci. 2015;562):1228–1237.
- Engelmann K, Friedl P. Optimization of culture conditions for human corneal endothelial cells. In vitro cellular & developmental biology : journal of the Tissue Culture Association. 1989;25(11):1065–1072. doi:10.1007/BF02624143.
- Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. In vitro cellular & developmental biology : journal of the Tissue Culture Association. 2004;45(6):1743–1751. doi:10.1167/iovs.03-0814.
- Navaratnam J, Utheim TP, Rajasekhar VK, Shahdadfar A. Substrates for expansion of corneal endothelial cells towards bioengineering of human corneal endothelium. J Funct Biomater. 2015;6(3):917–945. doi:10.3390/jfb6030917.
- Shao C, Fu Y, Lu W, Fan X. Bone marrow-derived endothelial progenitor cells: a promising therapeutic alternative for corneal endothelial dysfunction. Cells Tissues Organs. 2011;193(4):253–263. doi:10.1159/000319797.
- Joyce NC, Harris DL, Markov V, Zhang Z, Saitta B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis. 2012;18:547–564.
- Dai Y, Guo Y, Wang C, et al. Non-genetic direct reprogramming and biomimetic platforms in a preliminary study for adipose-derived stem cells into corneal endothelia-like cells. PLoS One. 2014;9(10):e109856. doi:10.1371/journal.pone.0109856.
- Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell–like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2014;23(12):1340–1354. doi:10.1089/scd.2013.0510.
- Chen P, Chen JZ, Shao CY, et al. Treatment with retinoic acid and lens epithelial cell-conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell-like cells. Exp Ther Med. 2015;9:351–360.
- Zavala J, Lopez Jaime GR, Rodriguez Barrientos CA, Valdez-Garcia J. Corneal endothelium: developmental strategies for regeneration. Eye (Lond). 2013;27:579–588.
- Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea. 2001;20:731–737.
- Peh GS, Chng Z, Ang HP, et al. Propagation of human corneal endothelial cells: a novel dual media approach. Cell Transplant. 2015;24:287–304.
- Spinozzi D, Miron A, Bruinsma M, et al. Improving the success rate of human corneal endothelial cell cultures from single donor corneas with stabilization medium. Cell Tissue Bank. 2018;19:9–17.
- Roy O, Leclerc VB, Bourget JM, Theriault M, Proulx S. Understanding the process of corneal endothelial morphological change invitro. InvestOphthalmolVisSc. 2015;2(56):1228–1237.
- Choi JS, Williams JK, Greven M, et al. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials. 2010;31:6738–6745.
- Lu X, Chen D, Liu Z, et al. Enhanced survival in vitro of human corneal endothelial cells using mouse embryonic stem cell conditioned medium. Mol Vis. 2010;16:611–622.
- Engelmann K, Friedl P. Optimization of culture conditions for human corneal endothelial cells. In vitro cellular & developmental biology : journal of the Tissue Culture Association. 1989;25:1065–1072.
- Choi JS, Kim EY, Kim MJ, et al. Factors affecting successful isolation of human corneal endothelial cells for clinical use. Cell Transplant. 2014;23:845–854.
- Joyce NC & Zhu CC. Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004;23:S8–S19 .
- Miyazaki T, Futaki S, Suemori H, et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun. 2012;3:1236.
- Nagase T, Ueno M, Matsumura M, et al. Pericellular matrix of deciduaderived mesenchymal cells: a potent human-derived substrate for the maintenance culture of human ES cells. Dev Dyn. 2009;238:1118–1130.
- Arnalich-Montiel F, Moratilla A, FuentesJulian S, et al. Treatment of corneal endothelial damage in a rabbit model with a bioengineered graft using human decellularized corneal lamina and cultured human corneal endothelium. PLoS One. 2019;14:e0225480.
- Peh GSL, Ang HP, Lwin CN, et al. Regulatory compliant tissue-engineered human corneal endothelial grafts restore corneal function of rabbits with bullous keratopathy. Sci Rep. 2017;7:14149.
- Spinozzi D, Miron A, Lie JT, et al. In Vitro Evaluation and Transplantation of human corneal endothelial cells cultured on biocompatible carriers. Cell Transplant. 2020;29:963689720923577.
- Teichmann J, Valtink M, Nitschke M, et al. Tissue engineering of the corneal endothelium: a review of carrier materials. J Funct Biomater. 2013;4:178–208.
- Mimura T, Yamagami S, Usui T, Seiichi HN, Amano S. Necessary prone position time for human corneal endothelial precursor transplantation in a rabbit endothelial deficiency model. Curr Eye Res. 2007;32:617–623.
- Mimura T, Yamagami S, Yokoo S, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004b;45:2992–2997.
- Mimura T, Yamagami S, Yokoo S, Usui T, Amano S. Selective isolation of young cells from human corneal endothelium by the sphere-forming assay. Tissue Eng Part C Methods. 2010;16:803–812.
- Numa K, Imai K, Ueno M, et al. Five-Year follow-up of first 11 patients undergoing injection of cultured corneal endothelial cells for corneal endothelial failure.Ophthalmology.2021 Apr 1;128(4):504–514.
- Xia X, Atkins M, Dalal R, et al. Magnetic human corneal endothelial cell transplant: delivery, retention, and short-term efficacy. Invest Ophthalmol Vis Sci. 2019;60:2438–2448.
- Ong HS, Peh G, Neo DJH, et al. A novel approach of harvesting viable single cells from donor corneal endothelium for cell-injection therapy. Cells. 2020;9:1428.
- Parikumar P, Haraguchi K, Senthilkumar R, Abraham SJ. Human corneal endothelial cell transplantation using nanocomposite gel sheet in bullous keratopathy. Am J Stem Cells. 2018;7:18–24.
- Price MO, Mehta JS, Jurkunas UV, Price FW Jr, et al. Corneal endothelial dysfunction: evolving understanding and treatment options. Prog Retin Eye Res. 2021;82:100904.
- McCabe KL, Kunzevitzky NJ, Chiswell BP, Xia X, Goldberg JL, Lanza R. Efficient generation of human embryonic stem cell-derived corneal endothelial cells by directed differentiation. PloS One. 2015;10:e0145266.
- Lovatt M, Yam GH, Peh GS, Colman A, Dunn NR, Mehta JS. Directed differentiation of periocular mesenchyme from human embryonic stem cells. Differentiation. 2017;99:62–69.
- Song Q, Yuan S, An Q, et al. Directed differentiation of human embryonic stem cells to corneal endothelial cell-like cells: a transcriptomic analysis. Experimental eye research. 2016;151:107–114.
- Raviola G. Schwalbe line’s cells: a new cell type in the trabecular meshwork of Macaca mulatta. Invest Ophthalmol Vis Sci. 1982;22:45–56.
- Yam GH-F, Seah X, Yusoff NZBM, et al. Characterization of human transition zone reveals a putative progenitor-enriched niche of corneal endothelium. Cells. 12 Oct. 2019;8(10):1244.
- Jirka S, Aartsma-Rus A. An update on RNA-targeting therapies for neuromuscular disorders. Current opinion in neurology. 2015;28(5):515–521.
- Hawkins PG, Santoso S, Adams C, Anest V, Morris KV. Promoter targeted small RNAs induce long-term transcriptional gene silencing in human cells. Nucleic Acids Res. 2009;37:2984–2995.
- Auffarth GU, Son HS, Koch M, et al. Implantation of an artificial endothelial layer for treatment of chronic corneal edema.Cornea.2021 Dec 31;40(12):1633–1638.