Abstract
Backgrounds
The role of O-GlcNAc transferase (OGT)-induced O-linked N-acetylglucosaminylation (O-GlcNAcylation) has been reported in multiple human diseases. However, its specific functions in osteoarthritis (OA) progression remain undetermined.
Objective
This study focused on the target proteins of OGT-induced O-GlcNAcylation in OA and the specific functional mechanism.
Methods
The levels of total O-GlcNAc and OGT were measured in both in vitro and in vivo OA models using western blot. The effects of OGT knockout on OA progression were detected through Safranin O staining, immunohistochemical staining and OARSI score evaluation. The effects of OGT silencing on LPS-induced chondrocyte injury were assessed by performing loss-of function assays. Co-immunoprecipitation (co-IP) was conducted to verify the effect of OGT-induced O-GlcNAcylation on the interaction between NEK7 and NLRP3. The role of OGT in modulating the O-GlcNAcylation and phosphorylation levels of NEK7 was analysed using western blot.
Results
The OGT-indued O-GlcNAcylation level was increased in both in vitro and in vivo OA models. Knockout of OGT mitigated OA progression in model mice. Additionally, silencing of OGT suppressed LPS-induced chondrocyte pyroptosis. Moreover, silencing of OGT inhibited the O-GlcNAcylation and enhanced the phosphorylation of NEK7 at S260 site, thereby blocking the binding of NEK7 with NLRP3.
Conclusion
OGT-induced NEK7 O-GlcNAcylation promotes OA progression by promoting chondrocyte pyroptosis via the suppressing interaction between NEK7 and NLRP3.
Keywords:
Introduction
Osteoarthritis (OA) is the most common bone joint disease worldwide, leading to disability in aged patients. As a degenerative disease, OA manifests itself in structural damage and functional failure of articular cartilage. With the increased ageing population in the whole world, the incidence of OA is increasing year by year [Citation1]. The current therapy for OA remains focused on alleviating the clinical symptoms. Despite the development in OA therapy, we still have no effective methods for alleviating the destruction of articular cartilage during OA development [Citation2].
Chondrocyte death is a crucial biological process for the pathogenesis of OA. As the only resident cell in cartilage, the articular chondrocyte takes responsibility for maintaining the equilibrium of the extracellular matrix. The morphology of chondrocytes in OA features hypertrophic change, mineralisation, and finally apoptosis [Citation3]. The apoptotic condition of chondrocytes in patients with OA is closely associated with the degradation of articular cartilage [Citation4]. Recent research has indicated that inhibiting the apoptosis of chondrocytes can mitigate cartilage degradation [Citation5–7]. Moreover, pyroptosis, a kind of cell inflammatory death, has been reported to be involved in the progression of OA [Citation8,Citation9]. Therefore, the current study aims to explore the novel mechanism affecting the pyroptosis of chondrocytes to uncover the molecular biomarker for OA patients.
O-linked N-acetylglucosaminylation (O-GlcNAcylation) is a post-translational modification of proteins, which can alter the activities of target proteins [Citation10,Citation11]. The balance of O-GlcNAcylation can be controlled by a pair of enzymes, including O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) [Citation10,Citation12]. Current studies have revealed the implication of O-GlcNAc cycling deregulation in the pathogenesis of various chronic diseases [Citation13,Citation14], including OA [Citation15,Citation16]. Nevertheless, the downstream targets of OGT-mediated O-GlcNAcylation in OA remain to be explored. This study focused on the role of OGT and O-GlcNAcylation in OA progression and the underlying functional mechanism.
In this study, we used OGT wild-type (OGT+/+) and OGT-knockout (OGT-/-) mice to determine OGT functions in OA. Additionally, we constructed an in vitro OA model to analyse the effect of OGT and OGT-induced O-GlcNAcylation on chondrocyte injury. The downstream targets and corresponding regulatory mechanisms were analysed by mechanism experiments. The current study may help to find novel molecular targets in OA therapy.
Materials and methods
Animal experiments
All procedures of animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Gannan Medical University. Twenty four 10-week-old male C57BL/6 mice weighing 25 g were used in this study. All mice were divided into two groups, including OGT+/+ mice and OGT-/- mice, obtained from the Jackson Laboratory (Bar Harbour, ME, USA). The above two groups of mice were separately divided into two groups randomly, including the sham group (negative control group; n = 6) and OA group (n = 6) to analyse the effect of OGT knockout on the cartilage injury of OA mice. OA model was generated by the medial meniscus (DMM) surgery to induce the surgical destabilisation. Briefly, the right knee joint of the mice underwent anaesthesia with an intraperitoneal injection of amobarbital sodium (25 mg/kg) and was exposed to the stereomicroscope through a medial capsular incision. After resection of the medial meniscus tibial ligament, the medial meniscus was displaced medially. At last, the incision was stitched and the skin was closed. Seventy days after surgery, all mice were sacrificed. The right knee joint of each mouse was collected for further histological detection.
Histological analysis
Knee joints were fixed in 10% formalin (CellPath, Newtown, UK) and decalcified with EDTA. Each joint was sectioned at 4 μm of thickness. Sections were stained with 0.2% Safranin O for 15 min to detect the proteoglycan content and cartilage fibrillation and erosion, as previously mentioned [Citation17]. Five random views of three sections per mouse were observed under an Olympus light microscope.
Immunohistochemical (IHC) staining
To analyse the levels of OA markers (MMP13 and Collagen II) in each mouse in different groups, sections were subjected to IHC staining. At first, cell endogenous peroxidase was inactivated by treating sections with peroxidase blocking solution for incubation at 37 °C for 30 min. The unspecific ligations were then blocked by treating sections with bovine serum albumin at 37 °C for 30 min. Afterwards, the sections were incubated with the primary antibodies, including anti-MMP13 (1: 2000 dilution, ab219620, Abcam, Cambridge, MA, USA) and anti-Collagen II (1: 200 dilution, ab307674, Abcam) at 4 °C overnight. The next day, the sections were washed and then incubated with the secondary antibody at 37 °C for 1 h. The sections were visualised using the diaminobenzidine (DAB) staining solution and observed under an Olympus light microscope. The levels of MMP13 and collagen II were quantified by two pathologists without prior knowledge of the data and grouping. The IHC score (0–12) was determined by multiplying the score for staining intensity with the score for positive area. The intensity scores ranged from 0 to 3: 0 (negative), 1 (weakly positive), 2 (moderately positive), and 3 (strongly positive). The positive area was scored as 0 (less than 5%), 1 (5–25%), 2 (26–50%), 3 (51–75%), and 4 (more than 75%).
OARSI scoring
As described previously [Citation18], a cartilage scoring system was applied to evaluate Safranin O-stained joint sections by using the summed score method (sum of 3 highest scores of each section or joint and a minimum of 9 sections per joint).
Cell culture and treatment
The chondrocytes (ATDC5 cells) purchased from the American Type Culture Collection (Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (FBS; Gibco) and 1% P/S (Gibco). ATDC5 cells were maintained in humid air (5% CO2+37 °C).
To establish an OA cell model, ATDC5 cells were stimulated with 10 µg/mL lipopolysaccharide (LPS, Sigma, St. Louis, MO, USA) for 12 h.
Short hairpin RNA (shRNA) adenovirus construction and infection
shRNA targeting OGT (sh-OGT) and its corresponding negative control (sh-NC) were acquired from GenePharma (Shanghai, China). HEK293 cells were cultured in DMEM supplemented with 10% FBS at 37 °C with 5% CO2. The shRNAs were inserted into the pShuttle-EGFP-U6 vector (SinoGenoMax, Beijing, China) to construct recombinant shuttle plasmids pShuttle-EGFP-U6-OGT-shRNA and pShuttle-EGFP-U6-NC-shRNA. These shuttle plasmids were inserted into pAdxsi adenovirus skeleton plasmid (SinoGenoMax) to synthesise pAdxsi-EGFP-U6-OGT-shRNA and pAdxsi-EGFP-U6-OGT-shRNA. These plasmids were amplified in HEK293 cells using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) to generate the replication-incompetent adenovirus. Then, the supernatant containing the virus was collected, and the viral titre of the adenovirus was determined by a limiting dilution assay.
ATDC cells were seeded in six-well plates and grown until 70% cell confluence. Infection was performed at a multiplicity of infection (MOI) of 50. After 48 h, cells were harvested.
Quantitative reverse transcription real-time polymerase chain reaction (RT-qPCR)
Total RNA isolation was completed using TRIzol reagent (Invitrogen). Total RNA (1 µg) was subjected to reverse transcription to generate cDNA by using the first-strand cDNA synthesis kit (Invitrogen). qRT-PCR was carried out on SmartChipTM Real-Time PCR system (TaKaRa, Dalian, China) with SYBR Premix Ex TaqTM II (TaKaRa). The 2−ΔΔCt method was applied to calculate and quantify relative RNA expression by taking GAPDH as the endogenous reference.
Cell counting kit 8 (CCK-8) assay
Cell viability of indicated ATDC5 cells was evaluated using the CCK-8 kit (Takara). Briefly, chondrocytes were seeded into a 96-well plate at a density of 5000 cells per well and incubated for 48 h. After that, each well was added with 10 μL of CCK-8 cocktail and subjected to further incubation at 37 °C for 2 h. For viability measurement, a microplate reader (BioTek, Biotek Winooski, Vermont, USA) was applied to test the absorbance value at 450 nm wavelength.
Flow cytometry
After being treated with indicated reagents or transfections, the pyroptosis of ATDC5 cells was evaluated with FLICA 660-YVADFMK according to the manufacturer’s protocols. To mark chondrocytes with membrane pores, chondrocytes were stained with PI and then measured by flow cytometry (BD Biosciences, San Jose, CA, USA). The results were analysed by FlowJo software (BD Biosciences).
Lactate dehydrogenase (LDH) release detection
LDH release in ATDC5 cells was detected using the LDH Cytotoxicity Assay Kit (Beyotime Biotech, Shanghai, China). Briefly, chondrocytes were cultured until 80–90% cell confluence. The samples were treated with LDH release reagent, mixed by pipetting, and incubated. When reached the predetermined time, the cell culture plate was subjected to centrifugation at 400 × g for 5 min. A microplate reader was applied to measure the absorbance at 590 nm and 680 nm using an aliquot of supernatant (120 μL).
Enzyme-linked immunosorbent assay (ELISA)
Removing the cells and other debris via centrifugation to collect the supernatant. The levels of IL-18 and IL-1β protein secreted into the cell culture supernatant were measured by using the IL-18/IL-1β Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) as per manufacturer’s instructions. Finally, the absorbance was assessed at 450 nm within 30 min.
Western blot
Total protein extraction was conducted using the radio-immunoprecipitation assay buffer (RIPA, Beyotime) and then quantified with the bicinchoninic acid (BCA) protein assay kit (Beyotime). Proteins were separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Membranes were blocked in 10 mM Tris-buffered saline (TBS) containing 5% non-fat milk for 2 h. After the incubation with primary antibodies, including anti-OGT (1: 1000 dilution, ab177941, Abcam), anti-OGA (1: 5000 dilution, ab124807, Abcam), anti-Caspase-1 (1: 1000 dilution, ab207802, Abcam), anti-GSDMD-N (1: 1000 dilution, ab215203, Abcam), anti-NIMA-related kinase 7 (anti-NEK7) (1: 1000 dilution, ab133514, Abcam), anti-NOD-like receptor family pyrin domain containing 3 (anti-NLRP3) (1: 1000 dilution, ab263899, Abcam), anti-RL2 (anti-O-GlcNAc, ab93858, Abcam) and the loading control anti-GAPDH (1: 5000 dilution, Proteintech, Wuhan, China) in 5% BSA dilution at 4 °C overnight, the membranes were washed in TBST and further incubated with a secondary antibody (1:5000, Proteintech) at room temperature for 1 h. After washing, the blots were detected by using an ECL (Millipore) system for visualisation.
Co-immunoprecipitation (Co-IP)
Briefly, the whole-cell lysates were prepared using RIPA lysis buffer containing protease inhibitors. The cell lysates were incubated with 5 μL indicated antibody (antu-NEK7 or anti-NLRP3) in 200 μL immunoprecipitation buffer for 4 h. Next, the samples were incubated with 20 μL immunomagnetic beads (Millipore) overnight. Finally, the enrichment of proteins was analysed by western blot which was performed as abovementioned.
Statistical analysis
Data obtained from experiments that were repeated at least three times were plotted as bar graphs showing the mean ± standard derivation (SD). Statistical analysis of data was made by using SPSS 18.0 (SPSS Inc., IBM, Armonk, NY, USA). Group comparison was performed using Student’s t-test (for two groups) or one-way ANOVA (for more than two groups). p < 0.05 was defined to be the threshold of statistical significance.
Results
The levels of total O-GlcNAcylation and OGT were increased in OA models
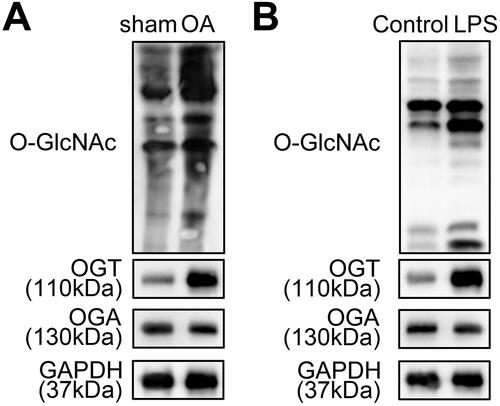
To analyse the involvement of O-GlcNAcylation in OA, we established an OA animal and cell models. Then, the level of total O-GlcNAc and the levels of O-GlcNAc cycling enzymes (OGT and OGA) in both in vitro and in vivo OA models were detected using western blot analysis. The results showed that the O-GlcNAc level was higher in the cartilage tissues of mice in the OA group compared with that in the sham group (). Meanwhile, the OGT level was found to be significantly increased in the cartilage tissues in OA models, whereas the OGA level was not changed (). Consistently, we found that O-GlcNAc and OGT levels were highly expressed in LPS-treated ATDC5 cells, compared with the control group (). Therefore, OGT-mediated O-GlcNAcylation may play a critical role in OA development.
Figure 1. The levels of total O-GlcNAcylation and OGT are increased in OA models. A. The level of total O-GlcNAc and the levels of O-GlcNAc cycling enzymes (OGT and OGA) were measured in the cartilage tissues of the OA mice model and the negative control sham mice using western blot. B. The levels of total O-GlcNAc, OGT, and OGA in ATDC5 cells treated with or without LPS were determined by western blot.
Knockout of OGT mitigates OA progression in model mice
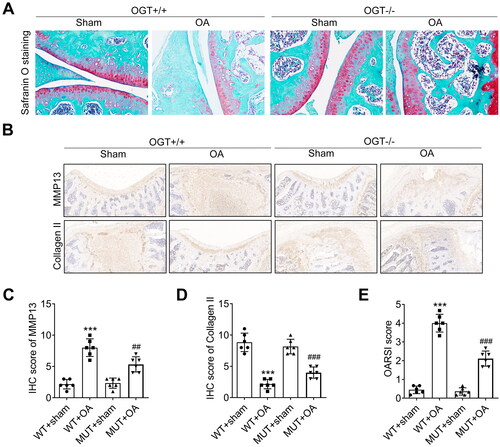
To identify the involvement of OGT in OA progression, we established the OA model using the wild-type (OGT+/+) and OGT-/- mice. Safranin O staining showed that there is no difference between the sham groups in OGT+/+ and OGT-/- groups. However, the DMM surgery notably reduced the proteoglycan content, cartilage fibrillation, and erosion both in OGT+/+ and OGT-/- groups. Interestingly, after DMM surgery, the proteoglycan content, cartilage fibrillation, and erosion in the OGT-/- group were higher than that in the OGT+/+ group (). Subsequently, IHC analysis of OA markers (MMP13 and Collagen II) showed that the expression level of MMP13 was increased, while Collagen II expression was downregulated in OA mice. Moreover, compared with the OGT+/+ mice, the expression level of MMP13 was significantly decreased while that of Collagen II was enhanced in OGT-/- mice (). Finally, we evaluated the OARSI score in different groups of mice. As indicated in , the increased OARSI score induced by OA was declined after OGT knockout. Collectively, OGT knockout alleviates the OA progression in vivo.
Figure 2. Knockout of OGT mitigates OA progression in model mice. OGT-knockout mice (OGT-/-) were generated for in vivo experiments, and mice with original OGT expression were used as wild-type (WT; OGT+/+). A. The proteoglycan content and cartilage fibrillation and erosion were detected in OGT-knockout OA or Sham mice by Safranin O staining by comparing with OGT+/+ mice. B. IHC analysis of OA markers (MMP13 and Collagen II) in OGT-knockout or sham mice. C. The level of MMP13 was quantified by the IHC score. D. The level of Collagen II was quantified by the IHC score. E. OARSI score was evaluated in different groups of mice. ***P < 0.001: OGT+/+ OA group vs OGT+/+ Sham group; ###P < 0.001 and ##P < 0.01: OGT-/- OA group vs OGT-/- Sham group.
Silencing of OGT suppresses LPS-induced chondrocyte injury
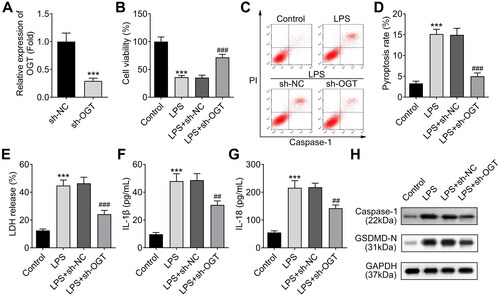
Subsequently, we investigated the effect of OGT on chondrocyte pyroptosis in OA progression in LPS-treated ATDC5 cells. First, we knocked down OGT expression in ATDC5 cells using sh-OCT adenovirus. RT-qPCR results indicated that the infection of adenovirus containing sh-OGT significantly down-regulated OGT expression in the chondrocytes (). As indicated by the CCK-8 data, silencing of OGT recovered chondrocyte viability that was inhibited by LPS (). The previous study has confirmed that pyroptosis of chondrocytes participates in the development of OA [Citation19]. Thus, we evaluated the pyroptosis of ATDC5 cells. The flow cytometry analysis using the marker of pyroptosis (caspase-1) indicated that the pyroptosis of chondrocytes was increased by LPS treatment, which was reversed by OGT silencing (). We next detected the LDH release of chondrocytes and found that LPS-induced LDH release could be inhibited by silencing of OGT (). Furthermore, we analysed the levels of inflammatory cytokines including IL-1β and IL-18 by ELISA, which are also the biomarkers of pyroptosis. According to the results, the levels of IL-1β and IL-18 were increased in LPS-induced chondrocytes, whereas silencing of OGT decreased their levels (). Finally, the levels of pyroptosis-related proteins were examined by western blot and the results showed that both levels of Caspase-1 and GSDMD-N were enhanced by LPS treatment and were reduced by OGT silencing (). In summary, OGT silencing could alleviate LPS-induced chondrocyte injury.
Figure 3. Silencing of OGT suppresses LPS-induced chondrocyte injury. A. OGT expression was knocked down in chondrocytes (ATDC5 cells) with the specific shRNA adenovirus (sh-OGT). The knockdown efficiency was measured using RT-qPCR. B. The viability of LPS-induced ATDC5 cells was assessed by CCK-8 assay after silencing of OGT. C, D. The pyroptosis rate of LPS-induced ATDC5 cells was measured by flow cytometry after silencing of OGT. E. The effect of OGT silencing on LPS-induced LDH release in ATDC5 cells was detected by using an LDH detection kit. F-G. The cellular level of inflammatory cytokines (IL-1β and IL-18) in LPS-induced ATDC5 cells was analysed through ELISA after OGT silencing. H. The levels of pyroptosis-related proteins were examined by western blot. ***P < 0.001: sh-OGT group vs sh-NC group; LPS vs Control group. ###P < 0.001 and ##P < 0.01: LPS + sh-OGT group vs LPS + sh-NC group.
OGT-mediated NEK7 O-GlcNAcylation promotes the interaction between NEK7 and NLRP3 by suppressing the phosphorylation of NEK7
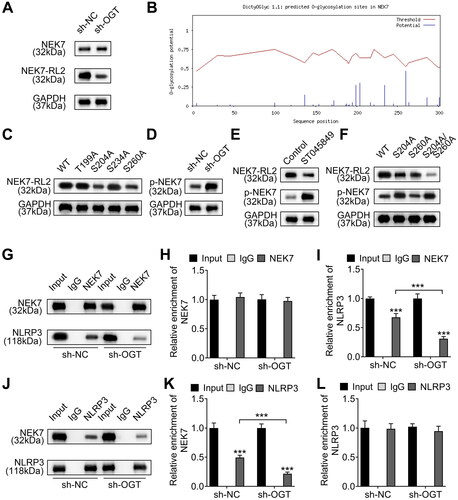
As mentioned above, O-GlcNAcylation plays a critical role in OA development. Moreover, we demonstrated that OGT silencing inhibits chondrocyte pyroptosis. Based on the catalytic effect of OGT on O-GlcNAcylation, the substrate modified by OGT was explored to elucidate its mechanism. NEK7 is a regulator of inflammation and pyroptosis [Citation20]. We evaluated whether OGT regulated NEK7 O-GlcNAcylation. We found that OGT knockdown decreased the O-GlcNAcylation level of NEK7 but did not change the NEK7 protein level (). To explore the functional O-GlcNAc sites, we predicted on DictyOGlyc-1.1 (https://services.healthtech.dtu.dk/services/DictyOGlyc-1.1/) and found several potential sites (). Then, we constructed four mutated vectors (T199A, S204A, S234A and S260A) to identify O-GlcNAcylation modification sites in NEK7 protein. The results indicated that mutation of S204 and S260 led to the decrease of O-GlcNAcylation level of NEK7, compared with wild type (WT) vector, indicating that S204 and S260 are two O-GlcNAc sites in NEK7 (). As OGT silencing does not influence the expression level of NEK7. We speculated whether OGT silencing change the phosphorylation of NEK7, for NEK7 phosphorylation was determined to regulate the activation of NLRP3 [Citation21]. As expected, we found that OGT silencing promoted the phosphorylation of NEK7 (). Next, to confirm the phosphorylation level of NEK7 could be promoted by O-GlcNAcylation inhibition, we used the O-GlcNAc inhibitor (ST045849) to inhibit O-GlcNAcylation. The treatment of ST045849 notably increased the phosphorylation and reduced the O-GlcNAcylation of NEK7 (). As mentioned above, we identified that S204 and S260 could be modified by O-GlcNAcylation. However, which sites involved in the dysregulated phosphorylation of NEK7 remain to be studied. Thus, we evaluated the O-GlcNAcylation and phosphorylation of NEK7 after mutation of S204 and S260 using western blot. It was uncovered that mutation of S204, S260, or both S204 and S260 (S204/S260) reduced the O-GlcNAcylation level of NEK7, whereas S204 and S204/S260 mutation upregulated phosphorylation of NEK7; however, S260 mutation did not affect its phosphorylation level (). Thus, we confirmed that OGT-induced NEK7 O-GlcNAcylation at S260 site could change the phosphorylation of NEK7. As reported, NEK7 interacts with NLRP3 to induce inflammation and the phosphorylation of NEK7 changes the interaction between them [Citation22]. Thus, we further detected whether OGT-mediated NEK7 O-GlcNAcylation affected the interaction between NEK7 and NLRP3 through co-IP assay. As shown in , silencing of OGT impeded the binding of NEK7 to NLRP3, as reflected by the reduced enrichment of NLRP3 and the unchanged enrichment of NEK7 in the immunoprecipitates of anti-NEK7 (). Similarly, OGT silence also hindered the binding of NLRP3 to NEK7 (), as indicated by the decreased enrichment of NEK7 and the unchanged enrichment of NLRP3 in the immunoprecipitates of anti-NLRP3 (). Here, we conclude that OGT-mediated NEK7 O-GlcNAcylation blocks its phosphorylation and its interaction with NLRP3, thus regulating the activation of pyroptosis.
Figure 4. OGT-induced NEK7 O-GlcNAcylation promotes the interaction between NEK and NLRP3 by suppressing the phosphorylation of NEK7. A. The O-GlcNAc level of NEK7 protein in OGT-silenced chondrocytes was measured using western blot. B. The several potential O-GlcNAc sites in NEK7 protein were predicted on DictyOGlyc-1.1. C. The O-GlcNAc level of NEK7 protein was measured in chondrocytes after the four sites were separately mutated. D. The phosphorylation level of NEK7 was detected in OGT-silenced chondrocyte. E. The O-GlcNAc and phosphorylation levels of NEK7 were measured in chondrocytes treated with the O-GlcNAc inhibitor (ST045849). F. The phosphorylation level of NEK7 was detected in chondrocytes after mutation of S204 and S260 using western blot. G. The effect of OGT silence on the binding of NLRP3 to NEK7 was detected through co-IP assay. H-I. The enrichment of NLRP3 or NEK7 in the immunoprecipitates of anti-NEK7. J. The effect of OGT silence on the binding of NEK7 to NLRP3 was detected through co-IP assay. K-L. The enrichment of NEK7 or NLRP3 in the immunoprecipitates of anti-NLRP3. ***P < 0.001.
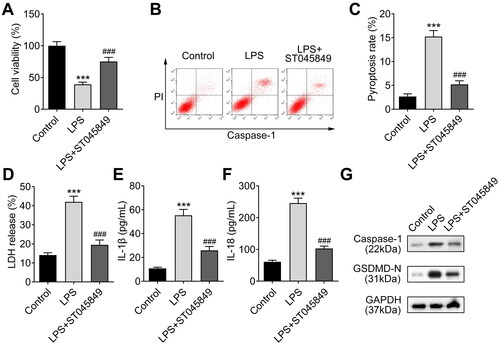
Inhibition of O-GlcNAcylation reverses LPS-induced pyroptosis of chondrocytes
To verify the involvement of O-GlcNAcylation in cell pyroptosis, we treated LPS-induced chondrocytes with the ST045849. Cell viability was detected through CCK-8 assay and the results uncovered that the viability of chondrocytes reduced by LPS was recovered by treating with ST045849 (). Moreover, from the results of flow cytometry, we found that LPS-induced increase in pyroptosis was reversed by ST045849 treatment (). Next, LPS-induced LDH release could be suppressed by ST045849 treatment (). Furthermore, ELISA data showed that the levels of IL-1β and IL-18 increased in LPS-induced chondrocytes were restored after treatment with ST045849 (). Meanwhile, the levels of pyroptosis-related proteins (Caspase-1 and GSDMD-N) were enhanced by LPS treatment and were reduced after ST045849 treatment (). These results suggest that inhibition of O-GlcNAcylation impedes chondrocyte pyroptosis caused by LPS.
Figure 5. Inhibition of O-GlcNAcylation recovers chondrocytes from LPS-induced injury. A. The viability of LPS-induced chondrocytes was assessed using CCK-8 assay after being treated with the O-GlcNAc inhibitor (ST045849). B-C. The pyroptosis rate of LPS-induced chondrocytes was examined using flow cytometry after ST045849 treatment. D. LPS-induced LDH release could be suppressed by ST045849 treatment, as detected by using an LDH detection kit. E-F. ELISA was used to detect the levels of IL-1β and IL-18 in LPS-induced chondrocytes treated with ST045849. G. The levels of pyroptosis-related proteins (Caspase-1 and GSDMD-N) were determined by western blot in LPS-induced chondrocytes treated with ST045849. ***P < 0.001: LPS group vs control group. ###P < 0.001: LPS vs LPS + ST045849 group.
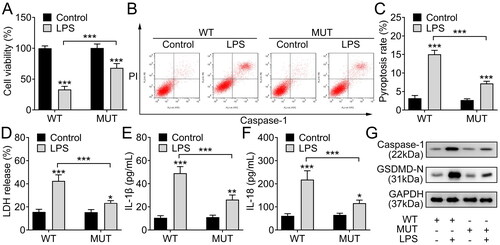
O-GlcNAcylation of NEK7 at S260 site promotes chondrocyte injury
To make further confirmation, we constructed WT and mutant S260 NEK7 vectors and transfected them into ATDC5 cells. Then, the chondrocytes were treated with LPS. The results indicated that compared with the WT group, chondrocytes with mutant NEK7 presented higher viability (), indicating that O-GlcNAcylation of NEK7 inhibited cell viability of LPS-stimulated chondrocytes. Moreover, the pyroptosis rate increased by LPS could be partially reduced after NEK7 S260 mutation (). Subsequently, the NEK7 S260 mutation could partially abolish the effect of LPS on LDH release (). The levels of IL-1β and IL-18 were lower in LPS-induced chondrocytes with S260 mutated NEK7 than those in chondrocytes with WT NEK7 (). Afterwards, the levels of pyroptosis-related proteins (Caspase-1 and GSDMD-N) were lower in LPS-induced chondrocytes with S260 mutated NEK7 than those in chondrocytes with WT NEK7 (). Therefore, we summarise that O-GlcNAcylation of NEK7 at S260 site inhibits viability and promotes pyroptosis of chondrocytes.
Figure 6. O-GlcNAcylation of NEK7 at S260 site promotes chondrocyte injury. A. The viability of LPS-induced chondrocytes with WT or S260 mutated NEK7 was measured by CCK-8 assay. B-C. The pyroptosis rate of LPS-induced chondrocytes with WT or S260 mutated NEK7 was analysed using flow cytometry. D. The LDH release condition of LPS-induced chondrocytes with WT or S260 mutated NEK7 was detected using LDH detection kit. E-F. The levels of IL-1β and IL-18 in LPS-induced chondrocytes with WT or S260 mutated NEK7 were measured through ELISA. G. The levels of pyroptosis-related proteins (Caspase-1 and GSDMD-N) in LPS-induced chondrocytes with WT or S260 mutated NEK7 were detected with western blot. ***P < 0.001: LPS + MUT group vs LPS + WT group.
Discussion
In our study, we established in vitro and in vivo OA models for exploring the role of O-GlcNAcylation-related mechanism in OA progression. The O-GlcNAc level and the levels of O-GlcNAc cycling enzyme OGT were increased in OA models compared with the control group, indicating the involvement of OGT-induced O-GlcNAcylation in OA. The role of OGT-induced O-GlcNAcylation has been unveiled in multiple human diseases by altering cell activities to regulate various biological processes, such as inflammation and necroptosis [Citation23], glucose metabolism in macrophages [Citation24], and liver cancer cell proliferation [Citation25]. In bone disease, OGT-induced O-GlcNAcylation has been reported as a modulator for osteoclast differentiation and bone loss [Citation26,Citation27]. As reported, O-GlcNAcylation protein modification is enhanced in the cartilage of patients with OA [Citation15]. However, the functions of OGT-induced O-GlcNAcylation in the cartilage injury of OA models and its target proteins remain to be investigated. To identify the effect of OGT on OA progression, we generated OGT-knockout mice and carried out animal experiments. According to the results, OGT knockout led to the retention of proteoglycan and less cartilage fibrillation and erosion. Moreover, the hyaline cartilage thickness was re-increased and the articular cartilage became ordered. OA marker MMP13 was down-regulated, and Collagen II was elevated in OGT-knockout mice. Meanwhile, the increase of OARSI score in OA mice was declined by OGT knockout. These results suggest that OGT-induced O-GlcNAcylation has a crucial role in the regulation of OA progression.
The apoptosis of chondrocytes is closely correlated with inflammation and bone injury in OA [Citation28]. Studies have shown that inhibition of inflammation-induced chondrocyte apoptosis can alleviate OA [Citation29–31]. To further determine the role of OGT in OA progression, we conducted loss-of-function assays in the LPS-induced OA cell model. The results demonstrated that silencing of OGT promoted cell viability and suppressed pyroptosis of LPS-treated chondrocytes. LDH release content indicated the injured conditions of cells [Citation32,Citation33]. Here, we determined that LPS-induced LDH release could be inhibited by silencing of OGT. The levels of inflammatory cytokines, including IL-1β and IL-18, are usually increased in OA [Citation34,Citation35]. We detected that the increased levels of IL-1β and IL-18 in LPS-induced chondrocytes were reduced after silencing of OGT. Moreover, caspase-1 and GSDMD-N are pyroptosis-related proteins, which have been verified to be involved in OA [Citation36,Citation37]. We found that levels of caspase-1 and GSDMD-N in LPS-treated chondrocytes were reduced by OGT silencing. These findings suggest that OGT silence alleviates chondrocyte injury caused by LPS. In addition, rescue experiment results showed that inhibition of OGT using ST045849 promoted cell viability and suppressed pyroptosis of LPS-stimulated chondrocytes, further confirming that chondrocyte injury can be alleviated by OGT loss. However, whether ST045849 can inhibit OGT to affect the progression of OA remains unclear. We have not identified the use of ST045849 in mice, such as the dose, time, and administration method. Hence, we will study the role of ST045849 in vivo in our future work.
NEK7 is a serine/threonine kinase, which is a crucial activator for NLRP3 inflammasome [Citation38]. A study has indicated that NEK7-mutant cells presented caspase-1 inactivation and less IL-1β production responding to NLRP3-activating stimuli [Citation39]. Thus, NEK7 is an important switch between inflammasome activation and cell activities. OGT-induced O-GlcNAcylation of target proteins can alter the phosphorylation levels. For example, O-GlcNAcylation of RYR1 can compete with NEK10-mediated phosphorylation to suppress phosphorylation [Citation40]. Hence, we further investigated whether OGT promotes OA progression by decreasing the phosphorylation level of NEK7 to strengthen NEK7/NLRP3 axis. Through bioinformatics analysis and western blot analysis, we confirmed that silencing of OGT inhibited the O-GlcNAcylation of NEK7 at S260 site to enhance its phosphorylation. Considering the coordination between NEK7 and NLRP3 in inflammation-induced injury [Citation41], we further carried out a mechanism investigation and identified that OGT promoted the interaction between NEK7 and NLRP3. These findings demonstrate that OGT-induced NEK7 O-GlcNAcylation promotes the interaction between NEK7 and NLRP3 by suppressing the phosphorylation of NEK7. Moreover, we found that mutation of NEK7 at S260 site facilitated cell viability and inhibited pyroptosis of chondrocytes, suggesting that silencing of OGT suppresses pyroptosis by inhibiting NEK7 O-GlcNAcylation.
To conclude, our findings demonstrate that OGT promotes OA progression by promoting chondrocyte pyroptosis. Mechanically, OGT-induced NEK7 O-GlcNAcylation enhances the interaction between NEK7 and NLRP3 by suppressing the phosphorylation of NEK7. All these findings suggest that OGT/NEK7/NLRP3 axis may be used as the therapeutic target in OA treatment.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. C H and Q W drafted the work and revised it critically for important intellectual content; Z Z, Y Y, H H and M H were responsible for the acquisition, analysis, or interpretation of data for the work; S L made substantial contributions to the conception or design of the work. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures of animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Gannan Medical University. All animal experiments should comply with the ARRIVE guidelines.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1–10.
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759.
- Blanco FJ, Guitian RN, Vazquez-Martul E, et al. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41(2):284–289.
- Thomas CM, Fuller CJ, Whittles CE, et al. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34.
- Chang JK, Chang LH, Hung SH, et al. Parathyroid hormone 1-34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009;60(10):3049–3060.
- Yan JY, Tian FM, Wang WY, et al. Parathyroid hormone (1-34) prevents cartilage degradation and preserves subchondral bone micro-architecture in Guinea pigs with spontaneous osteoarthritis. Osteoarthritis Cartilage. 2014;22(11):1869–1877.
- Sampson ER, Hilton MJ, Tian Y, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3(101):101ra93.
- Yang J, Hu S, Bian Y, et al. Targeting cell death: pyroptosis, ferroptosis, apoptosis and necroptosis in osteoarthritis. Front Cell Dev Biol. 2021;9:789948.
- Chang X, Kang Y, Yang Y, et al. Pyroptosis: a novel intervention target in the progression of osteoarthritis. J Inflamm Res. 2022;15:3859–3871.
- Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18(7):452–465.
- Chatham JC, Zhang J, Wende AR. Role of O-linked N-Acetylglucosamine protein modification in cellular (patho)physiology. Physiol Rev. 2021;101(2):427–493.
- Gloster TM, Vocadlo DJ. Mechanism, structure, and inhibition of O-GlcNAc processing enzymes. Curr Signal Transduct Ther. 2010;5(1):74–91.
- Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev Nutr. 2013;33(1):205–229.
- Koyama T, Kamemura K. Global increase in O-linked N-acetylglucosamine modification promotes osteoblast differentiation. Exp Cell Res. 2015;338(2):194–202.
- Tardio L, Andrés-Bergós J, Zachara NE, et al. O-linked N-acetylglucosamine (O-GlcNAc) protein modification is increased in the cartilage of patients with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):259–263.
- Someya A, Ikegami T, Sakamoto K, et al. Glucosamine downregulates the IL-1beta-Induced expression of proinflammatory cytokine genes in human synovial MH7A cells by O-GlcNAc modification-Dependent and -Independent mechanisms. PLoS One. 2016;11(10):e165158.
- Hu S, Zhang C, Ni L, et al. Stabilization of HIF-1alpha alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020;11(6):481.
- Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648.
- Yan Z, Da Q, Li Z, et al. Inhibition of NEK7 suppressed hepatocellular carcinoma progression by mediating cancer cell pyroptosis. Front Oncol. 2022;12:812655.
- Boal-Carvalho I, Mazel-Sanchez B, Silva F, et al. Influenza a viruses limit NLRP3-NEK7-complex formation and pyroptosis in human macrophages. EMBO Rep. 2020;21(12):e50421.
- Chen X, Liu G, Yuan Y, et al. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-kappaB signaling. Cell Death Dis. 2019;10(12):906.
- Cai H, Wang P, Zhang B, et al. Expression of the NEK7/NLRP3 inflammasome pathway in patients with diabetic lower extremity arterial disease. BMJ Open Diabetes Res Care. 2020;8(2):e001808.
- Li X, Gong W, Wang H, et al. O-GlcNAc transferase suppresses inflammation and necroptosis by targeting Receptor-Interacting serine/Threonine-Protein kinase 3. Immunity. 2019;50(3):576–590.e6.
- Shi Q, Shen Q, Liu Y, et al. Increased glucose metabolism in TAMs fuels O-GlcNAcylation of lysosomal cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell. 2022;40(10):1207–1222.e10.
- Raab S, Gadault A, Very N, et al. Dual regulation of fatty acid synthase (FASN) expression by O-GlcNAc transferase (OGT) and mTOR pathway in proliferating liver cancer cells. Cell Mol Life Sci. 2021;78(13):5397–5413.
- Li YN, Chen CW, Trinh-Minh T, et al. Dynamic changes in O-GlcNAcylation regulate osteoclast differentiation and bone loss via nucleoporin 153. Bone Res. 2022;10(1):51.
- Kim MJ, Kim HS, Lee S, et al. Hexosamine biosynthetic Pathway-Derived O-GlcNAcylation is critical for RANKL-Mediated osteoclast differentiation. Int J Mol Sci. 2021;22(16):8888.
- Huang R, Hui Z, Wei S, et al. IRE1 signaling regulates chondrocyte apoptosis and death fate in the osteoarthritis. J Cell Physiol. 2022;237(1):118–127.
- Ding Y, Wang L, Zhao Q, et al. MicroRNA‑93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF‑kappaB signaling pathway. Int J Mol Med. 2019;43(2):779–790.
- Bao J, Chen Z, Xu L, et al. Rapamycin protects chondrocytes against IL-18-induced apoptosis and ameliorates rat osteoarthritis. Aging (Albany NY). 2020;12(6):5152–5167.
- Zhang H, Zheng W, Li D, et al. miR-146a-5p promotes chondrocyte apoptosis and inhibits autophagy of osteoarthritis by targeting NUMB. Cartilage. 2021;13(2_suppl):1467S–1477S.
- Zu Y, Mu Y, Li Q, et al. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res. 2019;14(1):307.
- Tonomura H, Takahashi KA, Mazda O, et al. Glutamine protects articular chondrocytes from heat stress and NO-induced apoptosis with HSP70 expression. Osteoarthritis Cartilage. 2006;14(6):545–553.
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459–561419.
- Zhang L, Xing R, Huang Z, et al. Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis. Mediators Inflamm. 2019;2019:2165918–2165911.
- Qian J, Fu P, Li S, et al. miR-107 affects cartilage matrix degradation in the pathogenesis of knee osteoarthritis by regulating caspase-1. J Orthop Surg Res. 2021;16(1):40.
- Rozi R, Zhou Y, Rong K, et al. miR-124-3p sabotages lncRNA MALAT1 stability to repress chondrocyte pyroptosis and relieve cartilage injury in osteoarthritis. J Orthop Surg Res. 2022;17(1):453.
- Schmid-Burgk JL, Chauhan D, Schmidt T, et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J Biol Chem. 2016;291(1):103–109.
- Shi H, Wang Y, Li X, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17(3):250–258.
- Yan W, Cao M, Ruan X, et al. Cancer-cell-secreted miR-122 suppresses O-GlcNAcylation to promote skeletal muscle proteolysis. Nat Cell Biol. 2022;24(5):793–804.
- Hooftman A, Angiari S, Hester S, et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020;32(3):468–478.e7.
