Abstract
Background
Hemorrhoidal disease (HD) is defined as the symptomatic enlargement and/or distal displacement of anal cushions and is one of the most common proctological diseases. Sclerotherapy (ST) with 3% polidocanol foam induces an inflammatory reaction with sclerosis of the submucosal tissue and consequent suspension of the hemorrhoidal tissue. The aim of this study was to evaluate the short-term effectiveness and safety of ST with 3% polidocanol foam for the treatment of symptomatic second- and third-degree HD.
Methods
A total of 66 patients with symptomatic second- and third-degree HD underwent a single ST session between March 2017 and July 2018. A visual analog scale score was used to assess post-operative pain and patient satisfaction. The symptoms severity and anal continence were investigated through the Hemorrhoid Severity Score (HSS) and Vaizey score, respectively, at baseline, at 4 weeks and after 1 year.
Results
Fifty-seven out of 66 patients were male (86.3%), and the mean age was 52 (29–75; SD ± 12) years. The mean operative time was 4.5 (2–6; SD ± 1.23) minutes. No intraoperative complications and no drug-related side effects occurred. The overall success rate was 78.8% (52/66 patients) after a single ST session and 86% after two ST sessions (57/66 patients). The mean treatment effect, obtained comparing preoperative and 12 months symptom scores in each patient, showed a median change of 8 (p < 0.001). All patients resumed their normal daily activities the day after the procedures.
Conclusions
ST with 3% polidocanol foam is a safe, cost-effective and repeatable conservative treatment.
Background
Hemorrhoidal disease (HD) is defined as the symptomatic enlargement and/or distal displacement of anal cushions [Citation1] and is one of the most common proctological diseases [Citation2–4]; however, the true prevalence of the disease has never been precisely estimated [Citation2].
According to the severity of the disease, treatment options may include dietary and lifestyle modifications, medical treatment, office-based procedures or operative treatment [Citation5].
Sclerotherapy (ST) and rubber band ligation (RBL) are the most widely used non-surgical outpatient procedures for the treatment of first-, second- and third degree HD among patients who have failed conservative treatment [Citation5, Citation6].
The satisfactory results obtained in phlebology [Citation7, Citation8] have recently led to the use of several sclerosing agents [Citation9–11] for the treatment of HD.
In particular, foam ST with polidocanol was used for the first time in 2007 in the treatment of first-degree bleeding hemorrhoids [Citation12]. Afterwards, Moser et al. [Citation11] published the first randomized, controlled, single-blind, multicentre trial in 2013 and demonstrated the superiority of this treatment with polidocanol foam in terms of the success rate (88% vs 69%) and patient satisfaction (99% vs 84%) compared with treatment with fluid sclerosant in 130 patients with first-degree HD.
ST with polidocanol induces an inflammatory reaction with sclerosis of the submucosal tissue and consequent suspension of the hemorrhoidal tissue. Moreover, obliteration of the vascular support of the hemorrhoidal cushions leads to reduction in volume.
Unfortunately, even if this treatment is a reproducible and minimally invasive procedure, severe life-threatening complications, such as abdominal compartment syndrome, necrotizing fasciitis, impotence, retroperitoneal sepsis, prostatic abscess formation and rectourethral fistula formation, have been described after treatment with liquid polidocanol [Citation13–17]; however, the only complication described to date associated with the use of foam polidocanol was an episode of prostatitis [Citation11].
The present study was performed to establish the efficacy and safety of 3% polidocanol foam in the treatment of second- and third-degree symptomatic HD.
Methods
Study design
This was a retrospective single-center study and is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for cohort studies [Citation18]. Between March 2017 to July 2018, 66 patients with symptomatic second- and third-degree HD underwent to a single ST session with 3% polidocanol foam. Demographic data, the degree and symptoms of HD, patient satisfaction and operative details were collected in our prospective personal computer database.
Patients who had history of coagulopathy, cardiac disease, anticoagulant therapies, colorectal or anal neoplasia, inflammatory bowel disease (IBD), other proctological disease (anal fistulas and fissures), or pelvic radiotherapy were excluded. The inability to return for post-operative control visits and a well-known allergy to polidocanol were also considered exclusion criteria. Written informed consent was obtained from all the patients.
Proctological examination and proctoscopy were performed to assess the grade of HD according the Goligher classification [Citation19] and to exclude any associated anorectal pathology.
All patients underwent treatment performed by the same colorectal surgeon.
After the procedure the patients were observed for two hours before being discharged in order to exclude serious adverse drug reactions or post-operative complications. A visual analog scale (VAS) score was used to assess post-operative pain and patient satisfaction (Minimum score = 0, Maximum score = 0). Vaizey incontinence score [Citation20] (Minimum score 0 = perfect continence, Maximum score 24 = totally incontinent) was used to evaluate the anal continence, before the procedure, at 4 weeks and 1 year after surgery.
At each visit, patients were also asked about itching, tenesmus, soiling and thrombosis using a dichotomous parameter (yes or not) based on a clinical diary delivered at the time of discharge. Furthermore, the symptoms severity was investigated through the Hemorrhoid Severity Score (HSS) [Citation21] before the procedure, at 4 weeks and at the final follow-up visit 1 year after surgery.
This latter is a 5-item based questionnaire assessing the frequency of pain, discomfort, itching, soiling and need for manual reduction of hemorrhoids. Each item was graded on a 3-point scale (never = 0; less than once a week = 1; 1–6 times weekly = 2; every day = 3. Minumum score = 0, Maximum score = 15).
Recently, it has been successfully validated and considered as an essential tool for future hemorrhoid trials [Citation22].
The severity of bleeding was assessed by the number of bleeding episodes per day. Bleeding was defined as persistent in cases of more than 3 episodes after day 2 following ST. Conversely, we defined occasional as less than 3 episodes.
The occurrence of adverse drug reactions was recorded as well.
Patients were followed up by the same colorectal surgeon who had performed the procedures after 1 week (T1), 4 weeks (T2), 3 months (T3), 6 months (T4) and 12 months (T5).
Follow-up consisted of clinical external evaluation at T1, and clinical evaluation, rectal digital exploration and anoscopy from T2 to T5.
No antibiotics were prescribed during the post-operative period, but stool softeners were prescribed for 7 days, and simple analgesics were prescribed in cases of pain or discomfort (1000 mg of paracetamol and/or 10 mg of ketorolac tromethamine every 4–6 hours maximum).
Successful treatment was defined as the absence of any episode of persistent bleeding or prolapse within T5.
Autonomy was defined as the return to normal daily activities, including work.
Recurrences were defined as the presence of persistent bleeding in case of second-degree HD or persistent bleeding and prolapse in case of third-degree HD.
Technique
The foam was obtained following the Moser technique [Citation11], and the amount of foam injected for each single pile was 2 mL of 3% polidocanol. Before each injection, the foam already obtained was re-emulsified for 20 seconds.
All patients were treated in the Sims position (lateral decubitus position) in our outpatient clinic using a modified Blonde-Blanchard technique with injections of polidocanol foam in the three “classical” piles at 3, 7 and 11 o’clock [Citation23]. The patients were not sedated, and no local anesthetic was used.
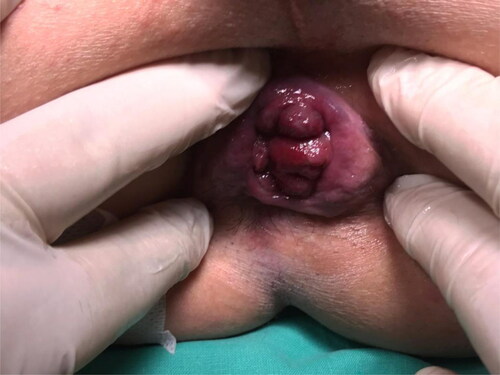
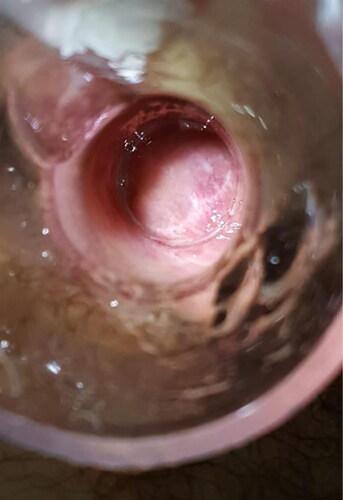
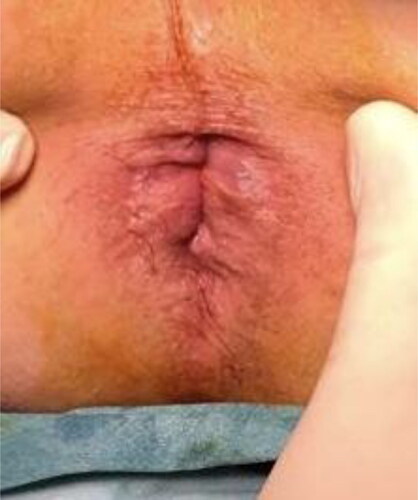
Different from previous techniques, we injected polidocanol into the hemorrhoidal piles and not into the submucosa. Furthermore, by injecting at the base of each hemorrhoidal pile above the dentate line to reduce post-operative pain, we ensured the maximum efficacy of the technique due to the fibrotic suspensory reaction ().
Figure 3. a. The injection of the 3% polidocanol foam above the dentate line. b. The swelling after the injection.
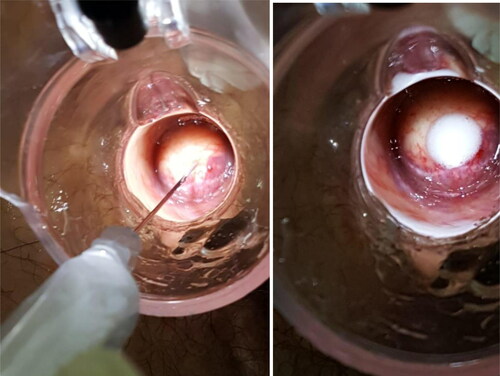
The inclination of the needle should be tangential, especially at the level of the right anterior pile (11 o’clock position) in order to avoid prostatic and urinary complications in male patients. At the end of the procedure, patients were asked to walk for approximately 20 minutes (before the pre-discharge check).
Statistical analysis
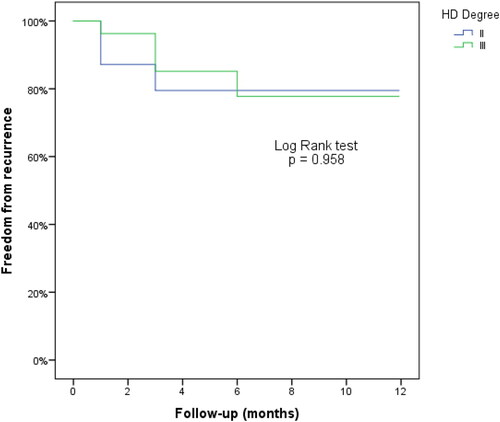
The results are reported as counts and percentages for categorical variables and as the means ± SDs (range) for continuous normally distributed variables, median [nterquartile range (IQR)] for ordinal categorical variables and for continuous not normally distributed variables. The chi-square test was used to compare the success rates between II- and III degree HD. The disease-free survival was evaluated as the time elapsed from the procedure to the relapse of disease. Kaplan-Meier curves were used to evaluate disease-free survival, and the log-rank test was used to evaluate differences between II- and III degree HD. The changes on HSS and Vaizey score over time have been analyzed with Friedman test, because that scores were not normally-distributed.
The results associated with a p-value <0.05 were considered statistically significant.
Statistical data analysis was performed using SPSS version 17.0 (Chicago, IL, USA) software for Windows.
Results
A total of 66 patients with second- (39 pts; 59%) and third-degree (27 pts; 41%) HD with a mean age of 52 (29–75; SD ± 12) years were consecutively enrolled and treated with 3% polidocanol foam. Fifty-seven out of 66 patients were male (86.3%). The mean operative time was 4.5 (2–6; SD ± 1.23) minutes. No intraoperative complications or drug-related side effects occurred. All patients were discharged 2 hours after the procedure. The procedural results are detailed in .
Table 1. Procedural results.
All patients were followed up at 12 months. The overall success rate after a single ST session was 78.8% (). There were no recurrences between 6 and 12 months (; and ). Furthermore, time to recurrences were not different between II and III degree HD and there was no significative correlation between the degree of HD and recurrences (p = 0.867).
Table 2. Cumulative percentage of success for II degree HD.
Table 3. Cumulative percentage of success for III degree HD.
The majority of patients (86%) were pain-free (VAS = 0). Nine patients reported post-operative pain (VAS: median 4, IQR 3–8) during defecation and during the first 5 post-operative days. There was 1 patient with thrombosis (after 7 days), 10 patients with tenesmus (all during the first 4 post-operative days), 6 patients with one episode of soiling during the first two days, and 6 patients with itching during the first three days ().
There were 10 patients with occasional post-operative bleeding. In 4 out of 10 patients, bleeding episodes became persistent, while the other 6 patients had resolution of this symptom. Post-operative bleeding was not associated with recurrences (p = 0.115).
No serious/life-threatening complications occurred. There was not statistically significant correlation between post-operative complications and recurrences, except for tenesmus ().
Table 4. Correlation between post-operative complications and recurrences.
All patients resumed their normal daily activities the day after the procedures.
Vaizey score significantly improved at T2 and T5, from a median preoperative value of 1 (IQR 0–3.75) to 0 (IQR 0–1) and 0 (IQR 0–0), respectively (). The treatment effect, obtained comparing preoperative and T5 symptom scores in each patient, showed a median change of 8 (IQR 7–9) (p < 0.001) ().
Table 5. Vaizey score.
Table 6. Hemorrhoid Severity Score.
Among the 14 recurrences, six (4 patients with second-degree HD and 2 patients with third-degree HD) were treated with a second ST session with an overall success rate of 86%, and the only patient with failure (third-degree HD) underwent successful dearterialization with mucopexy. The remaining 8 (2 patients with second-degree HD and 6 patients with third-degree HD) patients were treated with dearterialization and mucopexy. The second ST session was performed at least 4 weeks after the first injection.
Discussion
Polidocanol was first developed in 1931 as a detergent [Citation24], and it was introduced as anesthetic agent in Germany in 1936 [Citation25].
Currently, polidocanol is the most frequently used sclerosant for ST, and it is a nonionic surfactant that mainly targets endothelial cells.
In fact, injection of 3% polidocanol foam leads to marked vasospasm, damage to the hemorrhoidal endothelium and subsequent inflammatory reaction after only 2 minutes and induces a fibrotic reaction 30 minutes after administration [Citation26]. In particular, the foam formulation increases the proportion of active drug on the endothelium and leads to homogeneous distribution of drug microbubbles. This is a clear advantage of this formulation compared with variants of liquid sclerosing substances.
Recently, Fernandes et al. [Citation27] evaluated 2.000 consecutive patients, with II-IV degree HD, treated with polidocanol 2%. The authors reported a 98% success rate. However, this study is not directly comparable to our study for the following reasons: no validated scoring system used; the foam was obtained without a standardized system; different amount of foam (10 mL or 20 mL), different needle size (21 G) and different concentration of polidocanol (2%) have been used; follow-up was performed only at 4 weeks and in most patients by telephone.
Our overall success rate (78.8%) after one single ST session is lower than what Moser et al. [Citation11] reported. However, the results of their study are not comparable to those of our study because the authors considered only first-degree HD without the use of any validated symptoms score. Furthermore, the design of the study was completely different in the paper by Moser et al. [Citation11], which was a randomized, controlled, single-blind, multicentre trial.
Regarding the correlation between tenesmus and recurrences, further studies are needed to better specify these results. Anyway, this correlation may be probably addressed to the defecation stimulus due to the swelling of the piles induced by the injection of polidocanol.
After a second ST session in 6 patients with recurrence, our success rate reached 86%. In particular, 5 out of 6 patients responded well to the second treatment, which probably strengthened the effect of the first injection.
In our opinion, this concept is important to demonstrate the security and repeatability of the technique, considering the almost complete absence of discomfort that allowed a fast return to normal activities and the low cost of each vial of polidocanol (approximately 5 euros).
Regarding the symptomatic improvement, we cannot compare our data with other sclerotherapy-related studies but the median treatment effect of 8 () was higher than both in the rubber band ligation group of the HUBBLE trial [Citation28] and in the study by Nystrom et al. [Citation20] that compared stapled anopexy and diathermy excision, although the latter are randomized clinical trials.
The functional improvement demonstrated in 25/36 patients with a preoperative anal impairment has had a remarkable social and cost-saving effect as confirmed at the evaluation by the Vaizey score ().
In our experience, no life-threatening or major complications occurred in the perianal and prostatic area with our modified tangential approach. Furthermore, the low rate of post-operative pain was due to injection above the dentate line. In this context, the rational for the use of endoscopic ST is the improved visualization of the dentate line [Citation29]. Nevertheless, we consider the transanal approach to be superior.
There are at least two other applications of our procedure. In fact, HD is one of the most common causes of severe acute lower gastrointestinal bleeding [Citation30] requiring, sometimes, blood transfusion and haemodynamic management. In this context, we used polidocanol foam in two patients with II- and III degree HD (these patients were not considered because the follow-up period was very short) managing to get an immediate resolution of hemorrhoidal bleeding with recovery of hemoglobin values after few days of hospitalization.
Secondly, we must consider the possibility that this technique can be used as bridge to surgery. In our series 9 out of 14 recurrences successfully underwent a further minimally-invasive treatment, i.e., dearterialization and mucopexy, probably thanks to the improvement in the clinical scenario after injection of polidocanol.
Our study has some limitations. Although the data were collected prospectively, data analysis was performed retrospectively. The same surgeon performed all the procedures. Furthermore, the lack of a control group and of data on long-term effectiveness may reduce the impact of our results.
Nevertheless, to our knowledge, this is the first and largest series to date on the use of 3% polidocanol foam in the treatment of second- and third-degree HD. The high level of patient satisfaction and the complete evaluations of symptoms severity and continence function are its major strengths. Thus, further randomized trials with long-term follow-up should be designed, although no recurrences occurred between 6 and 12 months in this study.
Conclusions
ST with 3% polidocanol foam is a safe, cost-effective and repeatable conservative treatment for second- and third-degree HD. The use of this treatment as a bridge to surgery in patients with symptomatic hemorrhoids is a future area of research regarding this technique.
Authors’ contributions
GG: Substantial contributions to the conception and design of the work; acquisition, analysis, and interpretation of data for the work. Drafting the work and revising it critically for important intellectual content. Final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
PL, EN, MT contributed equally to this work: Substantial contributions to the conception and design of the work; acquisition, analysis, and interpretation of data for the work. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
RL, FP, MDS contributed equally to this work: substantial contributions to the acquisition of data for the work. Final approval of the version to be published.
AP: Drafting the work and revising it critically for important intellectual content; Final approval of the version to be published
Ethical approval
This study was approved by our local ethics committee (Comitato Etico Sezione Area Centro – Regione Calabria) and written informed consent was obtained from all patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request
| Abbreviations | ||
| HD | = | Hemorrhoidal Disease |
| ST | = | Sclerotherapy |
| RBL | = | Rubber band ligation |
| IBD | = | Inflammatory bowel disease |
| VAS | = | Visual analog scale |
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lohsiriwat V. Approach to hemorrhoids. Curr Gastroenterol Rep. 2013;15(7):332. doi:https://doi.org/10.1007/s11894-013-0332-6.
- Gallo G, Sacco R, Sammarco G. Epidemiology of hemorrhoidal disease. In: Ratto C, Parello A, Litta F (ed.), Hemorrhoids. Coloproctology. Vol 2. Cham: Springer; 2018:3–7.
- Johanson JF. Nonsurgical treatment of hemorrhoids. J Gastrointest Surg. 2002;6(3):290–294. doi:https://doi.org/10.1016/S1091-255X(01)00081-6.
- Serra R, Gallelli L, Grande R, et al. Hemorrhoids and matrix metalloproteinases: a multicenter study on the predictive role of biomarkers. Surgery 2016;159(2):487–494. doi:https://doi.org/10.1016/j.surg.2015.07.003.
- Gallo G, Martellucci J, Sturiale A, et al. Consensus statement of the Italian Society of Colorectal Surgery (SICCR): management and treatment of hemorrhoidal disease. Tech Coloprocol. 2020;24(2):145–164. doi:https://doi.org/10.1007/s10151-020-02149-1.
- Cocorullo GF, Tutino R, Falco N, et al. The non-surgical management for hemorrhoidal disease. A systematic review. G Chir. 2017;38(1):5–14. doi:https://doi.org/10.11138/gchir/2017.38.1.005.
- Uncu H. Sclerotherapy: a study comparing polidocanol in foam and liquid foam. Phlebology 2010;25(1):44–49. doi:https://doi.org/10.1258/phleb.2009.008064.
- Hamel-Desnos C, Desnos P, Wollmann JC, Ouvry P, Mako S, Allaert FA. Evalutaion of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg. 2003;29(12):1170–1175. doi:https://doi.org/10.1111/j.1524-4725.2003.29398.x.
- Takano M, Iwadare J, Ohba H, et al. Sclerosing therapy of internal hemorrhoids with a novel sclerosing agent. Comparison with ligation and excision. Int J Colorectal Dis. 2006;21(1):44–51. doi:https://doi.org/10.1007/s00384-005-0771-0.
- Yano T, Yano K. Comparison of injection sclerotherapy between 5% phenol in almond oil and aluminum potassium sulfate and tannic acid for grade 3 hemorrhoids. Ann Coloproctol. 2015;31(3):103–105.
- Moser K-H, Mosch C, Walgenbach M, et al. Efficacy and safety of sclerotherapy with polidocanol foam in comparison with fluid sclerosant in the treatment of first-grade haemorrhoidal disease: a randomized, controlled, single-blind, multicentre trial. Int J Colorectal Dis. 2013;28(10):1439–1447. doi:https://doi.org/10.1007/s00384-013-1729-2.
- Moser KH. Evaluation of efficacy and safety of polidocanol foam in the sclerotherapy of first degree bleeding haemorrhoids. Phlebol Rev (Przeglad Flebologiczny) 2007;15:103–106.
- Yang P, Wang YJ, Li F, Sun JB. Hemorrhoid sclerotherapy with the complication of abdominal compartment syndrome: report of a case. Chin Med J. 2011;124 (12):1919–1920.
- Schulte T, Fandrich F, Kahlke V. Life-threatening rectal necrosis after injection sclerotherapy for haemorrhoids. Int J Colorectal Dis. 2008;23(7):725–726. doi:https://doi.org/10.1007/s00384-007-0402-z.
- Namasivayam J, Payne D, Maguire D. Prostatic abscess following injection of internal haemorrhoids. Clin Radiol. 2000;55(1):67–68. doi:https://doi.org/10.1053/crad.1999.0066.
- Barwell J, Watkins RM, Lloyd-Davies E, Wilikins DC. Life-threatening retroperitoneal sepsis after hemorrhoid injection sclerotherapy: report of a case. Dis Colon Rectum. 1999;42(3):421–423. doi:https://doi.org/10.1007/BF02236364.
- Gupta N, Katoch A, Lal P, Hadke NS. Rectourethral fistula after injection sclerotherapy for haemorrhoids, a rare complication. Colorectal Dis. 2011;13(1):105. doi:https://doi.org/10.1111/j.1463-1318.2009.02156.x.
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi:https://doi.org/10.1016/j.ijsu.2014.07.013.
- Goligher JC, Duthie HL, Nixon HH. Surgery of the anus, rectum and colon. Vol 5. London: Baillière Tindall; 1984:98–149.
- Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut 1999;44(1):77–80. doi:https://doi.org/10.1136/gut.44.1.77.
- Nystrom PO, Qvist N, Raahave D, Lindsey I, Mortensen N. Randomized clinical trial of symptom control after stapled anopexy or diathermy excision for haemorrhoid prolapse. Br J Surg. 2010;97(2):167–176. doi:https://doi.org/10.1002/bjs.6804.
- Lee MJ, Morgan J, Watson A, Jones GL, Brown SR. A validated severity score for haemorrhoids as an essential prerequisite for future haemorrhoid trial. Tech Coloproctol. 2019;23(1):33–41. doi:https://doi.org/10.1007/s10151-019-01936-9.
- Lobascio P, Minafra M, Laforgia R, Giove C, Trompetto M, Gallo G. The use of sclerotherapy with polidocanol foam in the treatment of second-degree haemorrhoidal disease – a video vignette. Colorectal Dis. 2019; 21(2):244–245. doi:https://doi.org/10.1111/codi.14498.
- van der Vleuten CJ, Kater A, Wijnen MH, Schultze Kool LJ, Rovers MM. Effectiveness of sclerotherapy, surgery, and laser therapy in patients with venous malformations: a systematic review. Cardiovasc Intervent Radiol. 2014;37(4):977–989.
- Leopold CS, Maibach HI. Effect of cutaneously applied nonionic surfactants and local anesthetic bases on thermal sensations. Pharmazie. 2004;59(1):50–54.
- Orsini C, Brotto M. Immediate pathologic effects on the vein wall of foam sclerotherapy. Dermatol Surg. 2007;33(10):1250–1254. doi:https://doi.org/10.1111/j.1524-4725.2007.33261.x.
- Fernandes V, Fonseca J. Polidocanol foam injected at high doses with intravenous needle: the (almost) perfect treatment of symptomatic internal hemorrhoids. GE Port J Gastroenterol. 2019;26(3):169–175. doi:https://doi.org/10.1159/000492202.
- Brown SR, Tiernan JP, Watson AJM, et al. Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic second-degree and third-degree haemorrhoids (HubBLe): a multicentre, open-label, randomised controlled trial. Lancet. 2016;388(10042):356–364. doi:https://doi.org/10.1016/S0140-6736(16)30584-0.
- Ronconi M, Casiraghi S, Schieppati M. EndoTHeF: endo-luminal treatment of hemorrhoids with foam. Ann Colorectal Res. 2019;6(4):e86297. doi:https://doi.org/10.5812/acr.86297.
- Gayer C, Chino A, Lucas S, et al. Acute lower gastrointestinal bleeding in 1,112 patients admitted to an urban emergency medical center. Surgery 2009;146(4):600–606. doi:https://doi.org/10.1016/j.surg.2009.06.055.