Abstract
Background. Although there is agreement that post-diarrheal hemolytic uremic syndrome (D + HUS) is caused by Shiga toxin (Stx)–producing E. coli, little is known about factors that mediate the host response to these toxins and potentially contribute to pathogenesis. Nitric oxide (NO) is a candidate mediator by virtue of its antiplatelet and renal vasodilatory properties. Methods. We used a baboon model of HUS to measure plasma and urinary NO metabolites and expression of NO synthase (eNOS and iNOS) in renal tissue following the intravenous administration of Stx-1. Results. Plasma concentrations through 60 hours of observation did not differ significantly from controls. Urinary values (indexed against urinary creatinine) tended, however, to rise during the initial 12 hours following administration of Stx-1. This was followed by a sustained reduction that coincided with the development of hemolytic anemia (schistocytosis) and other features of HUS. However, immunohistochemical staining for eNOS and iNOS in tissue obtained immediately after death at a median of 59 hours showed similar levels in control and Stx-treated animals, despite the presence of a florid thrombotic microangiopathy and tubular injury in the Stx-treated group. Conclusion. We propose that urinary NO metabolite reduction was due to NO inactivation subsequent to its avid binding to free hemoglobin released from lysed red blood cells, and that this contributed to the acute renal failure by facilitating vasoconstriction and platelet aggregation and adhesion within the renal microvasculature.
Introduction
Acute renal failure (ARF) in post–diarrheal (D +) hemolytic uremic syndrome (HUS) is thought to be secondary to Shiga toxin (Stx)–mediated tubular and glomerular damage.Citation[1] Little is known, however, about factors that modulate the renal response to Stx. Nitric oxide (NO) is a substance that could play a role via its inhibitory effects on platelet aggregation and adhesion,Citation[2] neutrophil endothelial cell adhesion,Citation[3] and its vasodilatory action on renal vasculature.Citation[4] This latter property might be important because oligoanuric renal failure can occur in the absence of widespread occlusive glomerular thrombotic microangiopathy (TMA).Citation[5-7] In a mouse model of Shiga toxin–mediated HUS, inhibition of NO production worsened the acute nephropathy and increased lethality. This was prevented, however, by administration of the substrate of NO synthase (NOS) enzymes, L-arginine.Citation[8] There has, therefore, been speculation that administration of large quantities of L-arginine to humans with Shiga toxin–mediated (D +) HUS could be therapeutic.Citation[9] And in a different type of HUS (secondary to bone marrow transplantation), administration of a NO donor (i.e., transdermal isosorbide) appeared to be beneficial.Citation[10]
We have developed a baboon model of Stx-mediated HUS in which the intravenous administration of small amounts (50–200 ng/kg) of purified Stx-1 results in thrombocytopenia, microangiopathic hemolytic anemia, renal tubular injury, and glomerular TMA.Citation[11] This model bypasses the colitis phase of the disease with its associated unpredictable absorption of Stx and, thereby, provides the opportunity to study the host response to Stx in a defined and controlled manner.
In order to obtain additional insight into factors that modulate the host response to Stx, the baboon model was used to study NO metabolites, renal tissue NO synthetase expression, and to correlate these findings with clinical and histological features of HUS following the intravenous administration of Stx-1.
Methods
The animal model and Stx-1 were prepared as previously described.Citation[11] After obtaining baseline (hour zero) clinical measurements (e.g., temperature, heart rate, respiration rate, blood pressure) and blood and urine samples, 100 ng/kg of Stx-1 was intravenously administered as a single bolus dose (n = 6). For comparative purposes, five saline-injected animals served as negative controls. Blood samples were collected through a surgically placed femoral venous catheter; urine was collected via a Foley bladder catheter temporarily inserted through the urethra. Blood and urine samples were tested for the metabolic breakdown products of NO, and renal tissue was analyzed for endothelial NOS (eNOS) and inducible NOS (iNOS) expression at the time of necropsy. Complete blood counts, chemistry panels, urinalyses, and complete necropsies were also performed.
No Metabolites
Since NO is volatile and has a very short half-life, its two stable breakdown products (nitrate and nitrite) were measured. Total nitrite in plasma and urine was determined following the enzymatic conversion of nitrate to nitrite by nitrate reductase. Nitrite was detected colorimetrically as a colored azo dye product of the Griess reaction. A commercial total nitric oxide assay kit was used (Assay Designs, Inc., Ann Arbor, MI) and assays were performed according to the manufacturer's instructions. Urine concentrations were indexed against urine creatinine and expressed as micromoles/gram of creatinine. Plasma concentrations were expressed as micromoles/L.
NOS Tissue Expression
The expression of eNOS and iNOS in renal tissue was determined by immunohistochemistry in three Stx- and three saline-injected animals. A rabbit polyclonal antibody against human-iNOS and a mouse monoclonal antibody against the human-eNOS (Transduction Laboratories, Exeter, UK) were used. Immunoperoxidase staining was performed on 3-µm paraffin sections. After blocking nonspecific binding reactants with nonimmune horse serum (for eNOS) and nonimmune goat serum (for iNOS), slides were incubated overnight at 4 °C with the primary antibody (anti-eNOS 1:500, anti-iNOS 1:150) in PBS/1% BSA, followed sequentially by biotinylated horse antimouse IgG (for eNOS) or the biotinylated goat antirabbit IgG (for iNOS) and ABC solution (Vector Laboratories, Burlingame, CA), and developed with diaminobenzidine (for eNOS) or diaminobenzidine-nickel (for iNOS). For eNOS, sections were incubated before adding the ABC solution with antibiotin monoclonal antibody. The sections were counterstained with Harris hematoxylin. Negative controls included the omission of the primary antibody and replacement with a nonimmune antibody on a second section of the same tissue on each slide. Slides were observed on a DM/RB microscope by a pathologist blinded to the nature of the experimental group. Each section was scored for intensity of immunostaining [i.e., absent (0), faint (1), moderate (2), intense (3)]. At least 8–10 fields per section were examined. The final score (S) for each compartment (glomeruli, tubules, or vessels) per section were calculated as a weighted mean:where Ni (i = 0 to 3) is the number of glomeruli, tubules, or vessels that showed the level of staining intensity in that section.
Statistics
Since the sample sizes were small and values were, therefore, not normally distributed, the data were expressed as medians and ranges as well as percentage change from baseline. The Kruskal-Wallis test for nonparametric data was used to compare groups: a p < 0.05 was considered to be significant.
The animal experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Utah.
Results
Clinical Response
Clinical and laboratory variables (most abnormal) and survival duration, expressed as medians and ranges, are summarized in . As previously reportedCitation[12] the six animals that received the single 100 ng/kg bolus dose of Stx-1 showed no signs or symptoms of HUS for the first 24 hours. They then developed proteinuria, followed by hematuria, schistocytosis, and thrombocytopenia, followed by oligoanuric renal failure and death, with a median survival time of 59 hours. Necropsy showed typical features of glomerular TMA and tubular damage, as previously described.Citation[12] None of the animals injected with normal saline developed clinical or histological features of HUS.
Table 1. Clinical variables (most abnormal) in the various groups of animals, expressed as median values and ranges
Plasma Total Nitrites
Although plasma total nitrite concentrations (micromol/L) tended to rise above baseline within 12 hours of Stx-1 administration (not shown), the changes relative to baseline or saline control values did not achieve statistical significance.
Urine Total Nitrites and Renal Nitric Oxide Synthase (NOS) Expression
Medians and ranges of total urinary nitrite values for each group are shown in ; urine nitrite values (expressed as percentage change from baseline) are depicted in . Renal tissue NOS expression is shown in .
Table 2. Urinary NO values (µmol of total nitrities/g of creatinine) expressed as medians and ranges in the various groups of animals
Table 3. NOS Expression
Figure 1 Urine NO values (expressed as percentage change from baseline). † Significance relative to baseline values.* Significance relative to control animals.
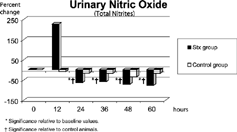
None of the saline-injected animals experienced a significant change in urinary NO metabolite excretion ( and ). Urine values of NO metabolites in animals that received Stx-1 rose 226% above baseline ( and ) by 12 hours (NS) and then fell to 37% of the baseline value by 24 hours (p = 0.025). Nitrite urinary values were also significantly lower than baseline at hours 48 and 60 and were significantly lower than values in control animals injected with saline from hours 24 through 60. However, renal eNOS and iNOS immunostaining of postmortem kidney tissue was similar to that of saline-injected animals ().
Discussion
Nitric oxide has several properties that could be important in the pathogenesis of Stx-mediated HUS: 1) It modulates the preglomerular vascular resistance and ultrafiltration coefficientCitation[4] and thus plays a major role in maintaining normal intrarenal hemodynamics and glomerular filtration, 2) it plays a significant role in inhibiting platelet aggregation and adhesion,Citation[2] 3) it functions as a leukocyte anti-adhesion factorCitation[3] via suppression of intercellular adhesion molecule-1 (ICAM-1).
Nitric oxide is produced from L-arginine under the catalytic control of nitric oxide synthases (NOS). Three NOS isoforms exist. Endothelial NOS (eNOS) and brain (bNOS) isoforms are responsible for basal, i.e., constitutive (cNOS) production, and are calcium dependent. The third isoform, eNOS, is found in renal glomerular, mesangial, and tubular cells. Although bNOS was first identified in brain tissue, it is also found in the macula densa of the kidney.Citation[13] Constitutive (eNOS and bNOS) NO production maintains normal (basal) control of vascular tone and inhibits platelet aggregation and adhesion. There is also an inducible isoform (iNOS) found in renal vascular endothelial,Citation[13] mesangial,Citation[14] and proximal tubularCitation[15] cells that is calcium independent, is receptor mediated, and is activated by a variety of agonists including LPS and TNF.Citation[16-18] In contrast to constitutive NOS, iNOS is thought to account for the increased NO production seen in pathological states. The NO's biological activity in the kidneyCitation[14] and on platelets is mediated via activation of guanylate cyclaseCitation[19] and subsequent production of its second messenger, cyclic guanosine monophosphate (cGMP), that is recognized as a measure of NO's biologic activity.Citation[20]
Reported experiments with tissue of human origin are very limited. The Stx down-regulates constitutive eNOS, but up-regulates the iNOS in cultured human aortic endothelial cells.Citation[21] The Stx-1 reduces basal NO production by both mesangial and human glomerular microvascular endothelial cells (GMVEC) in culture.Citation[22] The observation period in the earlier-cited studies was 24 hours or less. We, on the other hand, were able to study the chronological response to the administration of Stx-1 for up to 72 hours. There was a trend for urinary NO metabolite levels to increase during the initial 12 hours after infusion of Stx (prior to the onset of HUS). This may correspond to the rise in NO and/or NOS observed by many investigators in short-term experiments. The early and transient rise that we observed after Stx-1 administration was followed by a significant and sustained fall below baseline values.
Our inability to attribute falling urinary NO metabolite values to reduced renal expression of NOS isoenzymes is consistent with the observation of Bitzan et al.Citation[23] who found no reduction of eNOS expression in vascular endothelium exposed to Stx. On the other hand, we have previously shown that urinary cGMP, a recognized second messenger of NO and a marker of NO activity, is reduced during the acute phase of Stx-mediated (postdiarrheal) HUS in children, and that values return to normal during convalescence.Citation[24] There is also a report of a three-year-old with postdiarrheal HUS whose urinary NO metabolite levels were low and rose as her oligoanuric renal failure resolved.Citation[25]
Here we found that in animals injected with Stx, the fall in urinary NO metabolites at hour 24 coincided with the appearance of schistocytes (median value of 5.5% in animals given Stx vs. 0% in saline-injected control animals), but prior to the onset of oligo-anuric renal failure. This schistocyte value rose to a median of 20% at 48 hours (the longest time interval from Stx injection to survival of all animals), compared to a median value of 0.5% in control animals.
There are numerous observations in the literature to support the hypothesis that the biological activity of urinary and circulating NO in HUS is blocked by deoxyhemoglobin (deoxyHb) released subsequent to the brisk hemolysis. It has been known for many years that hemoglobin-nitric oxide combinations are among the fastest ligand-hemoglobin reactions. The formation of the NO-Hb complex occurs 5–20 times faster than does its reaction with oxygen. The affinity of NO for hemoglobin is approximately 1,500 times greater than that for carbon monoxide, and is primarily due to its slow rate of disassociation.Citation[26] In a porcine coronary artery model, hemoglobin produces a dose-dependent inhibition of NO-mediated vascular relaxation.Citation[27] This has also been shown to be true using rabbit aorta,Citation[28-30] as well as isolatedCitation[31&32] and intactCitation[33] canine arteries. Information from human models is very limited, but hemoglobin inhibits cyclic GMP production following stimulation of human endothelial cells by histamine.Citation[34] Moreover, the hemoglobin binding to NO is probably not limited to the circulation since heme is rapidly incorporated into endothelial cells,Citation[35] and there are ferrous heme proteins that are responsible for hemoglobin inhibition of NO activity.Citation[36]
In summary, our primate model observations, plus previous reports of reduced urinary cGMPCitation[37] and urinary NO metabolitesCitation[25] in children with postdiarrheal HUS, suggest that although renal NO production may initially increase following Stx, its activity is later reduced, probably via inactivation by hemoglobin. This inability to maintain a sustained increase in renal NO activity in response to Stx could contribute to the oligoanuric acute renal failure via intrarenal vasoconstriction, and by promoting platelet aggregation and adhesion to damaged renal endothelial surfaces.
Acknowledgments
This research was supported by the National Institutes of Health, grant number RO1 DK52083 awarded to Drs. Siegler, Pysher, Tesh and Taylor, and the National Institutes of Health grant number A1 34530 awarded to Dr. Tesh.
The authors gratefully acknowledge Andrew T. Pavia, M.D., Robert Oakes, Randall, M.D., Nathaniel D. Denkers, Brett D. Welch, Mary Ann Harmon, the personnel of the University of Utah's Animal Resource Center, and Jana Johnson who assisted with the experiments, data collection, statistical analysis, and preparation of this manuscript.
References
- Siegler, R L. The hemolytic uremic syndrome. Pediatr. Clin. North. Am. 1995, 42 (6), 1505–1529. [PUBMED], [INFOTRIEVE], [CSA]
- Radomski, M W.; Palmer, R M.J.; Moncada, S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987, 148, 1482–1489. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ikeda, M.; Ikeda, U.; Takahashi, M.; Shimada, K.; Minota, S.; Kano, S. Nitric oxide inhibits intracellular adhesion molecule-1 expression in rat mesangial cells.J. Am. Soc. Nephrol. 1996, 7, 2213–2218. [PUBMED], [INFOTRIEVE], [CSA]
- Deng, A.; Baylis, C. Locally produced EDRF controls preglomerular resistance and ultrafiltration coefficient.Am. J. Physiol. 1993, 264 (Renal Fluid Electrolyte Physiol. 33), F212–F215. [CSA]
- Argyle, J C.; Hogg, R J.; Pysher, T J.; Silva, F G.; Siegler, R L. A clinicopathological study of 24 children with hemolytic uremic syndrome. A report of the Southwest Pediatric Nephrology Study Group.Pediatr. Nephrol. 1990, 4, 52–58. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bohle, A.; Grabensee, B.; Fischer, R.; Berg, E.; Klust, H. On four cases of hemolytic-uremic syndrome without microangiopathy.Clin. Nephrol. 1985, 24 (2), 88–92. [PUBMED], [INFOTRIEVE], [CSA]
- Levy, M.; Gagnadoux, M F.; Habib, R. Pathology of hemolytic-uremic syndrome in children. In Hemostatis, Prostaglandins, and Renal Disease; Remuzzi, G., Mecca, G., de Gaetano, G., Eds.; Raven Press: New York, 1980; 383–397.
- Dran, G I.; Fernandez, G C.; Rubel, C J.; , et al. Protective role of nitric oxide in mice with Shiga toxin-induced hemolytic uremic syndrome.Kidney Int. 2002, 62 (4), 1338–1348. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jaradat, Z W.; Marquardt, R R. L-arginine as a therapeutic approach for the verotoxigenic Escherichia coli-induced hemolytic uremic syndrome and thrombotic thrombocytopenic purpura.Med. Hypotheses 1997, 49 (3), 277–280. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kajiume, T.; Nagita, A.; Yoshimi, S.; Kobayashi, K.; Kataoka, N. A case of hemolytic uremic syndrome improved with nitric oxide.Bone Marrow Transplant. 2000, 25 (1), 109–110. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Taylor, F B., Jr.; Tesh, V L.; DeBault, L.; , et al. Characterization of the baboon responses to Shiga-like toxin (descriptive study of a new primate model of toxic responses to Stx-1).Am. J. Pathol. 1999, 154 (4), 1285–1299. [PUBMED], [INFOTRIEVE], [CSA]
- Siegler, R L.; Pysher, T J.; Tesh, V L.; Taylor, F B., Jr. Response to single and divided doses of Shiga toxin-1 in a primate model of hemolytic uremic syndrome.J. Am. Soc. Nephrol. 2001, 12 (7), 1458–1467. [PUBMED], [CSA]
- Thorup, C.; Persson, A E.G. Macula densa derived nitric oxide in regulation of glomerular capillary pressure.Kidney Int. 1996, 49, 430–436. [PUBMED], [INFOTRIEVE], [CSA]
- Pfeilschifter, J.; Kunz, D.; Muhl, H. Nitric oxide: an inflammatory mediator of glomerular mesangial cells.Nephron. 1993, 64, 518–525. [PUBMED], [INFOTRIEVE], [CSA]
- McLay, J S.; Chatterjee, P.; Nicolson, A G. Nitric oxide production by human proximal tubular cells: a novel immunomodulatory mechanism? Kidney Int. 1994, 46, 1043–1049. [PUBMED], [INFOTRIEVE], [CSA]
- Walter, R.; Schaffner, A.; Schoedon, G. Differential regulation of constitutive and inducible nitric oxide production by inflammatory stimuli in murine endothelial cells.Biochem. Biophys. Res. Commun. 1994, 202 (1), 450–455. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Koide, M.; Kawahara, Y.; Tsuda, T.; Yokoyama, M. Cytokine-induced expression of an inducible type of nitric oxide synthase gene in cultured vascular smooth muscle cells.FEBS Lett. 1993, 318 (3), 213–217. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Breen, D.; Bihari, D. Acute renal failure as a part of multiple organ failure: the slippery slope of critical illness.Kidney Int. Suppl. 1998, 66, S25–S33. [PUBMED], [INFOTRIEVE], [CSA]
- Waldman, S A.; Murad, F. Cyclic GMP synthesis and function.Pharmacol. Rev. 1987, 39 (3), 163–196. [PUBMED], [INFOTRIEVE], [CSA]
- Williams, J M.; Lote, C J.; Thewles, A.; , et al. Role of nitric oxide in a toxin-induced model of haemolytic ureamic syndrome.Pediatr. Nephrol. 2000, 14, 1066–1070. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Matsunaga, T.; Nakajima, T.; Sonoda, M.; , et al. Reactive oxygen species as a risk factor in verotoxin-1-exposed rats.Biochem. Biophys. Res. Commun. 1999, 260 (3), 813–819. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Te Loo, M.; van Hinsbergh, V.; van der Velden, T.; Monnens, L.; van den Heuvel, L. Effects of Verocytotoxin-1 on Nitric Oxide Production by Human Glomerular Microvascular Endothelial and Mesangial Cells, 4th International Symposium and Workshop on “Shiga Toxin (Verocytotoxin)-Producing Escherichia Coli Infections,” Kyoto, Japan, 2000.
- Bitzan, M M.; Wang, Y.; Lin, J.; Marsden, P A. Verotoxin and ricin have novel effects on preproendothelin-1 expression but fail to modify nitric oxide synthase (ecNOS) expression and NO production in vascular endothelium.J. Clin. Invest. 1998, 101 (2), 372–382. [PUBMED], [INFOTRIEVE], [CSA]
- Siegler, R L.; Christofferson, R D.; Edwin, S S.; Mitchell, M D. Urinary cyclic GMP as a measure of endothelin derived relaxation factor (EDRF) in the hemolytic uremic syndrome. [abstract].JASN. 1991, 2, 274. [CSA]
- Dedeoglu, I O.; Feld, L G. Nitric oxide in the urine of a patient with hemolytic uremic syndrome. [letter].Pediatr. Nephrol. 1996, 10 (6), 812–813. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gibson, Q H.; Roughton, F J.W. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin.J. Physiol. 1957, 136, 507–526. [PUBMED], [INFOTRIEVE], [CSA]
- Evans, H G.; Ryley, H C.; Hallett, I.; Lewis, M J. Human red blood cells inhibit endothelium-derived relaxing factor (EDRF) activity.Eur. J. Pharmacol. 1989, 163 (2–3), 361–364. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Martin, W.; Villani, G M.; Jothianandan, D.; Furchgott, R F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta.J. Pharmacol. Exp. Ther. 1985, 232 (3), 708–716. [PUBMED], [INFOTRIEVE], [CSA]
- Edwards, D H.; Griffith, T M.; Ryley, H C.; Henderson, A H. Haptoglobin-haemoglobin complex in human plasma inhibits endothelium dependent relaxation: evidence that endothelium derived relaxing factor acts as a local autocoid.Cardiovas. Res. 1986, 20, 549–556. [CSA]
- Rioux, F.; Petitclerc, E.; Audet, R.; Drapeau, G.; Fielding, R M.; Marceau, F. Recombinant human hemoglobin inhibits both constitutive and cytokine-induced nitric oxide-mediated relaxation of rabbit isolated aortic rings.J. Cardiovasc. Pharmacol. 1994, 24 (2), 229–237. [PUBMED], [INFOTRIEVE], [CSA]
- Katusic, Z S.; Lee, H C.; Clambey, E T. Crosslinked hemoglobin inhibits endothelium-dependent relaxations in isolated canine arteries.Gen. Pharmacol. 1996, 27 (2), 239–244. [PUBMED], [INFOTRIEVE], [CSA]
- Katusic, Z S.; Marshall, J J.; Kontos, H A.; Vanhoutte, P M. Similar responsiveness of smooth muscle of the canine basilar artery to EDRF and nitric oxide.Am. J. Physiol. 1989, 257 (4 Pt 2), H1235–H1239. [PUBMED], [INFOTRIEVE], [CSA]
- Chou, S Y.; Ahmed, A.; Porush, J G. Renal vasoconstriction by endothelin (ET) is augmented by intravascular hemolysis and reversed by calcium antagonists. [abstract].J. Am. Soc. Nephrol. 1990, 1, 411. [CSA]
- White, D G.M.J.; Sumner, M J.; Watts, I S. The effect of endothelins on nitric oxide and prostacyclin production from human umbilical vein, porcine aorta and bovine carotid artery endothelial cells in culture.Br. J. Pharmacol. 1993, 109, 1128–1132. [PUBMED], [INFOTRIEVE], [CSA]
- Balla, G.; Vercellotti, G M.; Muller-Eberhard, U.; Eaton, J.; Jacob, H S. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species.Lab. Invest. 1991 64 (5), 648–655. [PUBMED], [INFOTRIEVE], [CSA]
- Motterlini, R.; Macdonald, V W. Cell-free hemoglobin potentiates acetylcholine-induced coronary vasoconstriction in rabbit hearts.J. Appl. Physiol. 1993 75 (5), 2224–2233. [PUBMED], [INFOTRIEVE], [CSA]
- Siegler, R L.; Christofferson, R D.; Cook, J B.; Edwin, S S.; Mitchell, M D. Urinary Cyclic GMP (cGMP) as a Measure of Nitric Oxide (NO) and Atrial Natriuretic Peptide (ANP) Activity in Post-Diarrheal HUS, 2nd International Symposium and Workshop on “Verocytotoxin (Shiga-like toxin)-producing Escherichia coli infections,” Bergamo, Italy, 1994.