Abstract
Vitamin B12 deficiency is a risk factor for bone disorders via mechanisms not fully understood. In this study, an increase in serum inorganic phosphorus (Pi) concentrations was associated with a vitamin B12 deficiency. Napi2a, a renal cotransporter for Pi reabsorption, accumulated on plasma membranes in a vitamin B12 deficiency suggests that vitamin B12 plays an important role in Pi homeostasis.
Key words:
Vitamin B12 deficiency is associated with various symptoms such as abnormal organic acid metabolism, megaloblastic anemia, and neurological dysfunction. We previously reported that vitamin B12 deficiency decreased the ratio of S-adenosyl methionine (SAM), a methyl donor for DNA methylation to S-adenosyl homocysteine (SAH) or SAM:SAH.Citation1) Thus, vitamin B12 has an important role in DNA methylation. Homocysteinemia, a known risk factor for heart, kidney, and brain diseases, is also a typical symptom of vitamin B12 deficiency. Recent reports revealed that elevation of homocysteine affected an osteoblast differentiation by in vitro experiment.Citation2,3) However, homocysteine concentration in bone tissue is unchanged in vitamin B12-deficient rats.Citation4) Moreover, a lower bone mineral density (BMD), another risk factor for disorders of bone, is more likely when vitamin B12 concentrations are low.Citation5) However, the mechanisms by which a vitamin B12 deficiency contributes to a lower BMD are not fully understood. In this study, we analyzed whether a vitamin B12 deficiency may have an impact on bone disorders other than just homocysteine-dependent bone disorders.
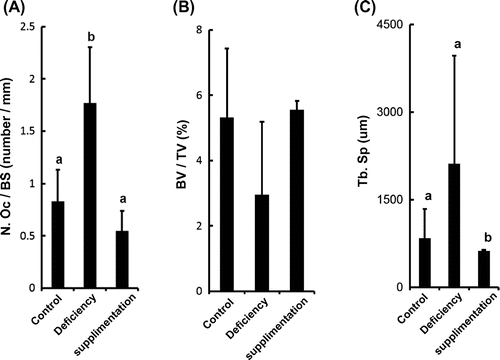
We first examined the effect of a vitamin B12 deficiency and supplementation on bone morphology in vitamin B12-deficient and vitamin B12-supplemented rats by using histomorphometric methods (OsteoMeasure, OsteoMetrics, USA). Data shown are represented as mean ± SD. Statistical significance was determined by one-way ANOVA followed by the Tukey–Kramer test for multiple comparisons. In brief, Wistar rats were weaned from dams, fed a vitamin B12-deficient diet during pregnancy, and lactation as we previously reported.Citation1) Further, these animals were subsequently divided into three groups: control, vitamin B12 deficient, and Cyano-vitamin B12 (CN-B12) supplemented (CN-B12; 1 μg/day) for seven days before sacrifice. The bone morphology results showed that osteoclasts play an important role in bone resorption, and the osteoclast number (OcN) was a rising trend in vitamin B12-deficient rats but rescued in CN-B12-supplemented rats (Fig. (A)). On the other hand, osteoblast plays an important role in bone formation, and osteoblast numbers were not affected by vitamin B12 status. (control vs. vitamin B12 deficient; 4.241 ± 1.551 vs. 4.845 ± 1.270 number/mm. Values are means ± SD, control and vitamin B12 deficient; n = 6, not significant.) These results indicated that the bone resorption was induced by elevation of OcN. Bone Volume Fraction (Bone Volume/Tissue Volume; BV/TV) in vitamin B12-deficient rats showed a trend toward being comparable to control rats (p < 0.14), whereas CN-B12-supplemented rats showed no such trend (Fig. (B)). Trabecular separation (TbSp) showed a trend toward increase in vitamin B12-deficient rats compared with the controls (p < 0.052). CN-B12-supplemented rats showed a decrease in TbSp compared to control and vitamin B12-deficient rats (Fig. (C)). Thus, from the bone morphology results, an increasing tendency of TbSp was associated with stimulated osteoclastogenesis. Previous reports have shown that bone tissue concentration of homocysteine is no different in vitamin B12-deficient rats.Citation4) Thus, these data suggest that a homocysteine (Hcy)-independent mechanism may play a role in bone disorders.
Fig. 1. Vitamin B12 deficiency affects bone morphology.
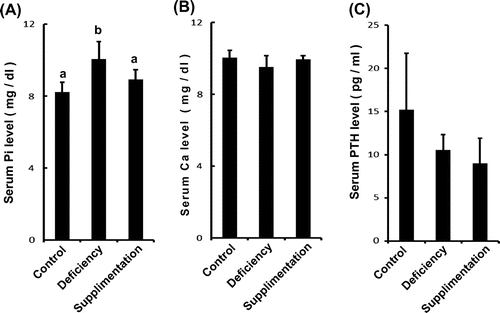
Inorganic phosphorus (Pi) has several important roles in the body and is a necessary component of bone, cell membranes, and nucleic acids.Citation5) Serum Pi concentrations are maintained by intestinal phosphorus absorption, the renal resorption system, bone, and intracellular fluid. Several hundred milligrams of Pi are absorbed daily in the intestine, and an equivalent amount is excreted in urine to maintain Pi homeostasis.Citation6) To analyze the effects of vitamin B12 on the serum Pi and calcium (Ca) homeostasis, serum Pi and Ca concentrations were measured using enzyme assays (Mitsubishi Chemical Medicine, Tokyo, Japan). Serum Pi was significantly increased in vitamin B12-deficient rats compared with controls. Supplementation with CN-B12 rescued the serum concentration of Pi which was not different from that in the control group (Fig. (A)). In contrast, serum Ca concentrations were not different among groups (Fig. (B)). Parathyroid hormone (PTH) is a Pi-regulatory hormone and controls Pi metabolism. Serum PTH concentration was measured using an electro-chemiluminescence immunoassays (Mitsubishi Chemical Medicine, Tokyo, Japan). However, the concentration of PTH was no different in vitamin B12-deficient rats compared with control (Fig. (C)). We confirmed that other Pi-regulating FGF23 hormones also remained unchanged (control vs. vitamin B12 deficient; 48.44 ± 7.01 vs. 48.44 ± 4.68 μg/ml. Values are means ± SD, control and vitamin B12 deficient; n = 4, not significant.). These results suggested that bone disorders occurring in the presence of a vitamin B12 deficiency could be caused by an elevated concentration of serum Pi.
Fig. 2. Serum Pi levels change in vitamin B12-deficient rats but not Ca and PTH levels.
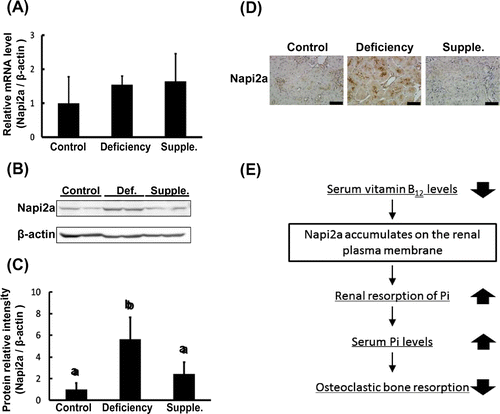
As described above, the renal resorption of Pi plays an important role in its retention in serum. Previous reports have shown that abnormal renal resorption, including in states of chronic kidney disease, induces bone disorders.Citation7) Therefore, renal metabolism is tightly involved in bone metabolism. Renal uptake occurs as serum Pi is filtered by the glomerulus and thereafter resorbed in the nephron. This resorption occurs in the proximal tubule by the sodium-dependent phosphate cotransporter (Napi2a). Napi2a is a major Pi cotransporter, which is localized in the apical brush border membrane of the proximal tubule, and is implicated in more than 70% of the renal uptake of Pi.Citation8,9) We assumed that the accumulation of serum Pi occurred as a result of Napi2a dysfunction perturbing the renal regulation of Pi resorption. Western blot and qPCR were performed to examine whether B12 concentrations affect Napi2a expression. mRNA levels were quantified using a Roche LightCycler real-time PCR machine. The following primer sequences were used: Napi2a forward 5′-TCCTCGTCAAGATGCTCAAC-3′; Napi2a reverse 5′-GAACACGGAACTGCTCTGG-3′; β-actin forward 5′-CTGACAGACTACCTCATGAAGATCC-3′; and β-actin reverse 5′-TAGCACAGCTTCTCTTTAATGTC-3′. RNA data are presented as relative mean vs. β-actin ± SD. mRNA levels of Napi2a were not observed to be different between vitamin B12-deficient rats and control (Fig. (A)). Napi2a antibodies were generated by immunize oligo peptide (CRPEPRSPQLPPRVFLEE) against rabbit. Napi2a protein levels were determined by western blotting. Protein levels of Napi2a significantly elevated in the vitamin B12-deficient rats compared with control rats. This elevation was rescued by supplementation with CN-B12 (Fig. (B) and (C)). To examine the effect of vitamin B12 on the localization of Napi2a, we performed light microscopic immunohistochemistry of Napi2a. The studies were performed as described previously.Citation10) Control rats showed a weak staining intensity of Napi2a in the renal proximal tubule (Fig. (D)). As indicated in Fig. (A) and (B), vitamin B12 deficiency showed a strong staining intensity of Napi2a and supplementation with CN-B12 decreased the intensity of Napi2a as well as control (Fig. (D)). These results suggest that the accumulation of serum Pi occurred as a result of Napi2a translocation inhibition in a B12-deficient state.
Fig. 3. Napi2a translocation is inhibited during vitamin B12 deficiency.
In Fig. , we also showed that serum concentration of Pi but not Ca accumulated according to vitamin B12 status (Fig. (A) and (B)). An increase in serum Pi concentration induced bone disorders in vitamin B12-deficient rats (Fig. ). Other reports have also indicated that vitamin B12 deficiency-induced bone disorders, such as osteopenia and osteoporosis, can increase fracture risk.Citation5,11) Thus, we suggest that an accumulation of excessive Pi in serum can cause bone disorders in a vitamin B12 deficiency state. Interestingly, the primary Pi-regulatory hormones including PTH, FGF23, and 1–25(OH)2D3, as well as dietary Pi, did not show any difference in response to vitamin B12 level (Fig. (C)). We consider that other mechanisms affect Pi dyshomeostasis during a vitamin B12 deficiency.
Renal resorption of Pi via Napi2a is important for Pi homeostasis.Citation12) Our immunohistochemistry data showed that apical expression of Napi2a was arrested by a vitamin B12 deficiency (Fig. ). The mRNA was not affected, but protein levels were elevated (Fig. ). These data suggest that Napi2a translocation is inhibited by a vitamin B12 deficiency. On the other hand, Napi2c is also known to exist on the renal plasma membrane since Napi2a has a similar topological structure to Napi2a. We hypothesize that Napi2c also accumulates on the plasma membrane in vitamin B12-deficient rats.Citation13) Translocation promotes interaction with endocytic receptors such as megalin that enables cellular internalization of nutrients.Citation14,15) Indeed, our previous studies indicated that intracellular trafficking of megalin was inhibited in the kidney by vitamin B12 deficiency.Citation10) It seems that trafficking of Napi2a was also arrested in the cell membrane with megalin. We found a novel pathway by which the inhibition of Napi2a translocation induced bone disorders in a state of vitamin B12 deficiency (Fig. (E)). Further studies are needed to examine Napi2a translocation mechanism. Here, we presented evidence of the role of vitamin B12 as a Pi-regulatory nutrient.
Authors’ contributions
Takahiro Shiga planned the experiments and wrote the draft of the article. Yoshifumi Kimira and Hiroshi Mano analyzed the data. Tetsunori Kawata contributed material tools. Tadahiro Tadokoro, Tsukasa Suzuki, and Yuji Yamamoto designed the research and approved the final version of the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Uekawa A, Katsushima K, Ogata A, et al. Change of epigenetic control of cystathionine beta-synthase gene expression through dietary vitamin B12 is not recovered by methionine supplementation. J. Nutrigenet. Nutrigenomics. 2009;2:29–36.
- Vacek TP, Kalani A, Voor MJ, et al. The role of homocysteine in bone remodeling. Clin. Chem. Lab. Med. 2013;51:579–590.
- Vaes BL, Lute C, Blom HJ, et al. Vitamin B(12) deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcif. Tissue Int. 2009;84:413–422.
- Herrmann M, Wildemann B, Wagner A, et al. Experimental folate and vitamin B12 deficiency does not alter bone quality in rats. J. Bone Miner. Res. 2009;24:589–596.
- Tucker KL, Hannan MT, Qiao N, et al. Low plasma vitamin B12 is associated with lower BMD: the framingham osteoporosis study. J. Bone Miner. Res. 2005; 20: 152–158.
- Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007;69:341–359.
- Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945–1953.
- Beck L, Karaplis AC, Amizuka N, et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA. 1998;95:5372–5377.
- Custer M, Lötscher M, Biber J, et al. Expression of Na-P(i) cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am. J. Physiol. 1994;266:F767–F774.
- Takahiro S, Tetsunori K, Tadasu F, et al. Elevation of urinary methylmolonic acid induces the suppression of megalin-mediated endocytotic cycles during vitamin B12 deficiency. Biochem. Biophys. Res. Commun. 2015;465:206–212.
- Melton ME, Kochman ML. Reversal of severe osteoporosis with vitamin B12 and etidronate therapy in a patient with pernicious anemia. Metabolism. 1994;43:468–469.
- Fukumoto S. Phosphate metabolism and vitamin D. Bonekey Rep. 2014;3:497.
- Ito M, Sakurai A, Hayashi K, et al. An apical expression signal of the renal type IIc Na+-dependent phosphate cotransporter in renal epithelial cells. Am. J. Physiol. Renal Physiol. 2010;299:F243–F254.
- Bachmann S, Schlichting U, Geist B, et al. Kidney-specific inactivation of the megalin gene impairs trafficking of renal inorganic sodium phosphate cotransporter (NaPi-IIa). J. Am. Soc. Nephrol. 2004; 15: 892–900.
- Bacic D, LeHir M, Biber J, et al. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006; 69: 495–503.
