Abstract
Background
Opioid prescription for inflammatory bowel disease (IBD)-related pain is on the rise. However, the use of strong opioids can result in severe complications, and even death, in IBD patients. This study aimed to define the role of fentanyl and morphine, two representative strong opioids, in the pathogenesis of dextran sodium sulfate (DSS)- and 2,4,6-trinitrobenzenesulfonic acid solution (TNBS)-induced colitis.
Method
DSS and TNBS models were induced in C57BL/6J and Balb/c mice, respectively. Disease activity index (DAI), histopathology, enzyme-linked immunosorbent assay (ELISA), multiplex ELISA, and flow cytometry were performed to evaluate the effects of fentanyl and morphine.
Result
Fentanyl exacerbated DSS- and TNBS-induced colitis, while morphine exhibited no significant immunomodulatory effect. Fentanyl and morphine had no obvious effects on the serum levels of adrenocorticotropic hormone (ACTH), glucocorticoid (GC), and prostaglandin E2 (PGE-2) in DSS and TNBS models. Fentanyl elevated the proportions of Th1 cells, μ-opioid receptor (MOR) + Th1 cells, and MOR + macrophages in the colonic mucosa of DSS-treated mice, and enhanced the proportions of Th1 cells, macrophages, MOR + Th1 cells, and MOR + macrophages in the colonic mucosa of TNBS-treated mice. We found that fentanyl upregulated the levels of inflammatory cytokines/chemokines in MOR + macrophages of the colonic lamina propria mononuclear cells (LPMCs) from DSS-treated mice, whereas it had no effect on the expression of most inflammatory cytokines/chemokines in MOR + macrophages in the colonic LPMCs from TNBS-treated mice.
Conclusion
Our findings suggest that fentanyl exacerbates murine colitis via Th1 cell- and macrophage-mediated mechanisms, while morphine exhibits no significant immunomodulatory effect.
Keywords:
Introduction
About 50–80% of patients with initial or exacerbated inflammatory bowel disease (IBD) have abdominal pain [Citation1–4]. Individuals with IBD may also suffer from musculoskeletal pain due to arthropathies and may have a higher risk of chronic pain and fibromyalgia [Citation5].
Opioids are a class of analgesic drugs used for moderate to severe pain. Fentanyl and morphine are representative opioids. In recent years, the prescription of opioid analgesics for chronic nonmalignant pain, including IBD-related pain, has increased [Citation6–8]. A recent retrospective study demonstrated a rise in the prescription of opioid analgesics for IBD patients in the UK from 1990 to 2013. Between 1990 and 1993, only 10% of patients with IBD used opioids. However, between 2010 and 2013, this percentage increased to 30% [Citation9]. Moreover, 5% of patients with IBD in Manitoba, Canada, had become severe users of opioids within 10 years after their diagnosis [Citation10]. There are many gastrointestinal side effects of opioid use, such as nausea, vomiting, and slowed intestinal peristalsis, which may cause toxic megacolon or hide IBD disease activity [Citation11–13]. These side effects are associated with increased hospitalization rates, mortality, and psychosocial problems [Citation6,Citation7,Citation14–16]. Frequent use of strong opioids has been relevant to increased mortality among IBD patients [Citation9,Citation10].
Opioids have immunomodulatory effects, but the underlying mechanisms remain unclear [Citation17]. There are three main types of opioid receptors, including μ-opioid receptor (MOR), δ-opioid receptor (DOR), and κ-opioid receptor (KOR). MOR, which is widely expressed and plays a critical role in neuroendocrine and immune regulation, is the main target receptor for potent opioids such as morphine and fentanyl [Citation18]. Opioids modulate immune system via a combination of direct actions on the immune cells, the hypothalamic-pituitary-adrenal (HPA) axis, or both [Citation19]. However, the immunomodulatory effect of opioids is a complex phenomenon, with studies reporting contradictory results. Morphine inhibits the secretion of inflammatory cytokines from peritoneal macrophages in mice [Citation20]. Cornwell et al. reported that morphine increased the number of circulating Tregs and Th17 activity in rhesus [Citation21]. In comparison with the control mice, the macrophages isolated from morphine-treated mice synthesized higher amounts of TNF-α, IL-6, and IL-10 after a stimulus of LPS, while fentanyl did not affect the production of the cytokines. However, the proportion of macrophages in the peritoneum of mice after administration of morphine and fentanyl decreased significantly [Citation22]. It has been observed that morphine inhibits immune responses in vitro and in vivo, and fentanyl inhibits immunoreactions in vitro [Citation23].
Increased use of strong opioids is associated with increased IBD mortality, but the mechanism of the underlying immune responses remains unclear. Thus, studying the role of strong opioids in the intestinal immune system is important for providing conclusive guidance on the use of strong opioids for IBD patients. The current study was to explore the influence of fentanyl and morphine, two strong opioids, on IBD mice, and exploring the underlying mechanisms thereof.
Materials and methods
Animals
C57BL/6J and Balb/c mice (male and female, 5–6 weeks old) were obtained from Charles River Laboratories (Beijing, China). Five mice were kept per cage in a specific pathogen-free (SPF) condition at the Tsinghua Animal Facility. Mice were allowed to adapt to the housing conditions for seven days before conducting the experiments. The experimental protocols were approved by the Animal Care and Use Committee of Tsinghua University (Protocol # 16-CZJ1).
Drugs
Fentanyl (Humanwell Pharmaceutical Co., Ltd., Yichang, China) and morphine (Northeast Pharm, Shenyang, China) were diluted in phosphate-buffered saline (PBS).
DSS and TNBS models
Dextran sodium sulfate (DSS)-induced acute colitis was triggered by administering 3% DSS (molecular weight 40 kDa; Alfa Aesar, Haverhill, MA, USA) (w/v) in drinking water to C57BL/6J mice for seven days. For 2,4,6-trinitrobenzenesulfonic acid solution (TNBS)- triggered acute colitis, 0.1 ml of TNBS solution (2 mg of TNBS in 50% ethanol) was administered intrarectally to Balb/c mice on day 0.
DSS model treated with fentanyl or morphine
To determine the effects of fentanyl or morphine on DSS-triggered acute colitis, DSS-treated mice (6–7 weeks old) were divided into four groups: (I) control mice received intraperitoneal PBS daily on days 0–6; (II) low-dose group mice received intraperitoneal fentanyl (0.1 mg/kg) or morphine (1 mg/kg) daily on days 0–6; (III) medium-dose group mice received intraperitoneal fentanyl (0.25 mg/kg) or morphine (2.5 mg/kg) daily on days 0–6; and (IV) high-dose group mice received intraperitoneal fentanyl (0.5 mg/kg) or morphine (7.5 mg/kg) daily on days 0–6. The mice were euthanized on day 7.
TNBS model treated with fentanyl or morphine
We divided the 6–7-week-old mice which were treated with TNBS into four groups: (I) control mice received intraperitoneal PBS on days 0 and 1; (II) low-dose group mice received intraperitoneal fentanyl (0.1 mg/kg) or morphine (1 mg/kg) on days 0 and 1; (III) medium-dose group mice received intraperitoneal fentanyl (0.25 mg/kg) or morphine (2.5 mg/kg) on days 0 and 1; (IV) high-dose group mice received intraperitoneal fentanyl (0.5 mg/kg) or morphine (7.5 mg/kg) on days 0 and 1. The mice were euthanized on day 2.
Evaluation of colitis
The colonic inflammation in mice was assessed using a scoring system of disease activity index (DAI), based on weight change (0–4), diarrhea (0–4), and hematochezia (0–4) [Citation24]. Colon length was measured and colonic mucosa was collected. Tissue sections were harvested and stained with hematoxylin and eosin (H&E). Histology activity index (HAI) was assessed using the following parameters, as previously described: epithelial damage (0–4) and infiltration (0–4) [Citation25]. Colonic mucosa was analyzed by multiplex enzyme-linked immunosorbent assay (ELISA) and flow cytometry.
Multiplex ELISA
The same quantity of MOR + macrophages sorted by flow cytometry were collected from each group (DSS control, DSS medium-dose fentanyl, TNBS control, and TNBS medium-dose fentanyl groups) and cultivated in 150 µL of complete RPMI 1640 medium containing penicillin (100 U/mL), streptomycin (100 mg/mL), and 10% heat-inactivated FBS (all purchased from Life Technologies, Carlsbad, CA, USA) in a 96-well plate. The supernatants of macrophage cultures and murine colonic mucosal extracts were analyzed for cytokines and chemokines by Multiplex ELISA (cat. #MCYTMAG-70K-PX32, Millipore, Burlington, MA, USA).
Flow cytometry analyses
Lamina propria mononuclear cells (LPMCs) were isolated from colons, as described by Melhem et al. [Citation26]. LPMCs from each group (control group and medium-dose fentanyl group of DSS model, and control group and medium-dose fentanyl group of TNBS model) were characterized using surface fluorochrome-conjugated antibodies. The surface fluorescent antibodies used were PE anti-mouse CD4 (cat. #100408; Biolegend, San Diego, CA, USA), PerCP anti-mouse F4/80 (cat. #123126; Biolegend), and PE anti-mouse CD11b (cat. #101207; Biolegend). Fixation/permeabilization and then intracellular staining of PecCP/Cy5.5 anti-mouse IFN-γ (cat. #505821; Biolegend) and ATTO-488 anti-mouse MOR (cat. #AOR-011-AG; Alomone Labs, Jerusalem, Israel) was performed using Transcription Factor Staining Buffer Set (cat. # 00-5523-00; eBioscience, Waltham, MA, USA) after surface markers were labeled according to the protocol provided by the manufacturer. F4/80 + CD11b + cells were recognized as macrophages; CD4 + IFN-γ+ cells as Th1 cells; F4/80 + CD11b + MOR + cells as MOR + macrophages; and CD4 + IFN-γ + MOR + cells as MOR + Th1 cells. Flow cytometry was carried out on the FACScalibur flow cytometer (BD, San Diego, CA, USA) and data were analyzed with FlowJo software.
Serum collection and ELISA
After euthanasia, blood was collected immediately through cardiac puncture into endotoxin-free silicone-coated tubes without additives. The collected blood was allowed to clot at 25 °C for 30 min followed by centrifuging (1800 × g, 4 °C, 15 min) to obtain serum. The serum specimens were preserved at −80 °C. Serum specimens were analyzed for adrenocorticotropic hormone (ACTH), glucocorticoid (GC), and prostaglandin E2 (PGE-2) levels by ELISA (Affandi-e, Shanghai, China).
Statistical analysis
The difference between the two groups was compared using Student’s t-test. The data were evaluated with one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for three or more study groups. All analyses were performed using GraphPad Prism 5 (GraphPad Software Inc, San Diego, CA, USA).
Results
Fentanyl aggravated DSS-triggered colitis
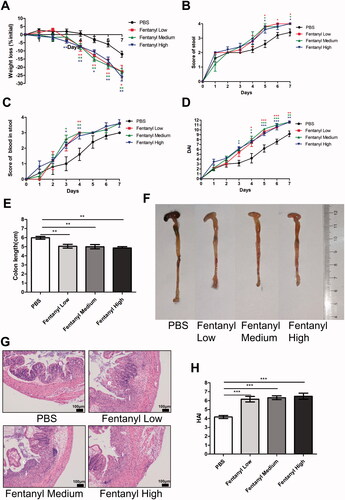
The role of fentanyl on DSS-induced acute colitis was examined. Using DAI as a routine score for colitis severity, we found a significant exacerbation of colitis after fentanyl administration (). When the three disease parameters (weight change, diarrhea, and hematochezia) were separately analyzed, we found that fentanyl aggravated all three parameters compared to the control group (). Fentanyl treatment significantly reduced the colon length (). Using H&E staining, it was observed that fentanyl caused more epithelial damage accompanied by greater inflammatory infiltration in the colons compared to PBS (). However, there was no obvious difference in the disease parameters among different doses of fentanyl treatment ().
Figure 1. Fentanyl aggravated dextran sodium sulfate (DSS)-triggered colitis. We fed 3% DSS water to mice on days 0–7. PBS (control) or fentanyl (0.1, 0.25, or 0.5 mg/kg) was intraperitoneally administered on days 0–6 (n = 10 each group). Euthanasia of the mice was performed on day 7. (A–C) Mice were assessed daily for weight, diarrhea, and hematochezia; (D) Disease activity index (DAI) was calculated on the basis of weight change, diarrhea, and hematochezia; (E) Colon lengths; (F) Progressive stages of colitis development; (G) 100× of representative hematoxylin and eosin (H&E)-stained sections of colon (structure: e, epithelial disruption; I, inflammatory infiltration); (H) Histology activity index (HAI) based on epithelial disruption and inflammatory infiltration. Data are mean ± SEM of experiments (10 mice per group). Asterisks represent significance in fentanyl treatment (0.1, 0.25, or 0.5 mg/kg) compared to the PBS control in A, B, C, and D, respectively. *p<.05; **p<.01; ***p<.001.
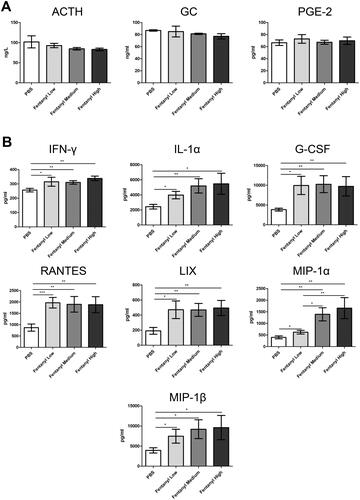
Fentanyl significantly increased the synthesis of multiple inflammatory cytokines/chemokines in the colonic mucosa of DSS model
Multiplex ELISA was performed on proteins isolated from the colonic mucosa of the mice for cytokine and chemokine analysis. Fentanyl upregulated the levels of IFN-γ, IL-1a, G-CSF, RANTES, LIX, MIP-1a, and MIP-1β. It is noteworthy that the elevation in the cytokine and chemokine levels, except of MIP-1a, was independent of the fentanyl dose ().
Figure 2. Fentanyl exhibited no obvious effects on the serum levels of adrenocorticotropic hormone (ACTH), glucocorticoid (GC), and prostaglandin E2 (PGE-2), while significantly increasing the synthesis of multiple inflammatory cytokines and chemokines in the colonic mucosa from dextran sodium sulfate (DSS) model. PBS (control) or fentanyl (0.1, 0.25, or 0.5 mg/kg) was intraperitoneally administered on days 0–6 (n = 10 each group). Euthanasia of the mice was performed on day 7. (A) The serum levels of ACTH, GC, and PGE-2 by enzyme-linked immunosorbent assay (ELISA); (B) The expression of inflammatory cytokines/chemokines in the colonic mucosa by multiplex ELISA. Data are mean ± SEM of experiments (10 mice per group). *p<.05; **p<.01; ***p<.001.
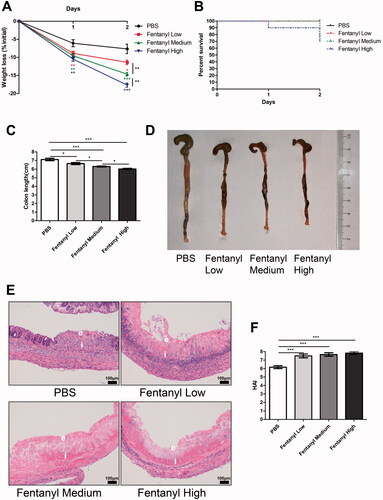
Fentanyl exacerbated TNBS-induced colitis
To determine if fentanyl has a pro-inflammatory effect on other cases of experimental colitis, we performed similar experiments using a TNBS model. Fentanyl-treated mice exhibited greater body weight loss and a worse colon appearance than PBS-treated mice (). One mouse from the high-dose fentanyl group died on day 1, whereas two mice from the medium-dose fentanyl group and two mice from the high-dose fentanyl group died on day 2 (). H&E staining showed greater epithelial damage as well as infiltration of inflammatory cells in the colons of fentanyl-administered mice when compared with PBS- administered mice. Moreover, colons showed more extensive necrosis in the medium-dose and high-dose fentanyl groups (). It should be noted that colitis was more severe with increasing doses of fentanyl in the TNBS model ().
Figure 3. Fentanyl exacerbated 2,4,6-trinitrobenzenesulfonic acid solution (TNBS)-triggered colitis. Murine acute colitis was triggered by TNBS on day 0. PBS (control) or fentanyl (0.1, 0.25, or 0.5 mg/kg) was intraperitoneally administered on days 0 and 1 (n = 10 each group). Euthanasia of the mice was performed on day 2. (A) Weight change; (B) Percent survival; (C) Colon lengths; (D) Progressive stages of colitis development; (E) 100× of representative hematoxylin and eosin (H&E)-stained sections of colon (structure: e, epithelial disruption; I, inflammatory infiltration); (F) Histology activity index (HAI) based on epithelial disruption and inflammatory infiltration. Data are mean ± SEM of experiments (10 mice per group). Asterisks represent significance in fentanyl treatment group (0.1, 0.25, or 0.5 mg/kg) compared to the PBS control group in A, respectively. *p<.05; **p<.01; ***p<.001.
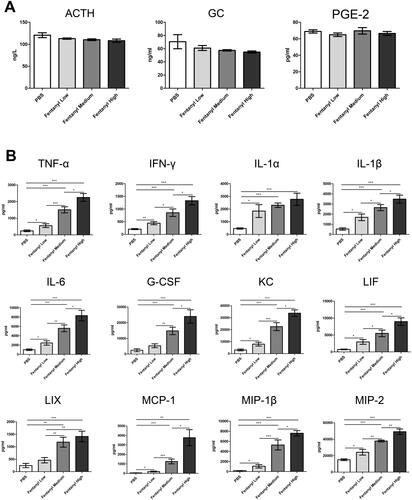
Fentanyl significantly elevated the secretion of multiple inflammatory cytokines/chemokines in the colonic mucosa of TNBS model
Fentanyl upregulated the production of TNF-α, IFN-γ, IL-1a, IL-1β, IL-6, G-CSF, KC, LIF, LIX, MCP-1, MIP-1β, and MIP-2 by multiplex ELISA. Moreover, the levels of most inflammatory cytokines and chemokines were enhanced with increasing fentanyl doses ().
Figure 4. Fentanyl exhibited no significant effects on the serum levels of adrenocorticotropic hormone (ACTH), glucocorticoid (GC), and prostaglandin E2 (PGE-2), while significantly increasing the synthesis of multiple inflammatory cytokines and chemokines in the colonic mucosa from 2,4,6-trinitrobenzenesulfonic acid solution (TNBS)-treated mice. Murine acute colitis was triggered by TNBS on day 0. PBS (control) or fentanyl (0.1, 0.25, or 0.5 mg/kg) was intraperitoneally administered on days 0 and 1 (n = 10 each group). Euthanasia of the mice was performed on day 2. (A) The levels of ACTH, GC, and PGE-2 in the serum by enzyme-linked immunosorbent assay (ELISA); (B) Expression levels of multiple inflammatory cytokines and chemokines in the colonic mucosa by multiplex ELISA. Data are mean ± SEM of experiments (7–10 mice per group). *p<.05; **p<.01; ***p<.001.
Fentanyl exhibited no significant effects on ACTH, GC, and PGE-2 serum levels in DSS- and TNBS-treated mice
We measured the levels of three hormones that are closely related to opioids and inflammation. ELISA revealed that fentanyl exhibited no obvious effects on the serum levels of ACTH, GC, and PGE-2 in DSS- and TNBS-treated mice ( and ).
Fentanyl enhanced the proportions of MOR + Th1 cells and MOR + macrophages in colonic LPMCs of DSS- and TNBS-treated mice
To identify the immune cells regulated by fentanyl in intestinal inflammation, flow cytometry was used to compare the cells isolated from DSS model treated with PBS with DSS model treated with fentanyl, and compare TNBS model treated with PBS with TNBS model treated with fentanyl. Fentanyl increased the proportion of Th1 cells in the LPMCs of DSS model and the proportions of Th1 cells and macrophages in the LPMCs of TNBS model. The proportions of MOR + Th1 cells and MOR + macrophages were found to be higher in the colonic LPMCs of both DSS and TNBS models treated with fentanyl compared to PBS ().
Figure 5. Fentanyl increased the proportions of μ-opioid receptor (MOR)+ Th1 cells and MOR + macrophages of lamina propria mononuclear cells (LPMCs) from the colonic mucosa of dextran sodium sulfate (DSS) and 2,4,6-trinitrobenzenesulfonic acid solution (TNBS) models. LPMCs of colonic mucosa were stained with CD4, IFN-γ, CD11b, F4/80, and MOR, followed by flow cytometry. CD4 + IFN-γ+ cells were considered as Th1 cells; F4/80+ CD11b + cells as macrophages; CD4 + IFN-γ+ MOR + cells as MOR + Th1 cells; and F4/80+ CD11b + MOR + cells as MOR + macrophages. (A) MOR + Th1 cells and MOR + macrophages were isolated from Th1 cells and macrophages of LPMCs from the colonic mucosa of DSS- and TNBS-administered mice exposed to PBS or medium-dose fentanyl (0.25 mg/kg; n = 5 each group), respectively; (B) Proportion statistics of Th1 cells, MOR + Th1 cells, macrophages, and MOR + macrophages of LPMCs. Data are mean ± SEM of experiments (5 mice per group). *p<.05; **p<.01; ***p < 0.001 (fentanyl-treated vs. PBS control in the DSS or TNBS model).
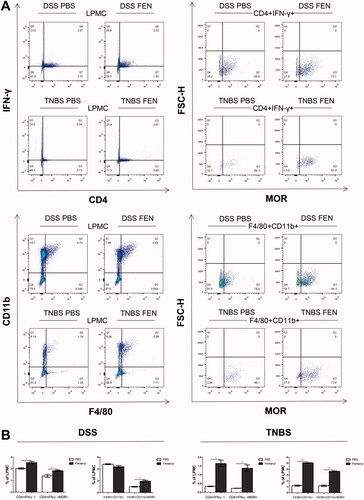
Fentanyl upregulated inflammatory cytokine/chemokine secretion in MOR + macrophages in the colonic LPMCs of DSS model, while it failed to upregulate the synthesis of most inflammatory cytokines/chemokines in MOR + macrophages in the colonic LPMCs of TNBS model
To evaluate the effects of fentanyl on the production of cytokines and chemokines in MOR + macrophages from colonic LPMCs of IBD mice, we performed multiplex ELISA on the culture media of MOR + macrophages. Fentanyl upregulated the production of IFN-γ, IL-1α, G-CSF, LIX, and MIP-1β in DSS model. However, in the TNBS model, only MIP-1β level was elevated in fentanyl-administered mice compared to the control mice ().
Figure 6. Fentanyl upregulated the production of inflammatory cytokines and chemokines in μ-opioid receptor (MOR)+ macrophages in the colonic lamina propria mononuclear cells (LPMCs) of dextran sodium sulfate (DSS) model, but failed to induce the synthesis of most inflammatory cytokines and chemokines from MOR + macrophages of the colonic LPMCs from 2,4,6-trinitrobenzenesulfonic acid solution (TNBS) model. The same quantity of flow-sorted MOR + macrophages were collected from each group (DSS control, DSS medium-dose fentanyl, TNBS control, and TNBS medium-dose fentanyl groups), and cultivated. The supernatant was analyzed using multiplex enzyme-linked immunosorbent assay (ELISA) after incubation for 24 h. The figure shows inflammatory cytokines and chemokines synthesized by F4/80 + CD11b + MOR + cells of LPMCs from each group. Experiments were repeated four times. *p<.05; **p<.01; ***p<.001 (fentanyl-treated vs. PBS control in the DSS or TNBS model).
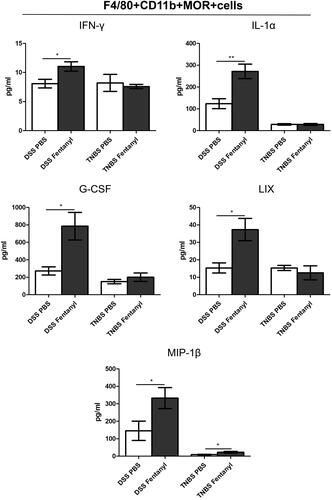
Morphine exhibited no significant effect on colitis in the DSS model
We assessed the effect of morphine on the murine IBD model. Morphine exhibited no significant effect on the disease parameters (weight loss, diarrhea, hematochezia, colon appearance, colon length, and H&E staining of colon section) or on serum levels of ACTH, GC, and PGE-2 in DSS-treated mice (). Only the level of IL-1β was upregulated in the medium- and high-dose morphine treatment groups compared to PBS treatment in the DSS model ().
Figure 7. Morphine exhibited no significant effect on the colitis of dextran sodium sulfate (DSS) model. We fed 3% DSS water to mice on days 0–7. PBS (control) or morphine (1, 2.5, or 7.5 mg/kg) was intraperitoneally administered on days 0–6 (n = 10 each group). Euthanasia of the mice was performed on day 7. (A–C) Mice were assessed daily for weight, diarrhea, and hematochezia; (D) Disease activity index (DAI) was calculated on the basis of weight change, diarrhea, and hematochezia; (E) Colon lengths; (F) Progressive stages of colitis development; (G) 100× of representative hematoxylin and eosin (H&E)-stained sections of colon (structure: e: epithelial disruption; i: inflammatory infiltration); (H) Histology activity index (HAI) based on epithelial disruption and inflammatory infiltration; (I) Serum levels of adrenocorticotropic hormone (ACTH), glucocorticoid (GC), and prostaglandin E2 (PGE-2) using enzyme-linked immunosorbent assay (ELISA); (J) Cytokine levels in the colonic mucosa by multiplex ELISA. Data are expressed as mean ± SEM of experiments (10 mice per group). Asterisk represents significance in morphine treatment (7.5 mg/kg) group compared to the PBS control group in A, respectively. *p<.05; **p<.01; ***p<.001.
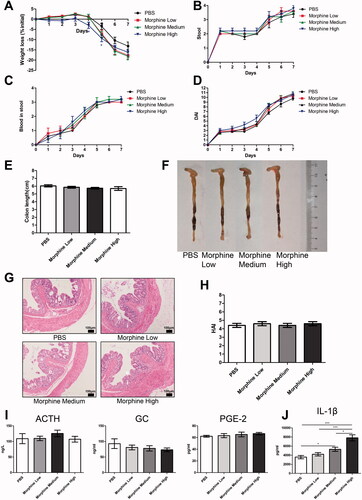
Morphine had no obvious effect on colitis in the TNBS model
Similar to the effect of morphine on DSS-treated mice, no significant effects on weight loss, colon appearance, colon length, H&E staining of colon section, and hormone levels (ACTH, GC, and PGE-2) were observed in TNBS-treated mice (). Only the level of IL-1α was enhanced in the high-dose morphine group compared to the TNBS-treated control group ().
Figure 8. Morphine exerted no significant effect on colitis induced by 2,4,6-trinitrobenzenesulfonic acid solution (TNBS). Acute colitis was triggered by TNBS on day 0. PBS (control) or morphine (1, 2.5, or 7.5 mg/kg) was intraperitoneally administered on days 0 and 1 (n = 10 each group). Euthanasia of the mice was performed on day 2. (A) Weight change; (B) Colon lengths; (C) Progressive stages of colitis development; (D) 100× of representative hematoxylin and eosin (H&E)-stained sections of colon (structure: e: epithelial disruption; i: inflammatory infiltration); (E) Histology activity index (HAI) based on epithelial disruption and inflammatory infiltration; (F) The serum levels of adrenocorticotropic hormone (ACTH), glucocorticoid (GC), and prostaglandin E2 (PGE-2) using enzyme-linked immunosorbent assay (ELISA); (G) Cytokine levels in the colonic mucosa by multiplex ELISA. Data are expressed as mean ± SEM of experiments (10 mice per group). *p<.05; **p<.01; ***p<.001.
Discussion
Opioids are increasingly being used for analgesic treatment worldwide [Citation27,Citation28]. Moreover, a significant rise in opioid prescription and frequent use of strong opioids for IBD patients has been observed, which is associated with an increase in the premature mortality rate [Citation9,Citation10]. However, the mechanisms by which frequent use of strong opioids increases the mortality of patients with IBD is unclear. We explored the immunomodulatory effects of fentanyl and morphine, two strong opioids, in IBD models, in this study. We found that fentanyl exacerbated murine colitis via Th1 cell- and macrophage-mediated mechanisms, while morphine exhibited no significant effect.
The immunomodulatory effect of opioids is complicated, the administration of different opioids plays different roles in the immune system, such as immunosuppression, immunostimulation, or dual effects [Citation17]. MOR is the main target receptor for fentanyl and morphine [Citation19]. MOR is different from the other two opioid receptors (DOR and KOR), and is expressed on the surface of a variety of immune cells [Citation18,Citation29]. We used three different doses of fentanyl and morphine. Fentanyl (0.1 mg/kg) and morphine (1 mg/kg) intraperitoneal injections had an analgesic effect, and higher doses of fentanyl and morphine had longer latency duration in mice [Citation30–32]. The three doses of fentanyl and morphine used in this study were selected in accordance with the dose conversion relationship between humans and mice.
We detected the key hormones of the HPA axis, ACTH and GC, and found that fentanyl and morphine exhibited no significant effects on their serum levels in both DSS- and TNBS-treated mice. The interaction between opioids and the HPA axis depends on the species and the duration of administration [Citation33]. Moreover, reports about HPA changes after long-term or chronic opioid use in human are scarce [Citation34]. In rodents, it is difficult to distinguish elevated cortisol induced by stress (due to animal treatment/injection) from opioid-induced changes [Citation33]. Neither fentanyl nor morphine had a significant effect on PGE-2 levels. Our future studies will include other inflammation-related endocrine hormones, which may be regulated by fentanyl and morphine.
We found a fentanyl dose-independent effect in DSS-treated mice, while a significant fentanyl dose-dependent effect was observed in TNBS-treated mice. We speculate that this may be related to the colitis model or the duration of drug administration. Fentanyl aggravated DSS- and TNBS-induced colitis, which is consistent with reports of increased mortality in IBD patients due to the use of strong opioids. Postoperative fentanyl use could increase the serum levels of IL-2 in humans [Citation23]. Intravenous fentanyl administration increased natural killer cell (NK) cytotoxicity in humans [Citation35]. These data suggest that fentanyl enhanced the in vivo immune response in both humans and mice. However, fentanyl played a different immune role in vitro [Citation23]. Fentanyl suppressed cytokines secreted by macrophages and T cells in vitro [Citation36]. This difference suggests that the immunomodulatory effects of fentanyl in vivo are related not only to the receptor but also to the neuroendocrine system, endogenous opioids, interactions with the immune system, and other factors [Citation19,Citation37]. Therefore, the observed immunomodulatory effects of fentanyl in vitro may not reflect the in vivo effects. Morphine caused immunosuppression and/or immunostimulation in non-IBD models [Citation20–23]. However, morphine did not show obvious immunomodulatory effects on the two IBD models in our present study. These observations suggest that the immunomodulatory effects of morphine may vary depending on the host, organ system (such as the digestive system), and experimental model.
We found that fentanyl could increase the proportions of MOR + Th1 cells and MOR + macrophages in the colonic LPMCs of DSS and TNBS models, and cause upregulation of inflammatory cytokine and chemokine secretion by Th1 cells and macrophages, which increase the inflammation. It is noteworthy that macrophage proportions of colonic mucosal LPMCs were unchanged in DSS model, but increased in TNBS model after fentanyl administration. Additionally, fentanyl upregulated inflammatory cytokine and chemokine production from MOR + macrophages in the colonic LPMCs of the DSS model, while it failed to induce the synthesis of most inflammatory cytokines and chemokines from MOR + macrophages in the colonic LPMCs of TNBS model. The differences between these two models may be related to the model or duration of fentanyl administration. Morphine showed no obvious immunomodulatory effect on IBD mice; only one of the 32 cytokines and chemokines was upregulated (IL-1β in DSS model and IL-1α in TNBS model). Even though the present study used fentanyl and morphine, which are both representative strong opioids, we observed a significant difference in the immunomodulatory effects on the IBD mice. Philippe et al. found that the MOR agonists could inhibit murine colitis. Additionally, MOR-deficient mice were more prone to inflammation progress compared to the wild-type mice [Citation38]. Therefore, we hypothesize that MOR is a receptor that can exert both immunosuppressive and immunostimulatory effects in IBD: when activated by endogenous opioid peptides, MOR mainly plays an immunosuppressive role, but the effect is different when it is activated by other strong exogenous opioids (such as morphine or fentanyl). The different immunomodulatory effects may be related to differences in ligand-receptor binding and effects on the neuroendocrine system caused by different ligands. Morphine had no obvious immunomodulatory effect in the IBD model, but its side effects still cannot be ignored. Our future studies will also evaluate the role of other strong opioids in IBD.
In conclusion, fentanyl exacerbates DSS- and TNBS-induced colitis via Th1 cell- and macrophage-mediated mechanisms, while morphine exhibits no significant effect. Although further clinical studies are required, this study provides guidance on the clinical application of fentanyl and morphine in IBD patients. We suggest that strong opioids should be divided into categories and that the immunomodulatory effects of each class on IBD should be carefully assessed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aghazadeh R, Zali MR, Bahari A, et al. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol. 2005;20(11):1691–1695.
- Nahon S, Ramtohul T, Paupard T, et al. Evolution in clinical presentation of inflammatory bowel disease over time at diagnosis: a multicenter cohort study. Eur J Gastroenterol Hepatol. 2018;30(10):1125–1129.
- Perler BK, Ungaro R, Baird G, et al. Presenting symptoms in inflammatory bowel disease: descriptive analysis of a community-based inception cohort. BMC Gastroenterol. 2019;19(1):47.
- Vegni E, Gilardi D, Bonovas S, et al. Illness perception in inflammatory bowel disease patients is different between patients with active disease or in remission: a prospective cohort study. J Crohns Colitis. 2019;13(4):417–423.
- Palm O, Moum B, Jahnsen J, et al. Fibromyalgia and chronic widespread pain in patients with inflammatory bowel disease: a cross sectional population survey. J Rheumatol. 2001;28(3):590–594.
- Foy R, Leaman B, McCrorie C, et al. Prescribed opioids in primary care: cross-sectional and longitudinal analyses of influence of patient and practice characteristics. BMJ Open. 2016;6(5):e010276.
- Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985.
- Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths – United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Burr NE, Smith C, West R, et al. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol. 2018;16(4):534–541.e6.
- Targownik LE, Nugent Z, Singh H, et al. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol. 2014;109(10):1613–1620.
- Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5(10):1126–1139.
- Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009;155(1–3):11–17.
- Pauly NJ, Michailidis L, Kindred MG, et al. Predictors of chronic opioid use in newly diagnosed Crohn's disease. Inflamm Bowel Dis. 2017;23(6):1004–1010.
- Banta-Green CJ, Von Korff M, Sullivan MD, et al. The prescribed opioids difficulties scale: a patient-centered assessment of problems and concerns. Clin J Pain. 2010;26(6):489–947.
- Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11(7):1063–1071.
- Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979–1986.
- Liang X, Liu R, Chen C, et al. Opioid system modulates the immune function: a review. Transl Perioper Pain Med. 2016;1(1):5–13.
- Sobczak M, Sałaga M, Storr MA, et al. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol. 2014;49(1):24–45.
- Al-Hashimi M, Scott SW, Thompson JP, et al. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111(1):80–88.
- Tomassini N, Renaud FL, Roy S, et al. Mu and delta receptors mediate morphine effects on phagocytosis by murine peritoneal macrophages. J Neuroimmunol. 2003;136(1–2):9–16.
- Cornwell WD, Lewis MG, Fan X, et al. Effect of chronic morphine administration on circulating T cell population dynamics in rhesus macaques. J Neuroimmunol. 2013;265(1–2):43–50.
- Filipczak-Bryniarska I, Nowak B, Sikora E, et al. The influence of opioids on the humoral and cell-mediated immune responses in mice. The role of macrophages. Pharmacol Rep. 2012;64(5):1200–1215.
- Liu Z, Gao F, Tian Y. Effects of morphine, fentanyl and tramadol on human immune response. J Huazhong Univ Sci Technolog Med Sci. 2006;26(4):478–481.
- Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249.
- Obermeier F, Kojouharoff G, Hans W, et al. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116(2):238–245.
- Melhem H, Spalinger MR, Cosin-Roger J, et al. Prdx6 deficiency ameliorates DSS colitis: relevance of compensatory antioxidant mechanisms. J Crohns Colitis. 2017;11(7):871–884.
- Lippold KM, Jones CM, Olsen EO, et al. Racial/ethnic and age group differences in opioid and synthetic opioid-involved overdose deaths among adults aged ≥18 years in metropolitan areas - United States, 2015–2017. MMWR Morb Mortal Wkly Rep. 2019;68(43):967–973.
- Smith BH, Fletcher EH, Colvin LA. Opioid prescribing is rising in many countries. BMJ. 2019;367:l5823.
- Zöllner C, Stein C. Opioids. Handb Exp Pharmacol. 2007;177:31–63.
- Chen W, Jin N, Lin Y, et al. Immunomodulatory effects of fentanyl or dexmedetomidine hydrochloride infusion after allogeneic heart transplantation in mice. Reg Anesth Pain Med. 2018;43(5):509–515.
- Molina-Martínez LM, González-Espinosa C, Cruz SL. Dissociation of immunosuppressive and nociceptive effects of fentanyl, but not morphine, after repeated administration in mice: fentanyl-induced sensitization to LPS. Brain Behav Immun. 2014;42:60–64.
- Newton PM, Kim JA, McGeehan AJ, et al. Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav. 2007;6(4):329–338.
- Vuong C, Van Uum SH, O’Dell LE, et al. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31(1):98–132.
- Facchinetti F, Grasso A, Petraglia F, et al. Impaired circadian rhythmicity of beta-lipotrophin, beta-endorphin and ACTH in heroin addicts. Acta Endocrinol. 1984;105(2):149–155.
- Yeager MP, Procopio MA, DeLeo JA, et al. Intravenous fentanyl increases natural killer cell cytotoxicity and circulating CD16(+) lymphocytes in humans. Anesth Analg. 2002;94(1):94–99.
- House RV, Thomas PT, Bhargava HN. In vitro evaluation of fentanyl and meperidine for immunomodulatory activity. Immunol Lett. 1995;46(1-2):117–124.
- Yardeni IZ, Beilin B, Mayburd E, et al. Relationship between fentanyl dosage and immune function in the postoperative period. J Opioid Manag. 2008;4(1):27–33.
- Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111(9):1329–1338.
