Abstract
The flavor deterioration of mayonnaise is induced by iron, which is released from egg yolk phosvitin under acidic conditions and promotes lipid oxidation. To prevent oxidative deterioration, natural components, rather than synthetic chemicals such as ethylenediaminetetraacetic acid have been required by consumers. In the present study, we evaluated the inhibitory effects of three egg white components with the same amino acid composition, namely egg white protein, hydrolysate, and the amino acid mixture, on lipid oxidation in mayonnaise and an acidic egg yolk solution as a model system. We found that the hydrolysate had the strongest inhibitory effect on lipid oxidation among the three components. The mechanism underlying the antioxidant effect was associated with Fe2+-chelating activity. Thus, egg white hydrolysate may have the potential as natural inhibitors of lipid oxidation in mayonnaise.
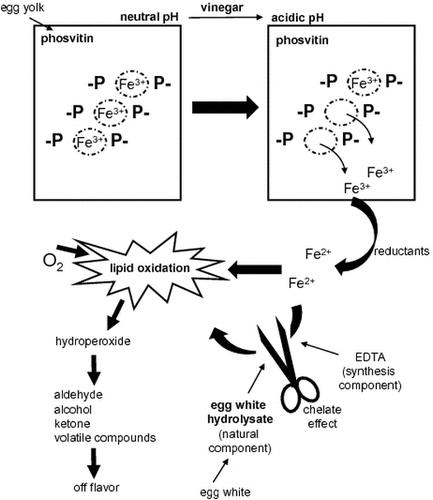
Mayonnaise is an acidic oil-in-water emulsion food that consists of vegetable oil, eggs, and vinegar. It contains nutrients such as polyunsaturated fatty acids and iron derived from egg yolk phosvitin,Citation1) which contains many phosphorylated serine residues.Citation2) Iron, which is released from egg yolk phosvitin because of the decrease in pH caused by acetic acid in vinegar, induces the oxidative degradation of mayonnaise.Citation3) In other words, phosvitin has little chelating activity in mayonnaise. To maintain the commercial value of mayonnaise, calcium disodium ethylenediaminetetraacetic acid (EDTA) has been used as a food additive over a long period in most countries. EDTA is an economical agent that is highly effective in strongly chelating iron. However, EDTA is also a chemical synthetic product that tends to give a negative image to consumers. Thus, antioxidants derived from a natural product are required as follows.Citation4–8) Previously, it was evaluated whether natural antioxidants such as phytic acid,Citation4) gallic acid,Citation5) tocopherol,Citation6) ascorbic acid,Citation7) and purple corn husk extractsCitation8) inhibited the oxidation of lipids in mayonnaise. Among these components, only purple corn husk extracts inhibited lipid oxidation in mayonnaise, but the color of mayonnaise turned purple. We have a concern that the purple color will not be acceptable to consumers.
Fig. 1. Volatile compounds and fluorescent products in the oxidation of acidic egg yolk solution as a model system of mayonnaise.
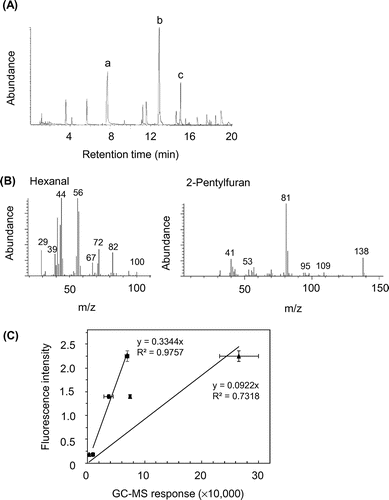
Fig. 2. Inhibitory effects of three egg white components on lipid oxidation in acidic egg yolk solution.
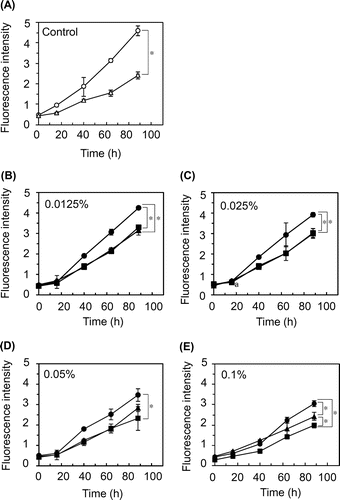
Fig. 3. DPPH radical-scavenging activity under acidic conditions.
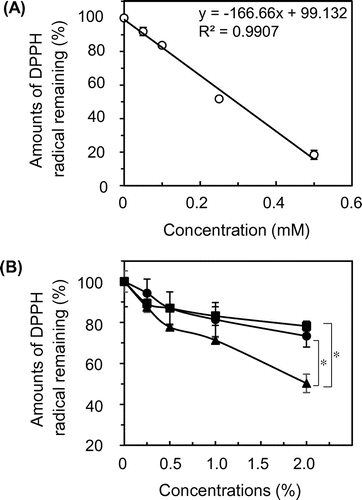
Fig. 4. Fe2+-chelating activity of three egg white components under acidic conditions.
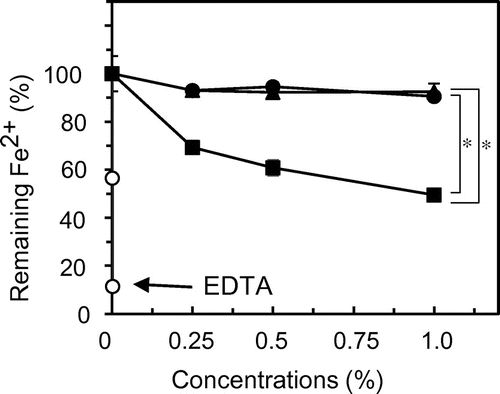
Fig. 5. Inhibitory effect of egg white hydrolysate on lipid oxidation in mayonnaise.
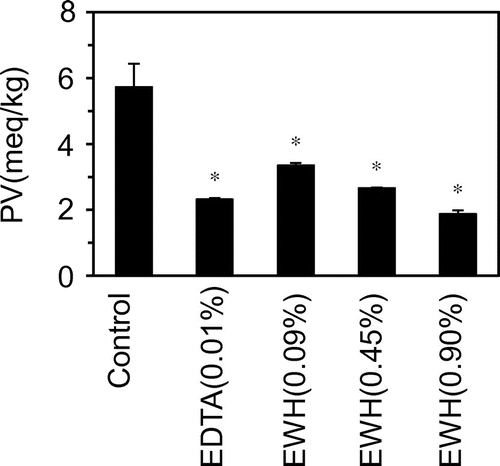
Fig. 6. Inhibitory effect of egg white hydrolysate on deterioration of mayonnaise by lipid oxidation.
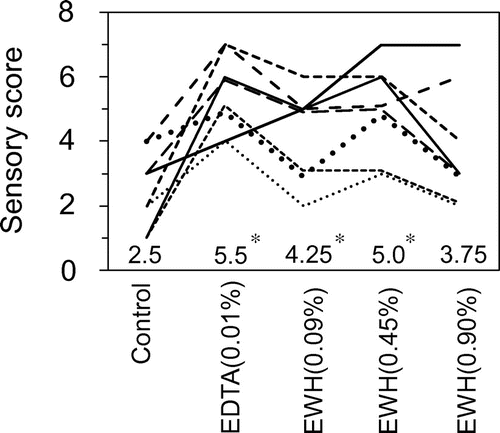
Fig. 7. Inhibitory effects of various acids on lipid oxidation in the acidic egg yolk solution.
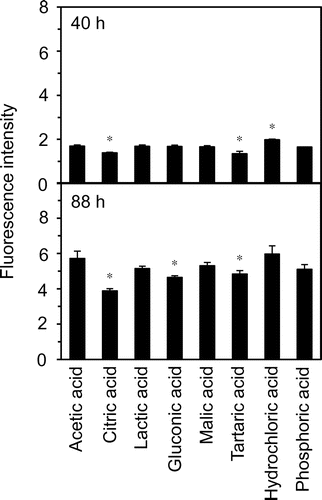
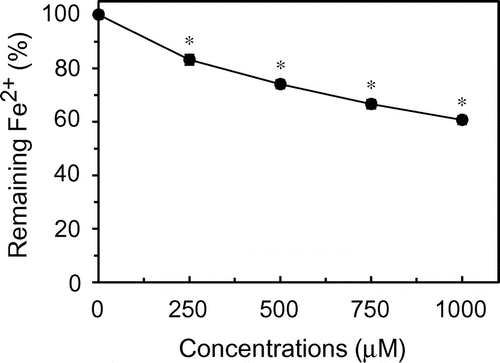
Fig. 8. Fe2+-chelating activity of citric acid under acidic conditions.
In addition to these natural antioxidants, some proteins and peptides are also known to function as antioxidants.Citation9) Egg albumin inhibited the iron-catalyzed oxidation of egg phosphatidylcholine.Citation10) Fat-free egg yolk protein exerted an antioxidant effect on ethanol/water or on cookies containing linoleic acid. Compared with egg-yolk protein, the hydrolysate exhibited a stronger antioxidant effect.Citation11) Because the color of these egg proteins/hydrolysate is white, the egg-derived components may prevent the oxidation of lipids in foods without changing the color of mayonnaise.
As described above, acetic acid caused the release of iron, subsequently leading to lipid oxidation. In addition, acetic acid itself may directly participate in lipid oxidation in mayonnaise because acetic acid was reported to accelerate the oxidation of soybean oil.Citation12) On the other hand, some organic acids have an antioxidative effect,12–14) and they may inhibit the lipid oxidation in mayonnaise.
Hence, in the present study, we determined the inhibitory effect of egg white components and organic acids, instead of acetic acid, on lipid oxidation using real mayonnaise and a model system, and the antioxidative mechanisms were also evaluated.
Materials and methods
Materials
Shelled eggs were purchased from a supermarket in Tokyo. Glacial acetic acid of food additive grade was purchased from The Nippon Synthetic Chemical Industry Co., Ltd. (Osaka, Japan). Calcium disodium EDTA was purchased from Maruzen Chemicals Co., Ltd. (Tokyo, Japan). Trolox and neocuproine were purchased from Sigma-Aldrich (St Louis, MO, USA). Egg white protein hydrolysate EP-1® is a product of Kewpie Co. (Tokyo, Japan). It is obtained by treating albumin with neutral protease of Aspergillus origin. Its average molecular weight is 1100. Citric acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), and 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid monosodium salt hydrate (ferrozine) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Ammonium iron (II) sulfate hexahydrate, FeCl3,100% trichloroacetic acid (TCA), and 5-sulfosalicylic acid dihydrate were purchased from Nacalai Tesque, Inc., (Kyoto, Japan). EDTA-2Na was purchased from Dojindo Laboratories (Kumamoto, Japan). All other chemicals were of analytical reagent grade.
The amino acid compositions of the egg white protein and hydrolysate, and preparation of the amino acid mixture
Egg white was freeze-dried and then pounded in a mortar. The resulting compound was used as egg white protein. The amino acid compositions of egg white protein and EP-1 were measured using a JLC/500V2 amino acid analyzer (Nippon Denshi, Tokyo, Japan) as follows. Egg white protein and EP-1 were hydrolyzed by HCl. Cysteine was oxidized to cysteic acid using performic acid prior to hydrolysis.Citation15) Tryptophan content was determined via barium hydroxide hydrolysis.Citation16) The amino acid contents of egg white protein and EP-1 are shown in Table . The findings were similar between the two.
Table 1. Amino acid composition of egg white protein and hydrolysate.
To prepare an amino acid mixture that has the same formulation ratio as EP-1, 18 amino acids were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan) and were then compounded.
Preparation of acidic egg yolk solution as a model system for lipid oxidation in mayonnaise
The solid content of egg yolk was adjusted to 43% via the addition of a small amount of egg white, according to the literature.Citation17) After the egg yolk mixture (10 g) was diluted with distilled water (130 g), the diluted egg yolk solution was heated at 60 °C for 3 min. Further, the egg yolk solution was adjusted to pH 4.0 using organic acids such as glacial acetic acid. The final solid content of egg yolk in the acidic egg yolk solution was 1%. Egg white components as candidate antioxidants were dispersed with a vortex mixer (model TM-151; AGC Techno Glass Co., Ltd. Shizuoka, Japan) in the distilled water. These components were added to the acidic egg yolk solution, and then the antioxidant action was evaluated as follows.
The acidic egg yolk solution (10 mL) in a 15-mL glass tube of 11 mm inner diameter with a screw cap was incubated at 55 °C in the dark. Lipid oxidation was evaluated by measuring the fluorescence intensity according to previous reports.Citation18–20) In brief, ether/ethanol (1:3, v/v) was added to an aliquot of the oxidized reaction mixture and then centrifuged at 1,200 g for 5 min at room temperature. The upper phase was measured at excitation and emission wavelengths of 360 and 440 nm, respectively, using a Hitachi F-2000 spectrophotofluorometer. The fluorescence intensity was expressed as a value relative to the standard value for 1 μg/mL quinine sulfate/0.1 N sulfuric acid set as 100.
GC-MS analysis
Volatile compounds produced by lipid oxidation from the acidic egg yolk solution were analyzed by GC-MS according to a previous report.Citation21) In brief, the acidic egg yolk solution (3 mL) was incubated in a sealed glass tube at 40 °C for 5 min. Volatile compounds were adsorbed via headspace solid phase microextraction using a polydimethylsiloxane/carboxen/divinylbenzene (Sigma-Aldrich) fiber at 40 °C for 20 min and then analyzed by GC using an Agilent 6890 gas chromatograph coupled to a 5973 mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) with electron impact ionization at 70 eV. Mass units were monitored from 29 to 290 m/z. Separation was performed on a Sol-Gel-Wax capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; SGE Analytical Science, Victoria, Australia) carrying a constant flow of helium (1.0 mL/min). The inlet temperature was set at 250 °C. The column oven temperature program consisted of an initial condition of 35 °C held for 5 min, followed by an increase to 120 °C at a rate of 5 °C/min and then 220 °C at a rate of 15 °C/min, followed by a final hold time of 6 min. The mass spectra of peaks were identified by comparison with the NIST database mass spectral library.
Preparation of mayonnaise
Materials for mayonnaise were compounded using the formulation shown in Table and then passed through the colloid mill of a Kewpie pilot plant (Kewpie Co.) at room temperature. Thereby, mayonnaise formulations having an average particle diameter of 2–5 μm were obtained. The particle diameter was determined using a laser diffraction particle size analyzer (model SALD-200 V; Shimadzu, Kyoto, Japan). The mayonnaise formulations (200 g) were enclosed in square bags (140 mm wide × 170 mm deep) of thin plastic made of nylon 15 μm/linear low-density polyethylene 60 μm.
Table 2. Formulation of mayonnaise.
Lipid oxidation in mayonnaise and taste sensory evaluation
Mayonnaise formulations were autoxidized at 55 °C for 7 days in the dark to measure the peroxide value (PV). After oxidization, lipids in the mayonnaise were extracted using the Bligh and Dyer method.Citation22) PV was determined according to the Japan Oil Chemists Society (JOCS) official method 2.5.2.2-2013.
In addition to the autoxidation, mayonnaise formulations were also photo-oxidized at 25 °C for 7 days under a fluorescent lamp at 500–600 lx for sensory testing. Taste, as evaluated by eight-trained panelists, was scored from 0 to 7, with higher numbers indicating better taste, on the basis of a score of 7 as the standard taste of mayonnaise stored at 4 °C for 7 days in the dark.
DPPH radical-scavenging activity
Egg white protein was dispersed in 1% (w/v) taurocholate and then sonicated with a probe-type homogenizer (Ultra S, VP-5S; Taitec, Saitama). Egg white protein hydrolysate (EP-1) and the amino acid mixture were dissolved in 6 N HCl/DMSO (0.016:1, v/v) and 6 N HCl/DMSO (0.056:1, v/v), respectively. The three resulting solutions were visually clear. The concentration of these samples was 20 mg/mL, and the negative controls and dilutions were prepared using the corresponding vehicles.
The scavenging activity of these samples on DPPH radicals was evaluated using a modification of a method described previously.Citation23) The solutions (20 μL) of egg white protein, egg white protein hydrolysate, and the amino acid mixture were incubated with DPPH working solution consisting of 90 μL of 100 mM DPPH-ethanol and 90 μL of 100 mM acetate buffer (pH 4.0) in each well of a 96-well plate for 30 min at room temperature. The supernatants (100 μL) were centrifuged at 1,600 g for 10 min at 4 °C, transferred to a new 96-well plate, and then diluted two-fold with distilled water. The DPPH content was analyzed at 510 nm using a microplate reader (Tecan Group, Männedorf, Switzerland). Trolox-ethanol was used as a positive control.
Fe2+-chelating activity
Fe2+-chelating activity was evaluated using a modification of a previously described method.Citation24,25) The solutions (75 μL) of egg white protein, egg white protein hydrolysate, the amino acid mixture, and negative controls and dilutions as described previously were mixed with 75 μL of ammonium iron (II) sulfate hexahydrate at 100 μM and 600 μL of 100 mM acetate buffer (pH 4.0). After incubation at 55 °C for 30 min, 7.5 μL of 100% TCA were added to 150 μL of the reaction mixture and then centrifuged at 9,600 g for 10 min at 4 °C. The supernatants (100 μL) were incubated with 80 μL of 10% (w/v) ammonium acetate and 20 μL of the ferrous iron color indicator consisting of 6.1 mM ferrozine and 14.4 mM neocuproine, which could be dissolved by the addition of several drops of 6 N HCl, in 96-well plates for 5 min at room temperature, and then analyzed at 560 nm using the microplate reader. EDTA-2Na was used as a positive control. Vehicle alone was used as the negative control for each sample.
During the incubation, there was a concern regarding reductions of Fe2+ content by autoxidation. Concomitant with the measurement of chelating activity, the oxidative stability of Fe2+ in each negative control solution was evaluated. In addition, due to the possible conversion of Fe2+ to Fe3+ caused by the egg white components, supernatants (100 μL) of samples were also mixed with 100 μL of 5-sulfosalicylic acid dihydrate at 200 μM in 96-well plates for 10 min at room temperature, followed by analysis at 510 nm using a microplate reader via a modification of a previously described method for evaluating Fe3+.Citation26) Because Fe3+ did not cause a color change in the reaction using the ferrous iron color indicator, the production of Fe3+ induced by the components might create the mistaken impression of effective Fe2+-chelating activity.
Statistical analysis
The data were analyzed using one-way ANOVA with Dunnett test or with Tukey-Kramer test. P-values < 0.05 were considered significant.
Results
Lipid oxidation in the acidic egg yolk solution
We measured the fluorescence of substances produced by the oxidation of acidic egg yolk solution as a mayonnaise model. The fluorescent substances are thought to be products of aldehyde groups in volatile compounds and amino groups as described below.
Figure 1(A) shows a GC chromatogram of the volatile compounds formed by oxidation in the acidic egg yolk solution at 55 °C for 72 h. The three main peaks were assigned by comparing MS spectra with those of the database library as follows: peak a, hexanal (Fig. 1(B)); peak b, 2-pentylfuran (Fig. 1(B)); and peak c, acetic acid (data not shown).
We plotted the fluorescence intensity of substances against GC responses of the first two compounds produced by the oxidation of acidic egg yolk solution at 55 °C for 72 h under variable conditions (pH 4.0 or 7.0 with EDTA at 0 or 25 μM). Positive correlations were found between the fluorescence intensity and the formation of hexanal (R2 = 0.9757) or 2-pentylfuran (R2 = 0.7318) (Fig. 1(C)). In particular, the fluorescence intensity exhibited a strong correlational relationship with the hexanal formation.
The inhibitory effects of egg white protein/hydrolysate/amino acid mixture on lipid oxidation in acidic egg yolk solution as a mayonnaise model
We evaluated the antioxidative effect of egg white components using acidic egg yolk solution as a mayonnaise model.
The fluorescence intensity in acidic egg yolk solution was increased during incubation in a time-dependent manner (Fig. 2(A), Control). EDTA (0.0047%), positive control, significantly decreased the intensity at 88 h (one-way ANOVA, p < 0.05) (Fig. 2(A)). Egg white protein, the hydrolysate, and the amino acid mixture also trended to decrease the fluorescence intensity in a concentration-dependent manner over the range of 0.0125–0.1% (Fig. 2(B–E)).
As shown in Fig. 2 (B–E), egg white hydrolysate and the amino acid mixture more strongly decreased the fluorescence intensity than egg white protein (The values at 88 h, p < 0.05). Among the three egg white components, the hydrolysate at 0.1% most effectively decreased the intensity (The values at 88 h, p < 0.05) (Fig. 2(E)).
DPPH radical-scavenging activity of egg white protein/hydrolysate/amino acid mixture
As described above, egg white components showed the antioxidative effect in acidic egg yolk solution as a mayonnaise model. Because the lipid oxidation is further accelerated by radicals produced from lipid peroxide,Citation27) radical-scavenging activity is thought as a possible mechanism for the antioxidative effect. DPPH was appropriately used for evaluating the activity as described below. EDTA showed no effect at all on the DPPH radical-scavenging activity (data not shown). Trolox is commonly used as a positive control. As shown in Fig. 3(A), the remaining levels of DPPH radicals (%) (Y) were plotted against the Trolox concentrations (mM) (X), revealing a negative correlation under the experimental condition used (R2 = 0.9907).
Egg white protein, the hydrolysate, and the amino acid mixture reduced DPPH radical levels in a concentration-dependent manner. The amino acid mixture had the strongest effect on DPPH radical among the three components at 2.0% (Fig. 3(B)). When the DPPH radical-scavenging activity of these components at 2.0% was evaluated based on that of Trolox using the formula Y = 166.66X, the values were 0.155 ± 0.011 mM for protein, 0.126 ± 0.004 mM for the hydrolysate, and 0.293 ± 0.026 mM for the amino acid mixture.
Fe2+-chelating activity of egg white protein/hydrolysate/amino acid mixture
As iron release caused lipid oxidation, the iron chelating effect would be one of the possible mechanisms of the antioxidative effect. An extremely low concentration (50 μM, 0.0186 mg/mL) of EDTA-2Na as a positive control chelated nearly all of the Fe2+ content (Fig. 4). Among the three egg white components, the hydrolysate exhibited the greatest Fe2+-chelating activity at 1.0% (Fig. 4), with approximately 50% of Fe2+ chelated after incubation for 30 min. Egg white protein and the amino acid mixture had little to no Fe2+-chelating activity.
No oxidation of Fe2+ to Fe3+ was observed during incubation for 30 min (data not shown).
The inhibitory effect of the hydrolysate on lipid oxidation in mayonnaise
We evaluated the antioxidative effect of egg white components on real mayonnaise. After incubation for 7 days in the dark at 4 °C, the PV in mayonnaise was approximately zero (data not shown), whereas the values at 55 °C reached approximately 6 (Fig. 5, Control). EDTA significantly decreased the PV. The hydrolysate (EWH) also significantly decreased the value over the range of 0.09–0.9% (Fig. 5).
Figure 6 shows the results of the sensory taste evaluation of mayonnaise after incubation for 7 days. Under the condition of 25 °C with light at 500–600 lx (Control), the average value of taste score was low (2.5). EDTA significantly inhibited the deterioration of mayonnaise, with the average score increasing to 5.5. The hydrolysate at 0.09 and 0.45% also significantly inhibited the deterioration (average scores, 4.25 and 5.0, respectively), but no effect was observed at a higher concentration (0.9%).
During the evaluating period, time-dependent viscosity changes were not significantly different among the mayonnaise samples (data not shown). Visible oil layer separation was also not observed in any samples (data not shown).
The effect of various acids on lipid oxidation in acidic egg yolk solution
In general, mayonnaise is adjusted to an acidic condition using vinegar containing acetic acid. However, as described above, acetic acid itself may accelerate the lipid oxidation. We evaluated the effect of various acids (six organic acids and two inorganic acids: hydrochloric acid and phosphoric acid) on lipid oxidation in the egg yolk solution and then compared them with acetic acid. Each egg yolk solution was adjusted to pH 4.0 with eight corresponding acids. Among the organic acids tested, citric acid and tartaric acid significantly reduced the fluorescence intensity after incubation for both 40 h and 88 h compared with the effect of acetic acid (Fig. 7).
Figure 8 presents the Fe2+-chelating activity of citric acid at pH 4.0. Citric acid significantly reduced the amounts of Fe2+. At 1000 μM, the amount of Fe2+ was reduced to approximately 60% of the initial level. Citric acid at the same concentrations showed no effect at all on the DPPH radical scavenging activity (data not shown).
Discussion
In the present study, we evaluated the effect of egg white-derived components such as egg white protein, the hydrolysate, and the amino acid mixture on lipid oxidation in mayonnaise.
We constructed a mayonnaise model using acidic egg yolk solution (pH 4.0) and then assessed the antioxidant action of egg white-derived components. The formation of fluorescent lipid peroxidation products was measured as an index of lipid oxidation in the acidic egg yolk solution. The fluorescent products are known to be produced from a Schiff base of an amino group and an aldehyde group.Citation20) Some volatile compounds were produced during the oxidation of acidic egg yolk solution. The amounts of hexanal (caproaldehyde) and 2-pentylfuran compounds among the volatile compounds were correlated with an increase in fluorescence intensity (Fig. 1(C)). Hexanal was reported to form fluorescent products with lysine.Citation28) Another volatile compound, 2-pentylfuran, was reported as one of the oxidative degradation products produced from linoleic acid,Citation29) a major polyunsaturated fatty acid in egg yolk, and as a potential marker of lipid peroxidation. However, it does not have an aldehyde group within its molecule. The production of 2-pentylfuran would be in appearance correlated with an increase in fluorescent products. Thus, in the present study, hexanal production would involve the production of fluorescent products.
Some proteins and amino acids are known as natural antioxidative components with iron-chelating and/or radical-scavenging activity. Amino acidsCitation30) such as histidine, lysine, and cysteine and proteins such as egg albumin,Citation31) soy protein,Citation10) casein,Citation32) and gelatinCitation33) inhibited the oxidation of linoleic acid in various model systems. In addition, the hydrolysate/peptides of some proteins were more effective in inhibiting lipid oxidation than their parental proteins. After the milk was treated with trypsin, the oxidation of milk fat was inhibited compared with before the treatment, suggesting that casein hydrolysate exerted stronger antioxidative effects.Citation34) Soy protein hydrolysate more effectively inhibited the oxidation of linoleic acid than soy protein.Citation35) Egg yolk protein hydrolysate displayed stronger antioxidative effects on the oxidation of linoleic acid in cookies than egg yolk protein and amino acids.Citation11) In agreement with these studies, we found that egg white protein hydrolysate most strongly inhibited lipid oxidation (Fig. 2(E)). The antioxidative effect of the hydrolysate was reported to be dependent on the variety of enzymes used to cleave peptide bonds, thereby indicating that the antioxidative effects of hydrolysate/peptides would be affected by the amino acid residues and the terminal sites.Citation36)
To investigate the mechanism underlying the inhibitory effect of egg white hydrolysate on the oxidation of acidic egg yolk solution, we evaluated the following two points: radical-scavenging and Fe2+-chelating activity. In the present study, among the three egg white components, amino acid mixture displayed the strongest DPPH radical-scavenging activity (Fig. 3(B)). Amino acids such as histidine,Citation37,38) and cysteineCitation39) were previously reported to have DPPH radical-scavenging activity. The amino acid mixture used in the present study contained these amino acids, and thus they also would exert the strongest effect under acidic conditions. As described previously, some studiesCitation11,34,35,40) reported that hydrolysate exhibited stronger inhibitory effects than the parental proteins on lipid oxidation and that the DPPH radical-scavenging activity of the hydrolysate was one of the mechanisms responsible for the stronger antioxidative effect. Egg white hydrolysate also displayed DPPH radical-scavenging activity. However, the effect tended to be weaker than that of protein, although there was no statistical difference between the two. The results suggested that the main inhibitory effect of the hydrolysate on lipid oxidation in the acidic egg yolk solution was not caused by radical-scavenging activity.
We evaluated Fe2+-chelating activity as another possible mechanism for the antioxidative action of egg white hydrolysate. The Fe2+-chelating activity tended to be in the order of hydrolysate >> amino acid mixtures = protein, suggesting that the Fe2+-chelating activity explained the antioxidative effect of the hydrolysate (Fig. 4). Thus, the results suggested that the inhibitory effect of the hydrolysate on lipid oxidation in the acidic egg yolk solution could be mainly due to its Fe2+-chelating activity. During the evaluation of Fe2+-chelating activity, Fe2+ was extremely stable under an acidic condition in the current study, in agreement with previous reports.Citation41) Thus, the autoxidation of Fe2+ did not interfere with the measurement of Fe2+-chelating activity.
The enzymatic hydrolysate of egg white albumin was previously reported to inhibit the oxidation of linoleic acid in ethanol/phosphate bufferCitation42) and corn oil emulsion.Citation25) The hydrolysate displayed strong Fe2+-chelating activity, and this activity was believed to explain the antioxidative effect, agreeing with our results. Some peptides in the hydrolysate have been considered to have antioxidative effects. Ala-His-Lys was previously identified as a candidate substance responsible for the antioxidative action of protein hydrolysate.Citation42) In the present study, the antioxidative and chelating activities of peptides in egg white hydrolysate were not clarified. Thus, a detailed determination of the amino acid residue involved in these effects requires further study.
We confirmed the inhibitory effect of egg white hydrolysate on lipid oxidation in real mayonnaise by determining the PV. The hydrolysate significantly inhibited lipid oxidation in a concentration-dependent manner. Hydrolysate at 0.45 and 0.9% exerted a similar effect as 0.01% EDTA, suggesting that the hydrolysate have the potential to inhibit lipid oxidation as an alternative of EDTA (Fig. 5). In addition to their effect on oxidative stability, the rating in the sensory evaluation of mayonnaise was also highest for the hydrolysate at 0.45% as well as EDTA at 0.01% (Fig. 6). Although the rating for hydrolysate at 0.9% was lower than that at 0.45%, off-flavors such as oxidized flavors were not observed in mayonnaise.
In mayonnaise containing egg white hydrolysate at 0.9%, the panelists sensed a bitter taste, which was significantly different from the standard taste. In fact, the bitter taste of egg white hydrolysate has been previously reported.Citation43) These results suggest that at the higher concentration, the taste of the hydrolysate themselves was responsible for the lower rating in the sensory evaluation. On the basis of these results, a hydrolysate concentration of 0.45% would be necessary to obtain the same inhibitory effect as 0.01% EDTA. The concentration of the hydrolysate did not affect the flavor, taste, color tone, or physical properties of mayonnaise. Thus, the egg white hydrolysate would be useful components as natural antioxidants in inhibiting deterioration of mayonnaise
Although acetic acid is generally used to make mayonnaise, it has pro-oxidative activity.Citation12) The use of other organic acids may further enhance resistance to lipid oxidation in mayonnaise, as organic acids such as citric acid have been reported to have antioxidative and/or iron chelating activity.Citation13,14) In the present study, we evaluated the antioxidant action of eight acids, namely six organic acids, hydrochloric acid, and phosphoric acid. Among the acids tested, citric acid effectively inhibited lipid oxidation in the acidic egg yolk solution (Fig. 7). Citric acid also displayed Fe2+-chelating activity under the acidic condition, although the effect was weaker than that of EDTA (Fig. 8). These results suggested that the effect of citric acid on lipid oxidation in the acidic egg yolk solution was caused by the chelating activity. From the point of view of microorganism propagation, in the processing of mayonnaise, the whole acetic acid cannot be replaced to citric acid because the antimicrobial action of citric acid was lower than that of acetic acid.Citation44) However, acetic acid may be partly replaced by citric acid as follows. It may be possible to use the lemon fruit juice, including the citric acid, for the production of mayonnaise. The oxidative stability of lipids in real mayonnaise using citric acid together with acetic acid deserves further study.
In conclusion, to estimate the inhibitory effect of egg white components on lipid oxidation in mayonnaise, we used acidic egg yolk solution as a simple model system. Oxidation was measured using fluorescence intensity, which was correlated with increasing hexanal formation during the incubation. Among the egg white components tested, hydrolysate displayed the strongest inhibiting effect. The antioxidant activity of the egg white hydrolysate could be mainly due to its Fe2+-chelating activity. The hydrolysate also inhibited lipid oxidation based on measurements of the PV in real mayonnaise and significantly suppressed the appearance of off-flavor in sensory taste experiments. The present study indicates that the egg white hydrolysate is a natural product with the potential to effectively inhibit the degradation of mayonnaise.
Authors contributions
H.K. designed the study. H.K. and E.K.-N. wrote the manuscript. R.S. and S.Y. performed lipid oxidation in mayonnaise and taste sensory evaluation and analyzed the data. E.K.-N. performed the experiments of DPPH and iron chelate and analyzed the data. All authors contributed to the critical revision of the manuscript.
Disclosure statement
The authors declare no conflicts of interest.
Acknowledgements
We thank the staff of Institute of Technology, Kewpie Co., Mineo Hasegawa and Mari Yamada for advice regarding the application of egg white hydrolysate in mayonnaise, Satoshi Teraoka for the preparation of mayonnaise, and Shiro Ogihara for the PV measurements. We also would like to thank Enago (www.enago.jp) for the English language review.
Notes
Abbreviations: DMSO, dimethyl sulfoxide; DPPH, 1,1-diphenyl-2-picrylhydrazyl; EWH, egg white hydrolysate; EDTA, ethylenediaminetetraacetic acid; PV, peroxide value; TCA, trichloroacetic acid.
References
- Cotterill OJ, Marion WW, Naber EC. A nutrient re-evaluation of shell eggs. Poult Sci. 1977;56:1927–1934.10.3382/ps.0561927
- Samaraweera H, Zhang WG, Lee EJ, et al. Egg yolk phosvitin and functional phosphopeptides-review. J Food Sci. 2011;76:R143–R150.10.1111/jfds.2011.76.issue-7
- Jacobsen C, Timm M, Meyer AS. Oxidation in fish oil enriched mayonnaise: ascorbic acid and low ph increase oxidative deterioration. J Agric Food Chem. 2001;49(8):3947–3956.10.1021/jf001253e
- Nielsen NS, Petersen A, Meyer AS, et al. Effects of lactoferrin, phytic acid, and EDTA on oxidation in two food emulsions enriched with long-chain polyunsaturated fatty acids. J Agric Food Chem. 2004;52:7690–7699.10.1021/jf0492316
- Jacobsen C, Hartvigsen K, Thomsen MK, et al. Lipid oxidation in fish oil enriched mayonnaise: calcium disodium ethylenediaminetetraacetate, but not gallic acid, strongly inhibited oxidative deterioration. J Agric Food Chem. 2001;49:1009–1019.10.1021/jf000729r
- Jacobsen C, Hartvigsen, K, Lund P, et al. Oxidation in fish-oil-enriched mayonnaise 2. Assessment of the efficacy of different tocopherol antioxidant systems by discriminant partial least squares regression analysis. Eur Food Res Techno. 2000;210:242–257.10.1007/s002179900070
- Jacobsen C, Adler-Nissen J, Meyer AS. Effect of ascorbic acid on iron release from the emulsifier interface and on the oxidative flavor deterioration in fish oil enriched mayonnaise. J Agric Food Chem. 1999;47:4917–4926.10.1021/jf990241u
- Li CY, Kim HW, Li H, et al. Antioxidative effect of purple corn extracts during storage of mayonnaise. Food Chem. 2014;152:592–596.
- Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr. 2008;48:430–441.10.1080/10408390701425615
- Nara E, Miyashita K, Ota T. Oxidative stability of liposomes prepared from soybean PC, chicken egg PC, and salmon egg PC. Biosci Biotechnol Biochem. 1997;61:1736–1738.10.1271/bbb.61.1736
- Sakanaka S, Tachibana Y, Ishihara N, et al. Antioxidant activity of egg-yolk protein hydrolysates in a linoleic acid oxidation system. Food Chem. 2004;86:99–103.10.1016/j.foodchem.2003.08.014
- Kajimoto G, Takashima R, Murakami C. Effects of antioxidant on the oxidative deterioration of oils in the presence of basic and acidic materials. J Jpn Oil Chem Soc (Japanese). 1998;47:591–597.10.5650/jos1996.47.591
- Fukuzawa K, Soumi K, Iemura M, et al. Dynamics of xanthine oxidase- and Fe3+-ADP-dependent lipid peroxidation in negatively charged phospholipid vesicles. Arch Biochem Biophys. 1995;316:83–91.10.1006/abbi.1995.1013
- Miwa S, Nakamura M, Okuno M, et al. Production of starch with antioxidative activity by baking starch with organic acids. Biosci Biotechnol Biochem. 2011;75:1649–1653.10.1271/bbb.110123
- Moore S. On the determination of cystine as cysteic acid. J Biol Chem. 1963;238:235–237.
- Pon NG, Schnackerz KD, Blackburn MN, et al. Molecular weight and amino acid composition of five-times-crystallized phosphoglucose isomerase from rabbit skeletal muscle. Biochemistry. 1970;9:1506–1514.10.1021/bi00809a005
- Strixner T, Kulozik U. Egg proteins. In: Phillips GO, Williams PA. Handbook of food proteins: egg proteins. Cambridge: Woodhead publishing; 2011. p. 150–209.10.1533/9780857093639.150
- Shimasaki H, Sato J, Hara I. Fluorescent spectrophotometry of autoxidized egg yolk lipoproteins. J Jpn Oil Chem Soc (Japanese). 1975;24:464–468.10.5650/jos1956.24.464
- Hara I, Shimasaki H, Sato J. Effect of oxidation on chemical spectral and immunochemical properties of egg yolk lipoprotein. Lipids. 1973;8:623–626.10.1007/BF02533145
- Shimasaki H. Assay of fluorescent lipid peroxidation products. Meth Enzymol. 1994;233:338–346.10.1016/S0076-6879(94)33039-5
- Yanagisawa T, Watanuki C, Ariizumi M, et al. Super chilling enhances preservation of the freshness of salted egg yolk during long-term storage. J Food Sci. 2009;74:E62–E69.10.1111/jfds.2009.74.issue-2
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917.
- Tanaka M, Nakagawa M. Antioxidant activity of thiocholesterol on copper-induced oxidation of low-density lipoprotein. Lipids. 1995;30:321–325.10.1007/BF02536039
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971;40:450–458.10.1016/0003-2697(71)90405-2
- Abeyrathne ED, Lee HY, Jo C, et al. Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates. Poult Sci. 2014;93:2678–2686.10.3382/ps.2014-04155
- Sharaf El-Din M, Schaur RJ, Schauenstein E. Uptake of ferrous iron histidinate, a promoter of lipid peroxidation, by Ehrlich ascites tumor cells. Biochim Biophys Acta. 1988;962:37–41.10.1016/0005-2760(88)90092-6
- Frankel EN. Lipid oxidation. Prog Lipid Res. 1980;19:1–22.10.1016/0163-7827(80)90006-5
- Stapelfeldt H, Skibsted LH. Kinetics of formation of fluorescent products from hexanal andl-lysine in a two-phase system. Lipids. 1996;31:1125–1132.
- Krishnamurthy RG, Smouse TH, Mookherjee BD, et al. Identification of 2-pentyl furan in fats and oils and its relationship to the reversion flavor of soybean oil. J Food Sci. 1967;32:372–374.10.1111/jfds.1967.32.issue-4
- Karel M, Tannenbaum SR, Wallace DH, et al. Autoxidation of methyl linoleate in freeze-dried model systems. III. Effects of added amino acids. J Food Sci. 1966;31:892–896.10.1111/jfds.1966.31.issue-6
- Goto M, Shibazaki K. Effect of the Oxidation of Oils on the Deterioration of Foods. J Jpn Soc Food Sci Technol (Japanese). 1971;18:277–283.10.3136/nskkk1962.18.277
- Rival SG, Boeriu CG, Wichers HJ. Caseins and casein hydrolysate. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J Agric Food Chem. 2001;49:295–302.10.1021/jf0003911
- Zirlin A, Karel M. Oxidation effects in a freeze-dried gelatin-methyl linoleate system. J Food Sci. 1969;34:160–165.10.1111/jfds.1969.34.issue-2
- Limi D, Shipe WF. Proposed mechanism for the antioxygenic action of trypsin in milk. J Dairy Sci. 1972;55:753–758.10.3168/jds.S0022-0302(72)85568-1
- Yamaguchi N, Yokoo Y, Fujimaki M. Studies on antioxidative activities of amino compounds on fats and oils part III. Antioxidative activities of soybean protein hydrolyzates and synergistic effect of hydrolyzate on tocopherol, J Jpn Soc Food Sci Technol (Japanese). 1975;22:431–435.10.3136/nskkk1962.22.431
- Yamaguchi N, Yokoo Y, Fufimaki M. Studies on antioxidative activities of amino compounds on fats and oils part II. Antioxidative activities of dipeptides and their synergistic effects on tocopherol. J Jpn Soc Food Sci Technol (Japanese). 1975;22:425–430.10.3136/nskkk1962.22.425
- Wu HC, Shiau CY, Chen HM, et al. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J Food Drug Anal. 2003;11:148–153.
- Gülçin I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids. 2007;32:431–438.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200.10.1038/1811199a0
- Noh DO, Suh HJ. Preparation of egg white liquid hydrolysate (ELH) and its radical-scavenging activity. Prev Nutr Food Sci. 2015;20:183–189.10.3746/pnf.2015.20.3.183
- Lambeth DO, Ericson GR, Yorek MA, et al. Implications for in vitro studies of the autoxidation of ferrous ion and the iron-catalyzed autoxidation of dithiothreitol. Biochim Biophys Acta. 1982;719:501–508.10.1016/0304-4165(82)90239-2
- Tsuge N, Eikawa Y, Nomura Y, et al. Antioxidative activity of peptides prepared by enzymatic hydrolysis of egg-white albumin. J Agric Chem Soc Jpn (Japanese). 1991;65:1635–1641.
- Cigić B, Zelenik-Blatnik M. Preparation and characterization of chicken egg white hydrolysate. Acta Chim Slov. 2004;51:177–188.
- Yamamoto Y, Higashi K, Yoshii H. Inhibitory activity of organic acids on food spoilage bacteria. J Jpn Soc Food Sci Technol (Japanese). 1984;31:525–530.10.3136/nskkk1962.31.8_525
