Abstract
Opt-out strategies have been shown to improve participation in cancer screening; however, there are ethical concerns regarding the presumed consent. In this study, we tested an alternative opt-in strategy, called: “enhanced active choice,” in which the response options summarize the consequences of the decision. The study was conducted as part of the Maltese colorectal cancer screening program, which offers men and women, aged 60–64, a “one-off” fecal immunochemical test (FIT). A total of 8349 individuals were randomly assigned to receive either an invitation letter that featured a standard opt-in strategy (control condition), or an alternative letter with a modified opt-in strategy (enhanced active choice condition). Our primary outcome was participation three months after the invitation was delivered. Additionally, we also compared the proportion who said they wanted to take part in screening. We used multivariable logistic regression for the analysis. Overall, 48.4% (N = 4042) accepted the invitation and 42.4% (N = 3542) did the screening test. While there were no statistically significant differences between the two conditions in terms of acceptance and participation, enhanced active choice did increase acceptance among men by 4.6 percentage points, which translated to a significant increase in participation of 3.4 percentage points. We conclude that enhanced active choice can improve male screening participation. Given the higher risk of CRC in men, as well as their lower participation screening, we believe this to be an important finding.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in Malta. Mortality from the disease has declined in recent years, due to the implementation of effective methods for prevention, early detection, and treatment.Citation1,Citation2 Free routine screening, for example, was introduced in 2012, with all residents aged between 60 and 64 years being offered a “one-off” fecal immunochemical test (FIT).Citation3 The Maltese Colorectal Cancer Screening Programme (CRSP) consists of two stages: a preliminary stage, in which all individuals eligible to participate in the program were sent a pre-invitation letter though the mail, and a second stage, where individuals who responded to the first stage to “opt in” were sent a free FIT kit and pre-addressed return envelope.Citation4
At present, screening uptake is below the international Benchmark for acceptable participation set out by the European guidelines for quality assurance in bowel cancer screening (i.e. 65%), with only 35.7% of the invited population accepting the screening test offer.Citation5 For screening programmes to be effective and cost-effective, it is imperative they achieve and maintain high participation. There is thus a strong public health mandate in Malta for low cost, evidence-based approaches to make screening more accessible.
Previous studies have shown that opt-out invitations, which automatically invite those who do not reply, result in higher participation than “opt-in” invitations, as individuals participate by default, without having to explicitly consent.Citation6–9 However, there have been concerns about the ethics of this practice.Citation10,Citation11 The opt-out approach is based on the status-quo bias and loss aversion, which result in individuals choosing the default option.Citation8,Citation12
While opt-out defaults have the advantage of including individuals who would not otherwise participate, there are some limitations for using them in CRC screening. Firstly, although as a result of inaction, participation may be enhanced in the short-term, screening programs with regular screening intervals require a longer commitment to screening to ensure public health benefits are achieved. Secondly, the provision of a single rigid "opt-out option" can disadvantage some individuals, if there is no single optimal behavior for everybody. In such situations, opt-out are less beneficial.Citation13 Thirdly, substantial waste and inefficiency may result from "no-shows" among those sent test kits as a consequence of a default policy.Citation13 Finally, unsolicited participation in the testing phase can be considered unethical, and may be legally challenged, since informed consent to participate was not obtained.Citation10,Citation11
Several studies, therefore, have championed alternative behavioral interventions that allow individuals to make an explicit decision.Citation14 A possible alternative approach consists of individuals affirmatively choosing between attending screening or not. Different to the opt-out or opt-in, this “active choice” approach focuses on individuals making an explicit choice. Several studies on organ donation, retirement planning and immunization and enrollment in a prescription automatic refill program proposed and investigated such active decisions.Citation13,Citation15–17
Spital found in representative surveys that individuals would support active choice approaches that force them to state whether they want to donate their organs and advocate a policy that would require individuals to record their decision.Citation16,Citation18,Citation19 Carroll and colleagues found in an observational study that required individuals to explicitly choose between enrolling and not enrolling in a retirement plan significantly increased enrollment compared to opt-in.Citation17
Keller and colleagues were the first to test the active choice approach in lab and field experiments in the context of flu shots and prescription refills.Citation13 They showed that the effectiveness of active choice can be enhanced by highlighting some of the key advantages and disadvantages of the two choice alternatives. Specifically, by framing the response options in such a way that they favor the desired option by highlighting its benefits and losses incumbent to the alternative, they found that active choice increased intentions and adherence compared to opt-in. They called their approach “enhanced active choice,” as it advantaged the option preferred by the communicator in the framing of the options.
Enhanced active choice targets procrastination, or decision avoidance, by appealing to regret aversion, or anticipated regret, from not choosing the desired option.Citation13 Using active choice approaches that require individuals to make an active decision about participating in a public health program could increase public commitment and public health.Citation10,Citation19,Citation20
In this study, we tested the effectiveness of enhanced active choice, against a standard opt-in approach, to promote acceptability and uptake in the Maltese CRSP. To the best of our knowledge, this is one of the first study to investigate enhance active choice in cancer screening. A previous study by Metha and colleagues did not find that enhanced active choices increased colonoscopy uptake among an employee population. Citation21
In line with the previous literature, we hypothesized that enhanced active choice would increase the likelihood of accepting the screening invite and participate in the program.
Methods
To investigate the effectiveness of enhanced active choice in a population-based CRC screening program, we conducted a randomized controlled trial (RCT) with two conditions. In total 8349 adults, aged between 60 and 64 (4287 [51.3%] screening naïve women and 4062 [48.7%] men), were invited in 2015 and individually randomized by a computer (2:1 allocation) to receive either the enhanced active choice condition (N = 5749) or the control condition (N = 2600). Unequal allocation was used to test within-group differences between individuals allocated to the enhanced active-choice condition.
As per usual care, the invitation mailing kit contained a pre-invitation letter with a short description of CRC and its screening methods. The mailing kit was in English and Maltese. The back of the letter contained a closed question about the person's interest in participating in the screening program and receiving a free FIT test by mail: “Would you like to participate and get screened for colorectal cancer?” The only difference between the two conditions was the framing of the response options to the question. In the English version, the control condition featured the response options “No” and “Yes,” while the enhanced active choice condition had "Yes, I want to participate and get screened for colorectal cancer to reduce the risk of suffering from this cancer later and to benefit from the effectiveness of early treatments in case of having this cancer" and "No, I would not like to participate in the colorectal cancer screening programme, even if it makes later medical treatments in case I should have this cancer more difficult." The content was developed with the Ministry for Health Malta and was aimed at reminding individuals, at the moment of making the screening decision, about the potential difficulties of late cancer diagnosis. The English and Maltese versions of the invitation can be found at OSF: https://osf.io/jrafg/.
Both mailings included a pre-addressed return envelope. The primary outcome of interest was participation within each study group, which was defined as returning the FIT kit. A secondary outcome of interest was acceptance of the screening invite, which was defined as communicating with the screening program that they want to receive a test kit.
Descriptive statistics were used to report the number of people who accepted the invite and participated by returning the kit within three months. Multivariable logistic regression was used to test for differences between the control and experimental condition, after adjusting for gender as a co-variate. Exploratory subgroup analyses were performed by gender, to test for independent effects for men and women. Sample size was calculated prior to data collection and based on real uptake and estimated effect sizes from the literature. The study was sufficiently powered to detect differences of at least 3% in participation between conditions, with a power of 80% and an alpha value of 0.05.Citation22 The study protocol was approved by the Health Ethics Committee.
Results
and show acceptance and participation across the two experimental conditions; 47.3% (N = 1229) in the control condition and 48.9% (N = 2813) in the enhanced active choice condition contacted the center to participate in screening, indicating that the new the enhanced active choice intervention did not significantly increase acceptance (adjusted Odds Ration [aOR] 1.07; 95% confidence intervals [CI]: 0.98–1.18, p = 0.141). Similarly, there was no statistically significant difference in participation between the two conditions; participation was 42.7% (N = 2452) and 41.9% (N = 1090) in the enhanced active choice and control conditions, respectively (aOR 1.03; 95% CI: 0.94–1.13, p = 0.565). Overall 48.4% accepted the screening invitation and 42.4% participated
Figure 1. Acceptance of the screening invite and participation in the screening programme in the two experimental conditions (N = 8349).
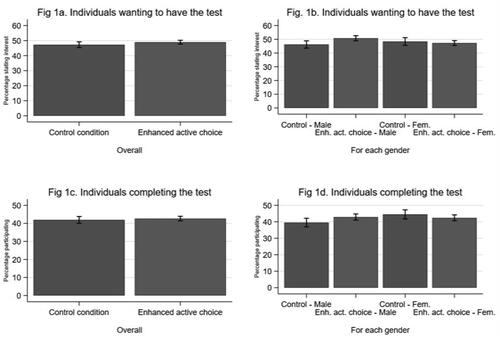
Table 1. Logistic regression on acceptance and participation (N = 8349).
The results of the interaction analysis demonstrate that enhanced active choice increased acceptance among men by 4.6 percentage points (50.8% vs 46.2%; OR 1.20; 95% CI: 1.05–1.37, p = 0.006), which translated to a significant 3.4 percentage point increase in participation (42.9% vs 39.5%, aOR 1.15; 95% CI: 1.01–1.31, p = 0.040). Among women, enhanced active choice did not affect acceptance or participation; 48.4% (N = 607) of women accepted the invitation in the control condition, while 47.3% (N = 1434) accepted it in the enhanced active choice condition (p = 0.502). While not statistically significant, participation for women was higher in the control condition (44.5%) than in the enhanced active choice condition and (42.4%, p = 0.214). In the control condition, female participation was significantly higher than male participation (44.5% vs 39.5%; OR 1.23; 95% CI: 1.05–1.43, p = 0.010).
Discussion
Our field experiment found that enhanced active choice did not increase overall screening acceptance or participation compared to the standard opt-in process. Enhanced active choice did, however, significantly increase acceptance and participation among men. While the results for the male population are in line with previous research, which shows a positive impact of an enhanced active choice on individual commitment, the almost statistically significant negative effect impact of enhanced active choice on female screening behavior merits some further investigation.Citation13 Some studies have argued that the higher participation rate may be caused by the increased perceived responsibility with the decision and anticipated regret.Citation13,Citation23,Citation24
The finding that active choice improved CRC screening acceptance and participation among men, specifically, is highly important. Men have higher risk of CRC and CRC death compared with women.Citation25 In line with previous studies we find that men are less likely to participate in the control condition than women.Citation25,Citation26 Adding active choice to the invitation letter, therefore, could improve participation and, thereby, the proportion of CRCs diagnosed in men at an early stage. This, in turn, would reduce differences in CRC deaths between the sexes.
Finally, while overall uptake was higher than in previously reported studies, it was still below the international Benchmark for acceptable participation, implying that other evidence-based interventions should be tested to make screening more accessible in Malta.Citation5
Strengths of our study include the use of a randomized controlled trial design and the use of objective data for acceptance and participation. Our study shows the importance of proof-of-concept experiments to generate reliable evidence of the interventions in other settings.Citation27 While enhanced active choice has been used successfully in the context of immunization and enrollment in a prescription automatic refill program, we found limited evidence that it affects cancer screening behavior.Citation13
A limitation of our study is that we only collected information about the participant’s gender for the analysis. Little is known about how other socio-economic variables, such as education and health literacy, would influence the perception of enhanced active choice, as Keller and colleagues only report univariate statistics, and do not provide information about the descriptive characteristics of their samples.Citation13 Future studies could test whether the effect of enhanced active choice affect is mitigated by socio-economic characteristics of the participants. A further limitation of this study is that the messages used as response options in the enhanced active choice condition were not co-developed with patients, but stakeholders in the screening program. It is, therefore, possible that the messages did not communicate the information accurately. We would, therefore, advocate using patient involvement and engagement to develop enhanced active choice interventions in future studies and implementation.
Conclusions
Our study finds that the enhanced active choice is an equivalent alternative to standard opt-in settings in terms of overall screening behavior. Its positive effect on male participation could reduce differences in screening participation and diagnosis. The study shows that enhanced active choice is relatively easy to implement and cost-neutral as it only involves changes to the framing of the participation question. This intervention could benefit many countries with similar CRC screening programs and differences in participation.
Open practices
The materials and data for the experiments are available at OSF: https://osf.io/jrafg/.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Funding
References
- Directorate for Health Information and Research, National Mortality Registry Annual Report. https://deputyprimeminister.gov.mt/en/dhir/Documents/Deaths/Annual%20Mortality%20Report%202015.pdf. Published 2015. Accessed March 20, 2020.
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi:https://doi.org/10.1136/gutjnl-2015-310912.
- Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi:https://doi.org/10.1136/gutjnl-2014-309086.
- Government of Malta. Colorectal Screening. National Colorectal Cancer Screening Programme. 2020. https://deputyprimeminister.gov.mt/en/phc/nbs/Pages/Screening-Programmes/Colorectal-Screening.aspx. Accessed March 20, 2020.
- Ponti A, Anttila A, Ronco G, Senore C, Basu P, Segnan N. 2017. Cancer screening in the European Union. Report on the Implementation of the Council Recommendation on Cancer Screening. Brussels: European Commission.
- Camilloni L, Ferroni E, Cendales BJ, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health. 2013;13(1):464. doi:https://doi.org/10.1186/1471-2458-13-464.
- Chapman GB, Li M, Colby H, Yoon H. Opting in vs opting out of influenza vaccination. Jama. 2010;304(1):43–44. doi:https://doi.org/10.1001/jama.2010.892.
- Johnson EJ, Goldstein DG. Medicine. Do defaults save lives? Science. 2003;302(5649):1338–1339. doi:https://doi.org/10.1126/science.1091721.
- Mehta SJ, Khan T, Guerra C, et al. A randomized controlled trial of opt-in versus opt-out colorectal cancer screening outreach. American Journal of Gastroenterology. 2018;113(12):1848–1854. doi:https://doi.org/10.1038/s41395-018-0151-3.
- Holm S, Ploug T. "Nudging" and informed consent revisited: why "nudging" fails in the clinical context. Am J Bioeth. 2013;13(6):29–31. doi:https://doi.org/10.1080/15265161.2013.781713.
- Ploug T, Holm S, Brodersen J. To nudge or not to nudge: cancer screening programmes and the limits of libertarian paternalism. J Epidemiol Community Health. 2012;66(12):1193–1196. doi:https://doi.org/10.1136/jech-2012-201194.
- Samuelson W, Zeckhauser R. Status quo bias in decision making. J Risk Uncertainty. 1988;1(1):7–59. doi:https://doi.org/10.1007/BF00055564.
- Keller PA, Harlam B, Loewenstein G, Volpp KG. Enhanced active choice: a new method to motivate behavior change. Journal of Consumer Psychology. 2011;21(4):376–383. doi:https://doi.org/10.1016/j.jcps.2011.06.003.
- Waller J, Macedo A, von Wagner C, et al. Communication about colorectal cancer screening in Britain: public preferences for an expert recommendation. Br J Cancer. 2012;107(12):1938–1943. doi:https://doi.org/10.1038/bjc.2012.512.
- Spital A. Mandated choice. The preferred solution to the organ shortage? Arch Intern Med. 1992;152(12):2421–2424. doi:https://doi.org/10.1001/archinte.152.12.2421.
- Spital A. Consent for organ donation: today and tomorrow. Semin Dial. 1993;6(4):264–267. doi:https://doi.org/10.1111/j.1525-139x.1993.tb00156.x.
- Carroll GD, Choi JJ, Laibson D, Madrian BC, Metrick A. Optimal defaults and active decisions. Q J Econ. 2009;124(4):1639–1674. doi:https://doi.org/10.1162/qjec.2009.124.4.1639.
- Spital A. Mandated choice. A plan to increase public commitment to organ donation. Jama. 1995;273(6):504–506. doi:https://doi.org/10.1001/jama.273.6.504.
- Spital A. Mandated choice for organ donation: time to give it a try. Ann Intern Med. 1996;125(1):66–69. doi:https://doi.org/10.7326/0003-4819-125-1-199607010-00010.
- Chouhan P, Draper H. Modified mandated choice for organ procurement. J Med Ethics. 2003;29(3):157–162. doi:https://doi.org/10.1136/jme.29.3.157.
- Mehta SJ, Feingold J, Vandertuyn M, et al. Active choice and financial incentives to increase rates of screening colonoscopy–a randomized controlled trial. Gastroenterology. 2017;153(5):1227–1229. doi:https://doi.org/10.1053/j.gastro.2017.07.015.
- Cohen J. 1988. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates.
- Cioffi D, Garner R. On doing the decision: effects of active versus passive choice on commitment and self-perception. Pers Soc Psychol Bull. 1996;22(2):133–147. doi:https://doi.org/10.1177/0146167296222003.
- Botti S, McGill AL. When choosing is not deciding: the effect of perceived responsibility on satisfaction. J Consum Res. 2006;33(2):211–219. doi:https://doi.org/10.1086/506302.
- White A, Ironmonger L, Steele RJ, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1):1–11. doi:https://doi.org/10.1186/s12885-018-4786-7.
- Hirst Y, Stoffel S, Baio G, McGregor L, von Wagner C. Uptake of the English Bowel (Colorectal) Cancer Screening Programme: an update 5 years after the full roll-out. Eur J Cancer. 2018;103:267–273. doi:https://doi.org/10.1016/j.ejca.2018.07.135.
- Stoffel S, Benito L, Milà N, et al. Testing behavioral interventions to optimize participation in a population-based colorectal cancer screening program in Catalonia. Spain. Preventive Medicine. 2019;119:58–62. doi:https://doi.org/10.1016/j.ypmed.2018.12.013.
