Abstract
Objective
Anxiety and depression have been linked to increased risk of cardiovascular disease (CVD). Whether anxiety is a risk factor independent from depression, and if associations are limited to specific CVD outcomes remains unclear. Design: Participants (N = 3135) of the prospective Osteoporotic Fracturs in Men Sleep ancillary study were community-dwelling men (age ≥ 65) living in the US. Main outcome measures: The Goldberg Anxiety and Depression Scales, coronary heart disease (CHD) and cerebrovascular disease (CER). We used Cox proportional hazards models to calculate adjusted hazard ratios and 95% confidence intervals. Results: During 12 years of follow-up, we accrued 612 cases of CHD and 291 cases of CER (incident or repeat-event). Overall, we observed no association between anxiety or depression and CER. Anxiety was significantly associated with CHD, but this effect was attenuated after controlling for depression and covariates. Depression was significantly associated with CHD after similar adjustments. For men without prior history of CVD, neither anxiety nor depression were associated with incident CHD. Conclusions: Anxiety was not a significant independent predictor of CHD or CER, suggesting that previous findings of anxiety as a risk factor of CVD might be attributed to failure to control for the effect of depression.
Introduction
Cardiovascular disease (CVD), including coronary heart disease (CHD) and cerebrovascular disease (CER), is the world’s leading cause of death (Piepoli et al., Citation2016). With some of the most established risk factors for CVD (e.g. smoking, high cholesterol levels, hypertension) on the decline (Piepoli et al., Citation2016), others, like obesity, are rising (Blüher, Citation2019), and researchers also acknowledge the importance of psychosocial risk markers including anxiety and depression (Albus, Citation2010; Cohen et al., Citation2015; Piepoli et al., Citation2016). Current CVD prevention guidelines suggest screening for both anxiety and depression to identify those at greatest risk (Piepoli et al., Citation2016), even though the status of anxiety as a risk factor of CVD is not as well established as it is for depression (Albus, Citation2010; Cohen et al., Citation2015). To close this gap, during the last decade, there has been an increased focus on studying the connection between anxiety and the development of CVD (Batelaan et al., Citation2016; Roest et al., Citation2010; Stewart et al., Citation2016; Tully et al., Citation2013). However, evidence remains mixed. Although meta-analyses (Emdin et al., Citation2016; Roest et al., Citation2010) have presented evidence of anxiety associated with an increased risk of CVD, the studies have been criticised for failing to take into account the effects of depression (Cohen et al., Citation2015; Tully et al., Citation2016). Anxiety and depression, both in terms of symptom reporting and diagnosis, are often comorbid, starting from childhood onwards, with a stronger tendency for anxiety being a precursor of depression than the other way around (Bruce et al., Citation2016; Cummings et al., Citation2014). Some researchers have suggested that depression is the main driver of the perceived relationship between anxiety and CVD risk due to the high comorbidity (Miloyan et al., Citation2016). Others have even suggested that anxiety has a protective effect on CVD risk (Hagger-Johnson et al., Citation2012; Langvik & Nordahl, Citation2014; Meyer et al., Citation2015; Parker et al., Citation2011; Pérez-Piñar et al., Citation2017). While anxiety and depression share a distinct neuroanatomical profile, specific regional brain volumes are differently associated with anxiety and depression (van Tol et al., Citation2010). Further, while depression is associated with determinantal health behaviors, anxiety is on the other hand positively associated with health protective behaviors in cardiac patients (Benyamini et al., Citation2013). Depression is linked to CVD both through behavioral factors and physiological mechanisms such as inflammatory response, platelet reactivity (Bucciarelli et al., Citation2020) and autonomic nervous system dysregulation (Tolentino & Schmidt, Citation2019). Research on pathophysiological mechanisms linking anxiety and CVD has been limited (Alvarenga & Byrne, Citation2016) and mixed (Ransing et al., Citation2017). Depression, but not anxiety is associated with cardiac risk factors like plasma triglycerides, glucose, and insulin resistance (Holt et al., Citation2013). For both platelet activation and inflammation, there is stronger evidence for the link to depression compared to anxiety (Huffman et al., Citation2010), further supporting an investigation of the role of anxiety as a risk marker independent of depression.
In one of the most comprehensive studies to date, a recent meta-analysis (Batelaan et al., Citation2016) that adjusted for depression found anxiety to be associated with an increased risk of CVD. While some have shown that anxiety is an independent risk factor of CVD events, when controlling for depression and conventional risk factors (Stewart et al., Citation2016), there is at present inconclusive evidence of an independent association of anxiety with incident CVD (Tully, Citation2017). Thus, there is a need for more studies examining the independent effect of anxiety on the risk of incident CVD, accounting for levels of depression.
Inconclusive or conflicting findings may also be attributed to different CVD outcomes combined as the main outcome variable, heterogeneous operationalisation of anxiety (i.e. different sub-types of anxiety having specific CVD-outcome relations), or the failure to differentiate between anxiety and depression as an etiological or prognostic marker (Tully et al., Citation2013). Research suggests that phobic anxiety has a stronger linkage to CVD than other sub-types, and that anxiety is unrelated to MI, but may be more important for other CVD outcomes (Alvarenga & Byrne, Citation2016). Further, gender differences are relevant not only for risk estimates, but also for the association between anxiety, depression and cardiovascular risk factors (Holt et al., Citation2013; Langvik & Nordahl, Citation2014). Sex differences in physiopathology and gender-differences in biological responses to mental stress (Bucciarelli et al., Citation2020) warrant the study of psychological risk-markers of CVD separately for men and women.
In the present study, we investigated whether anxiety is independently associated with CVD in an elderly, male population. In line with the reviewed literature, we hypothesised that depression increases risk of incident and repeat event CHD and CER, and that any association between anxiety and these outcomes will be attenuated when including depression in the model. In our models, we adjusted for common risk factors of CVD: age, BMI, cholesterol, blood pressure, physical activity, alcohol consumption, smoking status and diabetes (Piepoli et al., Citation2016; Reid & Owen, Citation2016). Other adjustments were included because their association with CVD risk: sleep quality (Cappuccio et al., Citation2011), education (Piepoli et al., Citation2020) and ethnicity (Kurian & Cardarelli, Citation2007). We included antidepressant use as a covariate, as it has been implicated in CVD risk (Glassman & Bigger, Citation2010; Nezafati et al., Citation2015).
Materials and methods
Sample and procedure
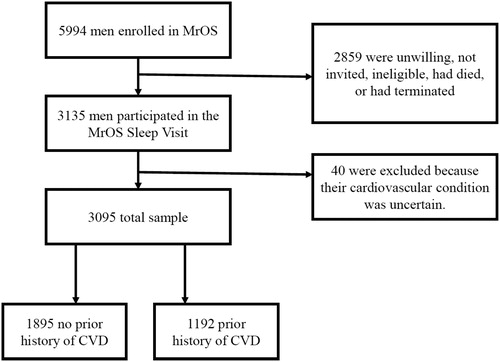
Participants (N = 5994) were recruited over a 25-month period from 2000 through 2002 as part of the Osteoporotic Fractures in Men Study (MrOS; http://mrosdata.sfcc-cpmc.net). The current study sample is based on the MrOS Sleep Study, an ancillary study that performed initial assessments from December 2003 to March 2005 among 3135 of the 5994 participants enrolled at MrOS baseline. Forty of these participants were excluded because they had missing values on central variables. The MrOS Sleep Study had a target recruitment number of 3000 participants (Mehra et al., Citation2007). See for a flow diagram of participants in the study. The study design, baseline characteristics and recruitment process have been previously described (Blank et al., Citation2005; Orwoll et al., 2005). In brief, the participants were community-dwelling men of 67 years or older at the time of the sleep study. As osteoporosis was the main focus in the MrOS study, participants had to be able to walk without assistance of another person and must not have had a bi-lateral hip replacement to be eligible. Participants were recruited from populations near six clinical sites across the US (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR, and San Diego, CA). Information about participants was gathered through self-administered questionnaires, interviewer-administered questionnaires and clinical examinations. The Sleep Study participants underwent comprehensive objective and subjective sleep assessments, and completed self-administered questionnaires related to anxiety and depression. This subset of the total sample formed the basis of the analyses in this article. All participants completed informed consent, and the study protocols were approved by Institutional Review Boards at each of the participating clinic sites.
Instruments
Anxiety and depression
The Goldberg Anxiety and Depression Scales (GADS) were used to measure anxiety and depression (Goldberg et al., Citation1988). Anxiety and depression were measured respectively using 9-item scales, where for each item participants indicated either the presence or the lack of symptoms. Examples of items were: “Have you felt keyed up, on edge?” (Anxiety) and “Have you had low energy?” (Depression). For each 9-item scale a summary score was generated by adding the number of items in which a presence of symptoms was indicated. A depression score of two or more or an anxiety score of five or more was used to indicate that the participant had a 50% chance of having a clinically important disturbance (Goldberg et al., Citation1988). The instrument has been recommended for use in epidemiological investigations as a short, valid and acceptable method of detecting heightened levels of anxiety and depression in elderly people (Koloski et al., Citation2008; Mackinnon et al., Citation1994).
Cardiovascular outcomes
Participants were followed for potential incident cardiovascular events by tri-annual questionnaire and/or phone. They were contacted every four months and had an overall response rate of more than 99%. Participants were asked about hospitalization or treatment for any CVD-related condition during the preceding 4-month interval. Medical records were obtained for all potential cases, and each case was centrally adjudicated by the MrOS Coordinating Center at the University of California, San Francisco and California Pacific Medical Center. Fatal events were further adjudicated by obtaining the death certificate or hospital records at the time of death, or by interview with the next of kin if the event did not result in hospitalization. The adjudicators were certified cardiologists using protocols that had successfully been employed at previous trials and studies of CVD (Grady et al., Citation1998). Follow-up time was through February 28th, 2015. The average time to a CHD event was 8.0 (SD = 3.4) years, 8.5 (SD = 3.0) years to a CER event. Coronary heart disease (CHD) includes any events in the following categories: acute myocardial infarction, coronary artery bypass surgery, ischemic congestive heart failure, mechanical coronary revascularization, ST and non-ST elevation MI, hospitalisation for unstable angina, sudden CHD death or other CHD event. Cerebrovascular (CER) events included stroke (residual after 24 hours) or transient ischemic attacks (TIA, no residual after 24 hours).
Model adjustment
Physical activity was measured using the Physical Activity Scale for the Elderly (PASE; Washburn et al., Citation1993). It was specifically designed to measure physical activity in the elderly, therefore including fewer items asking about sports and recreational activities, and more items about everyday activities. Participants were asked whether or to what degree they were involved in 12 types of activities during the last seven days, and weights were applied, reflecting the strenuousness of the activity. In the current sample, the scores on PASE ranged from 0 to 592. Sleep quality was measured using the Pittsburgh Sleep Quality Index (Buysse et al., Citation1989). Nineteen questions measured sleep quality, yielding a continuous scale from 0 to 21. Body mass index (BMI) was calculated based on measured height and weight taken of the participants at the sleep visit (kg/meters2). Serum samples were taken from the participants to measure oxidised LDL (cholesterol), measured in “milli units per litre” (Harrison, Citation2014). Resting blood pressure was measured using mercury sphygmomanometer. Self-reports were used to ascertain the number of alcoholic beverages a participant consumed a week (0-2, 3-13, more than 13), whether they had ever had diabetes or used antidepressants and their current cigarette smoking status (yes, no, or former).
Analysis
We used Stata/MP v. 15 for Windows to calculate Cox proportional hazards models. The dependent variable was time from clinic visit to fatal or nonfatal CVD endpoints. Separate models were run for CHD and CER outcomes. We performed three sets of analyses. In the first set we analysed the whole sample and added prior history of CVD as a covariate. In the second set, we analysed the subset of the sample that had no prior history of CVD (incident CHD and CER). In the third set, we analysed the subset of the sample that had experienced a previous CVD event (repeat event CHD and CER). As CHD and CER share many of the same risk factors, CVD was used as a general stratum instead of history of CHD or CER.
In Model 1, anxiety and depression were entered separately into the model to examine their individual effects. In Model 2 they were entered together to determine their independent effects in the presence of the other condition, while in Model 3 they were entered together in addition to all covariates. The covariates included an a-priori set of established risk markers of CVD according to current guidelines (Piepoli et al., Citation2016). Education, ethnicity, diabetes, use of antidepressants, smoking status and alcohol use were entered into the model as categorical variables, while age, blood pressure, cholesterol levels, BMI, sleep quality and activity were entered as continuous variables. Kaplan-Meier curves were produced to show the unadjusted probability of a CHD/CER event as a function of anxiety.
Results
The baseline characteristics of the sample are presented in , for the overall cohort, as well as stratified by outcome category (no CVD event, CHD event and CER event). The mean body mass index was 27.17 (SD = 3.83). At baseline (sleep visit 1), 3095 participated, and the mean age was 76.38 (SD = 5.54). Of those, 2078 participants did not experience any CVD events by the end of the follow-up period, while 612 experienced one or more CHD events and 291 experienced one or more CER events. Of the total sample, 277 (8.75%) had an anxiety score above 5, corresponding to a 50% chance of having a clinically significant disturbance. Among the no CVD event group, the proportion of participants with GADS-A ≥ 5 was 8.12%, compared to 11.11% and 8.65% in the groups with CHD events and CER events, respectively. Of the total sample, 1042 (33.81%) had GADS-D ≥ 2. The proportion having GADS-D ≥ 2 in the no CVD event group was 31.30%, while it was 43.42% in the CHD event group and 32.07% in the CER event group. A total of 215 (6.9%) men had both elevated anxiety and depression scores, and the association between anxiety and depression was significant, but moderate, χ2 = 282.59, V =.30, p < .001.
Table 1. Baseline characteristics of the sample.
Of the total sample, 38.61% had a previous diagnosis of CVD before the start of the study. Prevalence of prior diagnosis of CVD was 31.98% for the no CVD event group, 58.10% for the CHD event group and 47.93% for the CER event group.
Anxiety and risk of CHD
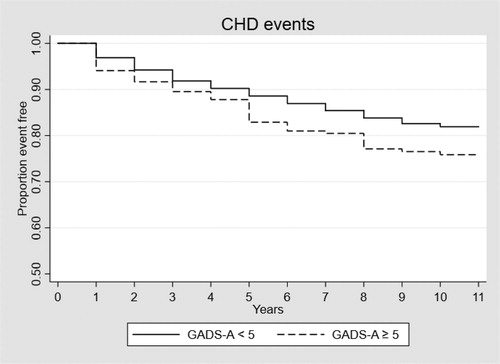
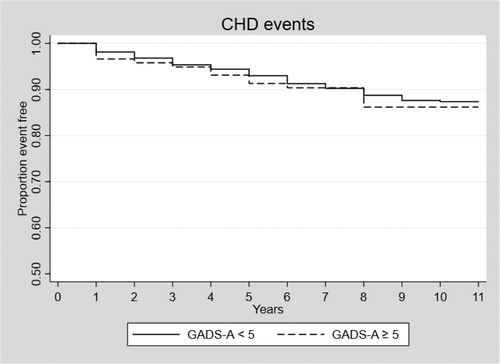
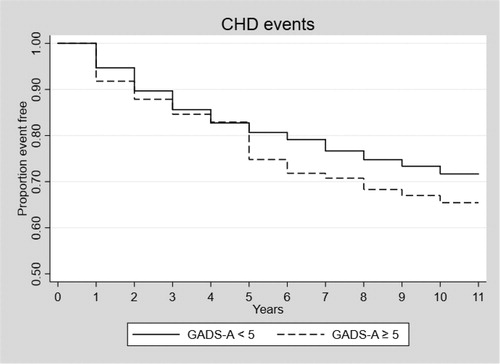
The results from the survival analyses can be seen in . feature the Kaplan-Meier survival curves showing the time to CHD event in Model 1 for those with and without anxiety, in the total sample and the two sub-sets.
Table 2. Hazard ratios for cardiovascular diseases in the total sample.
Table 3. Hazard ratios in participants with no previous diagnosis of CVD.
Table 4. Hazard ratios in participants with history of CVD.
In the unadjusted model (Model 1) for the total sample, anxiety was statistically significantly associated with the risk of having a CHD event, with a hazard ratio of 1.41, 95% CI [1.10, 1.83], p = .008. After controlling for depression (Model 2), the hazard ratio of anxiety decreased to 1.06, 95% CI [0.80, 1.39], p = .693. In this model, depression had a significant hazard ratio of 1.68, 95% CI [1.41, 1.99], p < .001. Results were similar in Model 3 after controlling for all covariates (age, education, race/ethnicity, diabetes, antidepressant use, BMI, cholesterol/oxidised low-density lipoprotein, smoking status, drinking habit, physical activity and sleep quality), in which no association was observed between anxiety and CHD (HR = 0.95, [0.71, 1.27], p = .730), and the association between depression and CHD remained significant but somewhat attenuated with a hazard ratio of 1.33, 95% CI [1.10, 1.60], p = .003. When restricting the analyses to men with a prior history of CVD at baseline, depression was associated with an increased risk of repeat event CHD when adjusting for all covariates, HR = 1.51, 95% CI [1.19, 1.93], p = .001. Depression was significantly associated with incident CHD among those with no history of CVD (HR = 1.33 [1.003, 1.773], p = .048), however, this effect was attenuated when controlling for other covariates. Anxiety was not significantly associated with either incident or repeat event CHD.
The inclusion of anxiety (Model 2) only marginally altered the HR of depression on CHD in the group with no history of CVD, from 1.32 to 1.33. There was no change in the HR of depression on CHD in the total sample nor in the group with a prior history of CVD.
Anxiety and risk of CER
Neither anxiety nor depression had a significant effect on CER in any of the three sets of analyses ().
Discussion
In the present study we found that anxiety increased the risk of CHD events among community dwelling older men. However, this effect was no longer significant when controlling for depression, as hypothesized. Depression significantly increased the risk of incident and repeat event CHD even when controlling for anxiety and prior history of CVD, and other relevant control variables. Among those with no prior history of CVD, neither depression nor anxiety was a significant predictor after controlling for other variables. In contrast, among those participants with prior history of CVD, depression, but not anxiety, was associated with risk of CHD. Neither depression nor anxiety had a significant effect on the likelihood of experiencing a CER event in any of the analyses.
These results differ from the conclusions by recent meta-analyses that show an effect of anxiety on CVD, when controlling for the effect of depression (Batelaan et al., Citation2016; Tully et al., Citation2013). Anxiety and depression can be difficult to properly differentiate due to their shared components (Clark & Watson, Citation1991) and comorbidity (Jacobson & Newman, Citation2017). Characteristics of the current sample and instruments might explain why we observed different results compared to recent meta-analyses. Anxiety and depression were measured using the Goldberg Anxiety and Depression Scale (GADS), an instrument not used by any of the studies included in Batelaan et al. (Citation2016), Emdin et al. (Citation2016), Pérez-Piñar et al., (Citation2017) or Roest et al. (Citation2010). Compared to other measures (e.g. the Hospital Anxiety and Depression scale; Zigmond & Snaith, Citation1983), GADS has a stronger focus on somatic symptoms, and has less strict cut-off values. Our sample had a mean age of 76 years. Comparatively, only 7 of the 32 studies included in Batelaan et al. (Citation2016) researched samples aged 65 years or older. Additionally, only men were included in the current study. When making comparisons to the meta-analyses, it should be mentioned that they all, with the exception of Emdin et al. (Citation2016), present substantial heterogeneity, implicating that divergent results between our study and the meta-analyses could be expected.
Depression was associated with an increased risk of CHD, and this effect persisted when looking only at participants with prior history of CVD, though it was not significant in the subset with no prior history of CVD when controlling for other variables. This is contrary to other studies showing depression to be an independent risk marker for incident CHD (Gan et al., Citation2014; Nicholson et al., Citation2006; Rugulies, Citation2002; Wu & Kling, Citation2016). Hence it is premature to conclude that depression is not an important risk factor for incident CHD. It might however suggest that the risk differs across populations. For instance, prior studies have found that depression was a stronger risk factor for women compared to men (Langvik & Nordahl, Citation2014). The current study indicates that risk factors of CVD may also differ across age groups, and that anxiety is less important compared to other risk markers among older men. The proportion of people who survive a CVD event is growing, though, which also makes knowledge about risk factors for a subsequent CVD event important.
The results underline the importance of specificity in research on the link between affective disorders and CVD, both in terms of the population of interest (prognostic vs. etiologic approach) and outcome. Depression was not associated with incident CHD in the group free of CVD at baseline, only with repeat event CHD. Further, the results varied across outcomes: While anxiety and depression variously showed some relation to CHD, an effect on CER was not observed. Studies variously use CVD or CHD as outcome, but the inclusion of CER events in a composite CVD outcome might obscure the results. The etiologies of CER and CHD are different (Widimský et al., Citation2013), hence affective disorders may affect them differently. For instance, 90% of MI patients share the underlying cause of their CHD (i.e. atherosclerotic plaque rupture with arterial thrombosis), while the causes of CER are more heterogenous (Widimský et al., Citation2013)
The majority of the previous studies on anxiety or depression have failed to control for the effect of the comorbid counterpart (Batelaan et al., Citation2016), making it difficult to draw conclusions about whether the effect is actually caused by the shared variance, or the other affective disorder. The study also supports increased specificity in choice of outcomes: While anxiety and depression variously showed some relation to CHD, an effect on CER was not observed.
One of the strengths of the study is the large sample used, another is that we controlled for all relevant confounders, both well-known ones such as age, smoking, cholesterol, blood pressure diabetes, BMI and physical activity (Piepoli et al., Citation2016) and more ambiguous ones such as antidepressant-use (Glassman & Bigger, Citation2010). Additionally, we controlled for the effect of the depression in analysing anxiety and performed separate analyses on participants with and without prior history of CVD. A limitation of this study is that it samples a rather homogenous population, namely older, primarily Caucasian men in the US, and the results therefore cannot be generalised to younger adults or women. The role of anxiety as an etiological and prognostic factor for women and in other age-groups warrants further investigation. Older men are more prone to CVD, which might bias the results compared to a more diverse sample. At the same time, the elderly are most likely to develop CVD, and thus, a particularly interesting group to study in the interest of reducing the general disease burden. Further, as psychological markers differ both in prevalence and CVD risk estimate among men and women, separate analysis for men and women are warranted (Langvik & Nordahl, Citation2014). Despite evidence of GADS having good psychometric qualities (Mackinnon et al., Citation1994), there are some limitations with the use of GADS. The discriminant validity has been questioned due to high correlations between the two subscales of GADS (Therrien & Hunsley, Citation2012). Further, it has been suggested that the cut-off scores are too low, especially for depression (Koloski et al., Citation2008). In our sample, the prevalence and ratio of depression to anxiety cases was higher than expected based on the general prevalence in most populations (Bandelow & Michaelis, Citation2015; Hasin et al., Citation2018), but our result are similar to other studies using GADS in older samples (Koloski et al., Citation2008).
Conclusion
In the current study, elevated symptoms of anxiety were not a significant independent risk factor for CHD or CER, regardless of prior history of CVD status among elderly men. This supports the notion that relationship between anxiety and CVD observed previously might be explained by comorbid depression, particularly among older men. Though further research is needed to confirm these findings, results suggest it may be prudent to focus on treatment of depression to reduce risk of CHD among older men. Treatment of anxiety symptoms may have little effect on risk of CVD outcomes.
Data findings
The data that support the findings of this study are openly available at MrOS Online: https://mrosdata.sfcc-cpmc.net.
Disclosure statement
Dr Stone reports grants from NIH, during the conduct of the study. The other authors have nothing to disclose.
Additional information
Funding
References
- Albus, C. (2010). Psychological and social factors in coronary heart disease. Annals of Medicine, 42(7), 487–494. https://doi.org/10.3109/07853890.2010.515605
- Alvarenga, M. E., & Byrne, D. (2016). Anxiety and cardiovascular disease: Epidemiology and proposed mechanisms. In M. E. Alvarenga & D. Byrne (Eds.), Handbook of psychocardiology (pp. 247–263). Springer. https://doi.org/10.1007/978-981-287-206-7_10
- Bandelow, B., & Michaelis, S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–335.
- Batelaan, N. M., Seldenrijk, A., Bot, M., Van Balkom, A. J. L. M., & Penninx, B. W. J. H. (2016). Anxiety and new onset of cardiovascular disease: Critical review and meta-analysis. The British Journal of Psychiatry, 208(3), 223–231. https://doi.org/10.1192/bjp.bp.114.156554
- Benyamini, Y., Roziner, I., Goldbourt, U., Drory, Y., & Gerber, Y. (2013). Depression and anxiety following myocardial infarction and their inverse associations with future health behaviors and quality of life. Annals of Behavioral Medicine, 46(3), 310–321. https://doi.org/10.1007/s12160-013-9509-3
- Blank, J. B., Cawthon, P. M., Carrion-Petersen, M. L., Harper, L., Johnson, J. P., Mitson, E., & Delay, R. R. (2005). Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemporary Clinical Trials, 26(5), 557–568. https://doi.org/10.1016/j.cct.2005.05.005
- Blüher, M. (2019). Obesity: Global epidemiology and pathogenesis. Nature Reviews. Endocrinology, 15(5), 288–298. https://doi.org/10.1038/s41574-019-0176-8
- Bruce, D. G., Davis, W. A., Dragovic, M., Davis, T. M. E., & Starkstein, S. E. (2016). Comorbid anxiety and depression and their impact on cardiovascular disease in type 2 diabetes: The fremantle diabetes study phase II. Depression and Anxiety, 33(10), 960–966. https://doi.org/10.1002/da.22523
- Bucciarelli, V., Caterino, A. L., Bianco, F., Caputi, C. G., Salerni, S., Sciomer, S., Maffei, S., & Gallina, S. (2020). Depression and cardiovascular disease: The deep blue sea of women's heart. Trends in Cardiovascular Medicine, 30(3), 170–176. https://doi.org/10.1016/j.tcm.2019.05.001
- Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 https://doi.org/10.1016/0165-1781(89)90047-4
- Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P., & Miller, M. A. (2011). Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. European Heart Journal, 32(12), 1484–1492. https://doi.org/10.1093/eurheartj/ehr007
- Clark, L. A., & Watson, D. (1991). Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100(3), 316–336. https://doi.org/10.1037/0021-843X.100.3.316
- Cohen, B. E., Edmondson, D., & Kronish, I. M. (2015). State of the art review: Depression, stress, anxiety, and cardiovascular disease. American Journal of Hypertension, 28(11), 1295–1302. https://doi.org/10.1093/ajh/hpv047
- Cummings, C. M., Caporino, N. E., & Kendall, P. C. (2014). Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin, 140(3), 816–845. https://doi.org/10.1037/a0034733
- Emdin, C. A., Odutayo, A., Wong, C. X., Tran, J., Hsiao, A. J., & Hunn, B. H. M. (2016). Meta-analysis of anxiety as a risk factor for cardiovascular disease. The American Journal of Cardiology, 118(4), 511–519. https://doi.org/10.1016/j.amjcard.2016.05.041
- Gan, Y., Gong, Y., Tong, X., Sun, H., Cong, Y., Dong, X., Wang, Y., Xu, X., Yin, X., Deng, J., Li, L., Cao, S., & Lu, Z. (2014). Depression and the risk of coronary heart disease: A meta-analysis of prospective cohort studies. BMC Psychiatry, 14(1), 371–311. https://doi.org/10.1186/s12888-014-0371-z
- Glassman, A. H., & Bigger, J. T. J. (2010). Depression and cardiovascular disease: The safety of antidepressant drugs and their ability to improve mood and reduce medical morbidity. In A. H. Glassman, M. Maj, & N. Sartorius (Eds.), Depression and heart disease (pp. 125–143). John Wiley & Sons. https://doi.org/10.1002/9780470972304.ch5
- Goldberg, D., Bridges, K., Duncan-Jones, P., & Grayson, D. (1988). Detecting anxiety and depression in general medical settings. BMJ, 297(6653), 897–899. https://doi.org/10.1136/bmj.297.6653.897
- Grady, D., Applegate, W., Bush, T., Furberg, C., Riggs, B., & Hulley, S. B. (1998). Heart and estrogen/progestin replacement study (HERS): Design, methods, and baseline characteristics. Controlled Clinical Trials, 19(4), 314–335. https://doi.org/10.1016/S0197-2456(98)00010-5 https://doi.org/10.1016/S0197-2456(98)00010-5
- Hagger-Johnson, G., Roberts, B., Boniface, D., Sabia, S., Batty, G. D., Elbaz, A., Singh-Manoux, A., & Deary, I. J. (2012). Neuroticism and cardiovascular disease mortality: Socioeconomic status modifies the risk in women (UK health and lifestyle survey). Psychosomatic Medicine, 74(6), 596–603. https://doi.org/10.1097/PSY.0b013e31825c85ca
- Harrison, S. (2014). MrOS oxidized LDL data. Retrieved from https://mrosdata.sfcc-cpmc.net/DataFolder/Datasets/OLSFEB14.zip
- Hasin, D. S., Sarvet, A. L., Meyers, J. L., Saha, T. D., Ruan, W. J., Stohl, M., & Grant, B. F. (2018). Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry, 75(4), 336–346. https://doi.org/10.1001/jamapsychiatry.2017.4602
- Holt, R. I. G., Phillips, D. I. W., Jameson, K. A., Cooper, C., Dennison, E. M., & Peveler, R. C. (2013). The relationship between depression, anxiety and cardiovascular disease: Findings from the Hertfordshire Cohort Study. Journal of Affective Disorders, 150(1), 84–90. https://doi.org/10.1016/j.jad.2013.02.026
- Huffman, J., Celano, C. M., & Januzzi, J. L. (2010). The relationship between depression, anxiety, and cardiovascular outcomes in patients with acute coronary syndromes. Neuropsychiatric Disease and Treatment, 6, 123–136. https://doi.org/10.2147/ndt.s6880
- Jacobson, N. C., & Newman, M. G. (2017). Anxiety and depression as bidirectional risk factors for one another: A meta-analysis of longitudinal studies. Psychological Bulletin, 143(11), 1155–1200. https://doi.org/10.1037/bul0000111
- Koloski, N. A., Smith, N., Pachana, N. A., & Dobson, A. (2008). Performance of the Goldberg Anxiety and Depression Scale in older women. Age and Ageing, 37(4), 464–467. https://doi.org/10.1093/ageing/afn091
- Kurian, A. K., & Cardarelli, K. M. (2007). Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethnicity & Disease, 17(1), 143–152. https://doi.org/10.13016/rsqw-ztls
- Langvik, E., & Nordahl, H. M. (2014). Anhedonic depression, history of depression, and anxiety as genderspecific risk factors of myocardial infarction in healthy men and women: The HUNT study. Health Psychology Open, 1(1), 205510291455765. https://doi.org/10.1177/2055102914557658
- Mackinnon, A., Christensen, H., Jorm, A. F., Henderson, A. S., Scott, R., & Korten, A. E. (1994). A latent trait analysis of an inventory designed to detect symptoms of anxiety and depression using an elderly community sample. Psychological Medicine, 24(4), 977–986. https://doi.org/10.1017/s0033291700029068
- Mehra, R., Stone, K. L., Blackwell, T., Ancoli Israel, S., Dam, T. T. L., Stefanick, M. L., & Redline, S. (2007). Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic fractures in men sleep study. Journal of the American Geriatrics Society, 55(9), 1356–1364. https://doi.org/10.1111/j.1532-5415.2007.01290.x
- Meyer, T., Hussein, S., Lange, H. W., & Herrmann-Lingen, C. (2015). Anxiety is associated with a reduction in both mortality and major adverse cardiovascular events five years after coronary stenting. European Journal of Preventive Cardiology, 22(1), 75–82. https://doi.org/10.1177/2047487313505244
- Miloyan, B., Bulley, A., Bandeen-Roche, K., Eaton, W. W., & Gonçalves-Bradley, D. C. (2016). Anxiety disorders and all-cause mortality: Systematic review and meta-analysis. Social Psychiatry and Psychiatric Epidemiology, 51(11), 1467–1475. https://doi.org/10.1007/s00127-016-1284-6
- Nezafati, M. H., Vojdanparast, M., & Nezafati, P. (2015). Antidepressants and cardiovascular adverse events: A narrative review. ARYA Atherosclerosis, 11(5), 295–304.
- Nicholson, A., Kuper, H., & Hemingway, H. (2006). Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal, 27(23), 2763–2774. https://doi.org/10.1093/eurheartj/ehl338
- Orwoll, E., Blank, J. B., Barrett-Connor, E., Cauley, J., Cummings, S., Ensrud, K., Lewis, C., Cawthon, P. M., Marcus, R., Marshall, L. M., McGowan, J., Phipps, K., Sherman, S., Stefanick, M. L., & Stone, K. (2005). Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–A large observational study of the determinants of fracture in older men. Contemporary Clinical Trials, 26(5), 569–585. https://doi.org/10.1016/j.cct.2005.05.006
- Parker, G., Hyett, M., Hadzi-Pavlovic, D., Brotchie, H., & Walsh, W. (2011). GAD is good? Generalized anxiety disorder predicts a superior five-year outcome following an acute coronary syndrome. Psychiatry Research, 188(3), 383–389. https://doi.org/10.1016/j.psychres.2011.05.018
- Pérez-Piñar, M., Ayerbe, L., González, E., Mathur, R., Foguet-Boreu, Q., & Ayis, S. (2017). Anxiety disorders and risk of stroke: A systematic review and meta-analysis. European Psychiatry, 41, 102–108. https://doi.org/10.1016/j.eurpsy.2016.11.004
- Piepoli, M. F., Abreu, A., Albus, C., Ambrosetti, M., Brotons, C., Catapano, A. L., Corra, U., Cosyns, B., Deaton, C., Graham, I., Hoes, A., Lochen, M.-L., Matrone, B., Redon, J., Sattar, N., Smulders, Y., & Tiberi, M. (2020). Update on cardiovascular prevention in clinical practice: A position paper of the European Association of Preventive Cardiology of the European Society of Cardiology. European Journal of Preventive Cardiology, 27(2), 181–125. https://doi.org/10.1177/2047487319893035
- Piepoli, M. F., Hoes, A. W., Agewall, S., Albus, C., Brotons, C., Catapano, A. L., Cooney, M.-T., Corrà, U., Cosyns, B., Deaton, C., Graham, I., Hall, M. S., Hobbs, F. D. R., Løchen, M.-L., Löllgen, H., Marques-Vidal, P., Perk, J., Prescott, E., Redon, J., Richter, D. J., Sattar, N., & Verschuren, W. M. M. (2016). 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis, 252, 207–274. https://doi.org/10.1016/j.atherosclerosis.2016.05.037
- Ransing, R. S., Patil, B., & Grigo, O. (2017). Mean platelet volume and platelet distribution width level in patients with panic disorder. Journal of Neurosciences in Rural Practice, 08(02), 174–178. https://doi.org/10.4103/jnrp.jnrp_445_16
- Reid, C., & Owen, A. (2016). Epidemiology of cardiovascular disease. In M. E. Alvarenga & D. Byrne (Eds.), Handbook of psychocardiology. (pp. 45–64). Springer. https://doi.org/10.1007/978-981-287-206-7_5
- Roest, A. M., Martens, E. J., de Jonge, P., & Denollet, J. (2010). Anxiety and risk of incident coronary heart disease. A meta-analysis. Journal of the American College of Cardiology, 56(1), 38–46. https://doi.org/10.1016/j.jacc.2010.03.034
- Rugulies, R. (2002). Depression as a predictor for coronary heart disease: A review and meta-analysis. American Journal of Preventive Medicine, 23(1), 51–61. https://doi.org/10.1016/S0749-3797(02)00439-7
- Stewart, J. C., Hawkins, M. A. W., Khambaty, T., Perkins, A. J., & Callahan, C. M. (2016). Depression and anxiety screens as predictors of 8-year incidence of myocardial infarction and stroke in primary care patients. Psychosomatic Medicine, 78(5), 593–601. https://doi.org/10.1097/PSY.0000000000000315
- Therrien, Z., & Hunsley, J. (2012). Assessment of anxiety in older adults: A systematic review of commonly used measures. Aging & Mental Health, 16(1), 1–16. https://doi.org/10.1080/13607863.2011.602960
- Tolentino, J. C., & Schmidt, S. L. (2019). Association between depression and cardiovascular disease: A review based on QT dispersion. European Journal of Preventive Cardiology, 26(14), 1568–1563. https://doi.org/10.1177/2047487319833509
- Tully, P. J. (2017). Anxiety and incident cardiovascular disease: Is the jury still out? The American Journal of Cardiology, 120(3), e21. https://doi.org/10.1016/j.amjcard.2016.06.027
- Tully, P. J., Cosh, S. M., & Baune, B. T. (2013). A review of the affects of worry and generalized anxiety disorder upon cardiovascular health and coronary heart disease. Psychology, Health & Medicine, 18(6), 627–644. https://doi.org/10.1080/13548506.2012.749355
- Tully, P. J., Harrison, N. J., Cheung, P., & Cosh, S. (2016). Anxiety and cardiovascular disease risk: A review. Current Cardiology Reports, 18(120), 1–8. https://doi.org/10.1007/s11886-016-0800-3
- van Tol, M.-J., van der Wee, N. J. A., van den Heuvel, O. A., Nielen, M. M. A., Demenescu, L. R., Aleman, A., Renken, R., van Buchem, M. A., Zitman, F. G., & Veltman, D. J. (2010). Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry, 67(10), 1002–1011. https://doi.org/10.1001/archgenpsychiatry.2010.121
- Washburn, R. A., Smith, K. W., Jette, A. M., & Janney, C. A. (1993). The Physical Activity Scale for the Elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology, 46(2), 153–162. https://doi.org/10.1016/0895-4356(93)90053-4
- Widimský, P., Kožnar, B., Vaško, P., Peisker, T., & Štětkářová, I. (2013). Acute myocardial infarction and acute stroke: What are the differences? Focus on reperfusion therapy. Cor et Vasa, 55(2), e111–e116. https://doi.org/10.1016/j.crvasa.2013.02.002
- Wu, Q., & Kling, J. M. (2016). Depression and the risk of myocardial infarction and coronary death: A meta-analysis of prospective cohort studies. Medicine, 95(6), e2815. https://doi.org/10.1097/MD.0000000000002815
- Zigmond, A. S., & Snaith, R. P. (1983). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x