Abstract
Oral immunotherapy (OIT) is a promising treatment of food allergy. To administer an appropriate oral dose of an allergenic component as OIT to individuals sensitized with a food allergen may prevent inducing food allergic inflammation in them. So we attempted to establish a mouse model to evaluate efficacy for oral administration of food allergen after sensitization. In BALB/c mice sensitized by injecting ovalbumin (OVA) with alum twice, OVA was administered before inducing inflammation by feeding the mice with egg white (EW) diet. Severe inflammatory responses, such as enteropathy, weight loss, IL-4 production, and increase of IgE antibody levels, were suppressed by administration with 4 mg of OVA 7 times before feeding EW diet. OVA administration alone induced a slight Th2 response, but no symptoms. The current study demonstrated that severe food allergic enteropathy could be prevented by pre-administration with appropriate dose of OVA to sensitized mice.
Weight loss by EW-diet-feeding was rescued by OVA-feeding after sensitization in a food allergy model.
Food allergy is a growing public health problem and it sometimes threatens life of patients.Citation1) Oral immunotherapy (OIT) is noted as promising approach for food allergy. Recently, the causative allergenic component in a food allergen has been well explored and used for diagnostics for food allergies.Citation2) OIT has been applied to food allergic patients, but the treatment may cause severe allergic responses including diarrhea, vomiting, anaphylactic shock, and so on. Recently, a clinical study of primary prevention of egg allergy using pre-administration of egg powder for infants with eczema was reported.Citation3) They indicated that the trial was effective in prevention of egg allergy without side effects to high-risk infants with eczema (participants) concluding sensitized infants, not showing allergic symptoms, but having high titer of serum IgE. It was suggested that the efficacy was obtained by technical superiority of the trial, i.e., to use heated egg-powder, not raw egg, to use a two-step protocol starting with a low and safe dose (50 mg powder per day), and to maintain skin care for eczema. However, in spite of accumulation of the evidence in preventive effects of OIT for egg allergy in addition to previous evidence for peanut allergy,Citation4) the mechanism of the efficacy remains unknown. So we attempted to establish a mouse model of egg allergy to evaluate the efficacy of pre-administration of OVA, a major component of EW, and the mechanisms of OIT in the sensitized case.
Previously, we have established a mouse model for food allergy exhibiting intestinal inflammation, Th2 response and weight loss with increase in serum ovalbumin (OVA)-specific IgE levels.Citation5) This model sensitized with intra-peritoneal injection of OVA with alum causes food allergy by subsequent feeding EW-diet containing OVA. In this study, we examined whether previous oral administration of an appropriate dose of OVA could prevent severe symptoms of food allergy using this model.
Materials and methods
Animals
Female BALB/c mice were purchased from Clea Japan Inc (Tokyo, Japan). All experiments were performed in accordance with guidelines for the care and use of laboratory animals of the University of Tokyo.
Experimental schedule
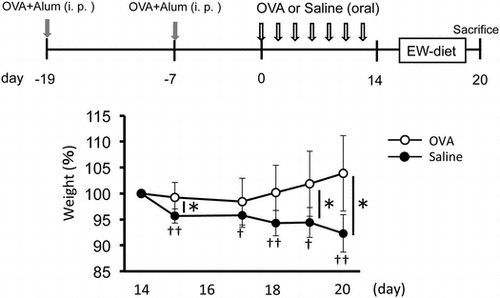
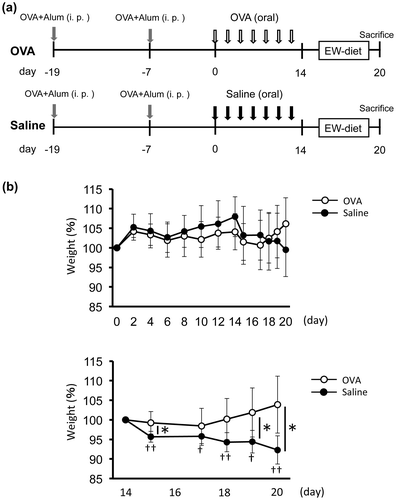
BALB/c mice (six-week-old) were sensitized with 50 μg OVA with 1 mg of alum (Imject® Alum; Pierce Biotechnology Inc., Rockford, IL, USA) in PBS by intraperitoneal injection on day -19 and -7. From day 0, every other day for 14 days, mice were divided into two groups (n = 8 each) and administrated 4 mg OVA in 200 μL saline (OVA group; 20 mg/mL) or saline only (control group). From day 14, 2 groups of mice (n = 5 each) were fed with EW diet containing egg white protein as its protein source (Funabashi farm, Funabashi, Japan) for inducing food allergic inflammation during 6 days. On day 14 (3 mice/group) and 20 (5 mice/group), mice were sacrificed for analysis of IgE antibody level, cytokine production, cell proliferation and histology (Fig. (a)).
Fig. 1. Weight loss by EW-diet-feeding was rescued by OVA-feeding after sensitization in a food allergy model.
Cell culture for analysis of cytokine production and cell proliferation
Spleen (SPL) and mesenteric lymph node (MLN) lymphocytes of mice were pooled in each experimental group and CD4+ T cells purified (>95%) using MACS cell separation system (Miltenyi Biotec, Bergisch Gladbach, Germany). For measurement of cytokines, CD4+ T cells (4 × 105 cells/well) were cultured with mitomycin C-treated BALB/c splenocytes as antigen-presenting cells (8 × 105 cells/well), and different concentrations of OVA in 96-well plates using RPMI 1640 medium containing 5% FCS. Culture supernatants were collected for cytokine measurements 72 h later. Cytokine concentrations (IL-2, IL-4, and IFN-γ) were evaluated by ELISA as described previously.Citation6) For measurement of cell proliferation, tritiated thymidine (18.5 kBq/well) was added to plates cultured for 72 h. After 24 h, cells were harvested by Micro96 Cell Harvester (Skatron Instruments Inc., Sterling, VA, USA), and incorporation of tritirated thymidine was counted by 1450 Microbeta® TriLux (Perkin Elmer Wallac Inc., Gaithersburg, MD, USA).
Histological analysis for intestine
Harvested jejunum were fixed with 10% formalin, and embedded in paraffin. Embedded tissues were sliced to 5 μm thick sections, and stained with hematoxylin and eosin for histological analysis.Citation5) The ratio of villous height to crypt depth was analyzed as typical and quantitative parameter of enteropathy as described previouslyCitation7) in the analyses after EW feeding.
IgE antibody level
OVA-specific IgE antibody titers in sera were measured by ELISA. Immunoplates were coated with rat anti-mouse IgE antibody (BD Pharmingen, San Jose, CA, USA). Sera and standards were added after blocking with BSA. Specific antibody levels were detected by biotinylated OVA, followed by streptavidin–alkaline phosphatase and enzyme substrate (p-nitrophenylphosphate).
Statistical analysis
Results were shown as mean ± SD. The Mann-Whitney U test was used for serum IgE analysis. The Student t test was used weight change, cytokine production, and villous height/crypt depth ratio analysis. Difference was considered significant at p < 0.05.
Results
Weight loss by EW-diet-feeding was rescued by OVA-feeding after sensitization in a food allergy model
We investigated whether food allergy symptoms were suppressed by oral administration of OVA using our OVA/alum-sensitized food allergic mice model. In our model, food allergic symptoms were induced by EW-diet-feeding. So we examined whether oral administration of OVA after immunization with OVA/alum had efficacy for suppressing food allergy elicited by subsequent EW-diet-feeding. Brandt et al. have reported that oral administration of 10 mg OVA induced diarrhea using OVA/alum-sensitized mice.Citation7) Thus, we speculated that oral administration of OVA with lower doses may rather suppress food allergic symptoms without inducing symptoms by itself. We firstly examined whether oral administration of 1 or 2 mg OVA before EW-diet-feeding suppressed food allergic symptoms using our mouse model. But we could not get stable results of suppressed weight loss by OVA-feeding (data not shown).
When we administrated repeated doses of 4 mg OVA to OVA/alum-sensitized mice (OVA group) before EW-diet-feeding (Fig. ), similar to the saline group, the OVA group did not show any weight loss during period of pre-treatment with OVA administration (from day 0 to 14). Compared with the body weight on day 14, significant weight loss was observed by EW-diet-feeding in the saline group, but not in the OVA group. These results indicate that oral administration of 4 mg OVA have the potential of suppressing food allergic symptoms in our model.
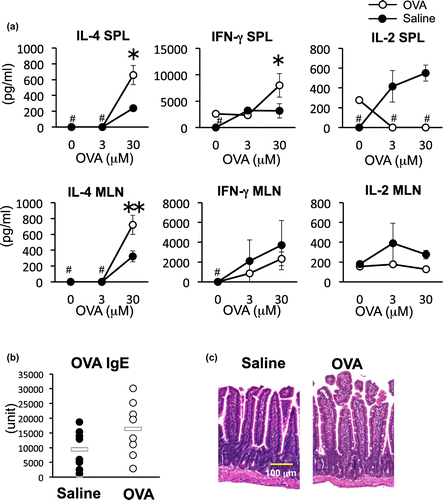
OVA-feeding enhanced IL-4 production but not weight loss, IgE antibody levels and intestinal inflammation
To evaluate whether pre-administration of OVA would affect immune responses of the mice, we next investigated CD4+ T cell cytokine production in SPL and MLN, serum IgE antibody levels and intestinal inflammation in the mice of the OVA group and saline control group on day 14 before inducing inflammation. IL-4 productions of OVA group in both SPL (*p < 0.05) and MLN (**p < 0.01) were significantly higher than that of saline group (Fig. (a)). IFN-γ production increased in the SPL, but not in local MLNs, indicating activation of T cells was systemically promoted by pre-administration of OVA (*p < 0.05). IgE antibody production tended to be elevated in the OVA group, but was not significantly different compared to the control group (Fig. (b)). Intestinal inflammation was not observed in control groups and was minimal in the OVA group (Fig. (c)). Typical signs of inflammation, such as villous branching, crypt elongation, thickened muscular layer, and goblet cell hyperplasia were not observed, but goblet cell infiltration into the mucosal epithelium was slightly induced by OVA administration. These results indicate that oral administration of 4 mg OVA induced Th2 response but did not induce allergic symptoms.
Fig. 2. Cytokine response, IgE antibody levels and intestinal histology analysis of OVA-administrated mice.
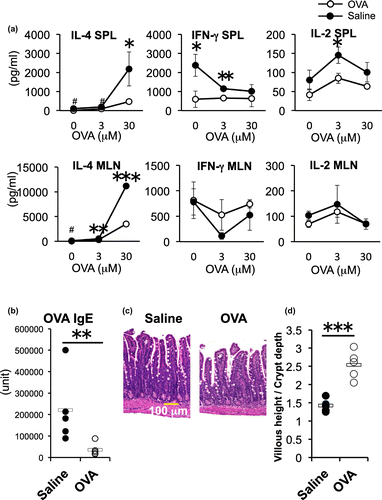
IL-4 production and IgE antibody response after feeding of EW diet was suppressed by OVA-feeding
We next examined whether oral administration of 4 mg OVA influenced the Th2 inflammatory responses elicited by EW-diet-feeding. The IL-4 production of OVA group was significantly lower compared to that of the control group in both SPL and MLN (*p < 0.05 [SPL] **p < 0.01 and ***p < 0.001 [MLNs], Fig. (a). The CD4+ T cell proliferative response was also lower in the OVA group than that of the control group (Supplemental Fig. 1). IFN-γ and IL-2 production were significantly inhibited in SPLs of OVA group (**p < 0.01), and the productions in MLNs of both groups were similarly maintained at lower levels in response to different concentrations of OVA. The low production in MLNs was the result of EW-diet-feeding, and was not significantly affected by pre-administration of OVA The serum OVA-specific IgE level of the OVA group was significantly lower than that of saline group (Fig. (b)). The histology analysis showed that the OVA group had milder enteropathy without crypt elongation and thickening of submucosa compared with severe enteropathy in the saline group (Fig. (c)). To estimate quantitatively morphological changes (crypt elongation, villous atrophy, and goblet cell hyperplasia) of the jejunum of the two groups after EW-diet-feeding (OVA-treated and saline-treated), we measured villous height and crypt depth as a typical parameter for monitoring morphology in more than 10 randomly selected villi per mouse, calculated the ratio (villous height to crypt depth), and evaluated the inflammatory changes of the jejunum. The ratio significantly increased in EW-fed OVA-group than that in EW-fed saline group, showing the inflammatory changes observed in EW-fed control group was resolved by OVA administration in OVA-group mice (Fig. (d)). These data indicate that oral administration of 4 mg OVA has the ability of suppressing systemic and local Th2 response, weight loss and enteropathy elicited by EW-diet-feeding.
Fig. 3. Effects of oral administration of OVA on the cytokine response, IgE titer and intestinal inflammation after EW-diet-feeding.
Discussion
OIT may be utilized not only for food allergic patients but also for prevention of allergy in sensitized individuals. If oral immunotherapy with continuing administration of lower dose of allergens could be established for sensitized individuals, it would be possible for them to avoid inducing allergic symptoms at later intake of allergen, and it may become a new form of immunotherapy.Citation3) In this study, we investigated the efficacy of OVA as a component of EW for OIT after sensitization using the OVA/alum food allergic enteropathy model. Our data indicated that multiple 4 mg OVA administrations to OVA-sensitized mice had the ability to suppress Th2 inflammatory responses, and weight loss elicited by EW-diet-feeding. Weight loss was the major symptom in this allergy model, likely induced by both Th2 inflammation and impaired absorption. Although a slight Th2 response was induced during OVA administration, symptoms were not elicited. The EW-diet used in this study contains 10% OVA which results in approximately 200 mg daily intake of OVA. Our results indicated that a small amount of component administration before ingesting a large quantity of the same antigen could induce tolerance. Repetitive moderate stimulation of T cells may have led to T cell tolerance. This model should be useful for establishing the protocol like PETITCitation3) and elucidating the mechanisms of OIT in sensitized individuals. Some previous studies have evaluated the efficacy of OIT in mouse models of food allergy after sensitization and before allergen challenge.Citation8–10) However, these studies have not focused on the effects of the OIT procedure alone and/or have evaluated effects on immediate hypersensitivity.
In the study of Brandt et al., diarrhea was induced by intragastric challenge of 10 mg OVA after sensitization.Citation11) We also checked effects of 10 mg OVA administration in our sensitized model, and diarrhea was induced at the second and seventh 10 mg OVA feeding (data not shown). In the present study, OVA administration of 4 mg dose to sensitized mice did not induce symptoms by itself, and attenuated enteropathy elicited by subsequent EW-diet-feeding. Although it may appear that weight gain of the OVA group was suppressed from day 0 to 14, there was no significant difference between OVA and saline groups, and in a repeated experiment, weight gain was observed in the OVA group during this period. Nevertheless, there is a possibility of a slight allergic reaction that did not result in symptoms as suggested by significant IL-4 production of spleen and MLN cells in the OVA group (Fig. ). The administration of doses lower than 4 mg after sensitization (1 or 2 mg, data not shown) did not result in stable effects. Our interests are concentrated on differences among detailed responses induced by administration of 1 or 2 mg, that of 4 mg, and that of 10 mg of OVA. Moderate Th2 activation followed by slight allergic reaction without inducing symptoms, but not insufficient or excess activation, may be required for efficient and safe induction of tolerance,Citation12) as shown in current results. Although the exact mechanisms are unknown, the activated Th2 cells may be anergized or clonally deleted by repetitive antigen stimulation, followed by subsequent induction of regulatory T cells. Thus, for OIT, appropriate choices of antigen dose depending on patient’s conditions such as sensitization should be important. To clarify markers exhibited by appropriate stimulation with oral-administered allergens in each high-risk infant or patient, we should compare and examine biochemical and immunological mediators or responses to different doses of OVA (1, 2, 4, and 10 mg) by using this mouse model hereafter.
In this study, we demonstrated that severe food allergic enteropathy and IgE responses could be attenuated by pre-administration with appropriate dose of OVA in an OVA-sensitized model. As indicated in egg or peanut allergy, studies of efficient prevention for sensitized or high-risk infants of food allergy by OIT are now emerging. Our mouse model showing efficacy of pre-administration after sensitization could contribute to development of safe and effective OIT for sensitized individuals and the elucidation of mechanisms.
Author contributions
EH planned and performed the experiments, analyzed the results and wrote the paper. MM planned and performed the experiments, analyzed the results and revised the manuscript. HH, SY, and JT contributed to the planning and performing the experiments. NK, OK, and TH contributed to the histological analyses. WD and KO-D contributed to the planning of the study, interpretation of the results and revised the manuscript. HN-A and SH planned the study, analyzed the results, wrote, and revised the manuscript. All authors read and approved the manuscript.
Disclosure statement
SH received a grant from Torii Pharmaceutical.
Funding
This work was partly supported by Agri-health Translational Research Project from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Supplemental material
Supplemental material for this article can be accessed at https://doi.org/10.1080/09168451.2017.1361806.
HiraideMorinagaFigS1.pdf
Download PDF (54.1 KB)Notes
Abbreviations: EW, egg white; MLN, mesenteric lymph node; OIT, oral immunotherapy; OVA, ovalbumin; SPL, spleen
References
- Oyoshi MK, Oettgen HC, Chatila TA, et al. Food allergy: insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol. 2014;133:309–317.
- Sato S, Yanagida N, Ohtani K, et al. A review of biomarkers for predicting clinical reactivity to foods with a focus on specific immunoglobulin E antibodies. Curr Opin Allergy Clin Immunol. 2015;15:250–258.10.1097/ACI.0000000000000162
- Natsume O, Kabashima S, Nakazato J, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:276–286.10.1016/S0140-6736(16)31418-0
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813.10.1056/NEJMoa1414850
- Burggraf M, Nakajima-Adachi H, Hachimura S, et al. Oral tolerance induction does not resolve gastrointestinal inflammation in a mouse model of food allergy. Mol Nutr Food Res. 2011;55:1475–1483.10.1002/mnfr.201000634
- Sato A, Hashiguchi M, Toda E, et al. CD11b+ Peyer’s patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171:3684–3690.10.4049/jimmunol.171.7.3684
- Nakajima-Adachi H, Ebihara A, Kikuchi A, et al. Food antigen causes Th2-dependent enteropathy followed by tissue repair in T-cell receptor transgenic mice. J Allergy Clin Immunol. 2006;117:1125–1132.10.1016/j.jaci.2006.01.016
- Jiménez-Saiz R, Rupa P, Mine Y. Immunomodulatory effects of heated ovomucoid-depleted egg white in a BALB/c mouse model of egg allergy. J Agric Food Chem. 2011;59:13195–13202.10.1021/jf202963r
- Rupa P, Mine Y. Oral immunotherapy with immunodominant T-cell epitope peptides alleviates allergic reactions in a BALB/c mouse model of egg allergy. Allergy. 2012;67:74–82.10.1111/all.2011.67.issue-1
- Leonard SA, Martos G, Wang W, et al. Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012;129:1579–1587.10.1016/j.jaci.2012.04.009
- Brandt EB, Strait RT, Hershko D, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677
- Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–157.10.1080/17402520600876804
