Abstract
Megalo-type isomaltosaccharides are an enzymatically synthesized foodstuff produced by transglucosylation from maltodextrin, and they contain a mid-chain length polymer of D-glucose with α-1,6-glycoside linkages. The injection of a solution of megalo-type isomaltosaccharides (1–4%(w/v), average DP = 12.6), but not oligo-type isomaltosaccharides (average DP = 3.3), into the intestinal lumen dose-dependently reduced the transport rates of tight junction permeable markers in a ligated loop of the anesthetized rat jejunum. Application of the megalosaccharide also suppressed the transport of tight junction markers and enhanced transepithelial electrical resistance (TEER) in Caco-2 cell monolayers. Cholesterol sequestration by methyl-β-cyclodextrin in the Caco-2 monolayers abolished the effect of megalosaccharide. Treatment with anti-caveolin-1 and a caveolae inhibitor, but not clathrin-dependent endocytosis and macropinocytosis inhibitors, suppressed the increase in TEER. These results indicate that isomaltosaccharides promote the barrier function of tight junctions in the intestinal epithelium in a chain-length dependent manner and that caveolae play a role in the effect.
Megalo-type isomaltosaccahride interacts to caveolae components, and initiates signals to the tight junction for enhancing barrier function of the intestinal epithelium.
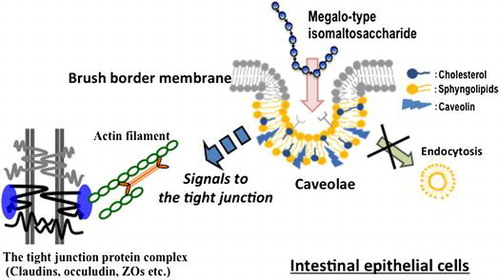
The intestinal tight junction seals the apical junction between epithelial cells to form a physical barrier, and it consists of several proteins, claudins, occludin, and junctional adhesion molecules. Extracellular loops of claudins mainly contribute to the barrier function of the tight junction [Citation1], and the intracellular domains of these proteins are anchored to actin filaments via zonula occluden proteins. The permeability of the intestinal epithelium for non-nutrient solutes is closely associated with the barrier function of the tight junction, which is controlled by the contraction of the cytoskeleton, composed of non-muscle myosin, and the turnover of tight junction proteins [Citation2]. These factors are associated with cell signaling pathways, including myosin-light chain kinase [Citation3] or Rho GTPase/Rho-associated coiled-coil kinase [Citation4–6].
Prolonged dysfunction of the tight junction barrier results in a condition called “leaky gut,” which exposes body tissues to harmful compounds in the intestinal lumen, including lipopolysaccharides, other materials derived from the intestinal microbiota, or toxic molecules in foods. Increasing the permeation of these compounds through the intestinal epithelium induces chronic inflammation in the abdominal adipose tissues and the liver, which impairs insulin sensitivity [Citation7] and causes steatohepatitis that develops from non-alcoholic fatty liver disease with the over-activation of the immune systems [Citation8–11].
Several studies have found that luminal factors, including food ingredients, affect the barrier function of the intestinal epithelium via the tight junction. Consuming a high-fat diet was shown to impair intestinal barrier function and reduce claudin expression, and the increased levels of bile acids resulting from the high-fat diet partly contributed to this impairment [Citation12]. Medium-chain fatty acids were shown to increase tight junction permeability in Caco-2 cell monolayers, and the action depended on phospholipase C and IP3 signaling [Citation13]. In contrast, short-chain fatty acids have been shown to reduce tight junction permeability. The long-term application of butyrate suppressed tight junction permeability through lipoxygenase expression in Caco-2 cell monolayers [Citation8,14]. Acetate and propionate, but not butyrate, rapidly reduced the permeability in rat intestine and Caco-2 monolayers, and the effect was dependent on caveolae [Citation15]. Long-chain n-3 polyunsaturated fatty acids and L-glutamine are important for maintaining the intestinal barrier function [Citation16,17]. Flavonoids can also suppress tight junction permeability and strengthen the epithelial barrier function [Citation18,19].
We developed an enzymatically synthesized megalo-type isomaltosaccharide consisting of a mid-chain length D-glucose polymer with continuous α-1,6-glycoside linkages [Citation20]. This newly developed saccharide increased the water solubility of low molecular weight soluble bioactive compounds such as flavonoids, especially quercetin, and their glycosides. This saccharide promoted the intestinal absorption of a quercetin glycoside by increasing its water solubility [Citation21]. The effect of this megalo-type isomaltosaccharide may depend on molecular interactions between the saccharide and the hydrophobic parts of flavonoids with flexible α-1,6-glycoside linkages. We postulated that the megalo-saccharide has some physiological effects on intestinal function through its interactions with epithelial surface molecules, and the results indicated that the saccharide reduces the permeability of the intestinal epithelium through tight junctions. The aims of the present study were to examine the effects of this saccharide on epithelial tight junctions and to define the associated mechanisms of action.
Materials and methods
Chemicals
A linear α-1,6-glucosaccharide mixture was prepared from maltodextrin using a bacterial glucosyltransferase (dextrin dextranase from Gluconobacter oxydans ATCC 11894, EC 2.4.1.2). The mixture was then fractionated by 50–90% methanol precipitation into megalo-type isomaltosaccharides (IMM) with an average DP = 12.6 and oligo-type isomaltosaccharides (IMO) with an average DP = 3.3. Lucifer Yellow dipotassium salt and nystatin were purchased from Wako Pure Chemical Industries Co., Ltd. (Osaka, Japan). FD20S (FITC dextran; average molecular weight 20,000), methyl-β-cyclodextrin, chlorpromazine hydrochloride, and 5-(N-ethyl-N-isopropyi)-amiloride (EIPA) were obtained from Sigma-Aldrich (St Louis, MO, USA). Dynasore and anti-caveolin-1 were purchased from Santa Cruz Biotechnology, (Dallas, Texas, USA) and Cell Signaling Technology, Inc. (Danvers, MA, USA), respectively. All other reagents and chemicals were the highest commercially available grade.
Animals for the small intestinal loop experiments
Male Sprague-Dawley rats weighing about 200 g (7 weeks old; Japan SLC, Shizuoka, Japan) were housed in individual stainless-steel cages with wire-mesh bottoms in a room with controlled temperature (22 ± 2 °C), relative humidity (40–60%), and light (12 h-light/dark cycle at 8:00–20:00). All rats were acclimated for 7 d with free access to water and a standard AIN93G diet formulation [Citation22]. The study was approved by the Hokkaido University Animal Committee, and the animals were maintained in accordance with the Hokkaido University guidelines for the care and use of laboratory animals.
The rats were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg body weight; Ketaral, Daiichi Sankyo, Tokyo, Japan) containing xylazine (12 mg/kg body weight; MP Biomedicals, Irvine, CA, USA) to perform experiments involving a ligated intestinal loop after overnight fasting. Briefly, an abdominal midline incision was made, and a ligated small intestinal loop (15 cm in length) was prepared in the jejunum in each rat. The jejunal segments were washed with saline and were ligated between 5 and 20 cm distal from the ligament of Treitz. A test solution (1.5 mL) containing L-glutamine (6 mmol/L) and a permeable marker of tight junctions, Lucifer yellow (0.2 μmol/L, molecular weight 444) or FD-20S (5 μg/L, Average molecular weight is 20,000), in MOPS buffer (pH 6.5) with or without 1–4%(w/v) IMM or IMO was injected into the ligated intestinal segment with a syringe and an injection needle. The isotonicity of the solutions was adjusted with NaCl. The jejunal loops with the luminal contents were removed from the rats 20 min after the injection, and the luminal contents including the mucosal tissue was collected to measure the remaining permeable markers. During the experiment, body temperature was maintained with a heating pad. The concentration of Lucifer yellow or FD-20S in the homogenate of the luminal solution and the mucosa was measured by fluorescence at 430-nm excitation/530-nm emission or 491-nm excitation/519-nm emission, respectively. The fluorescence in a blank loop homogenate was subtracted from those in the sample homogenates.
Caco-2 cell monolayer experiments
Caco-2 cells (HTB-37; American Type Culture Collection, Rockville, MD, USA) were cultured in DMEM containing 10% FBS with 1 mmol/L sodium pyruvate, 50,000 U/L penicillin and 50 mg/L streptomycin adjusted to pH 7.4. The cells were seeded into polyester membrane filter (Transwell, 12 mm insert diameter, 0.4-μm pore size; Corning Costar Co., Cambridge, MA, USA) at a density of 1.12 × 105 cells/cm2. Transepithelial electrical resistance (TEER) was monitored by using a commercial apparatus (Millicell-ERS; Millipore Co., Billerica, MA, USA). Experiments were conducted on days 3–4 after confluence, which were determined by reaching maximum and stabilizing TEER values. The medium was refreshed every 3 d. For all experiments, Caco-2 monolayers that reached 700–1000 Ω/cm2 TEER were used. Caco-2 cells were used for experiments between passage 35 and 55.
Transport rate of Lucifer yellow and TEER across the cell monolayer were measured to assess tight junction permeability. Higher TEER indicates lower permeability in tight junction and increased epithelial barrier function. After washing Caco-2 cell monolayers with HBSS (134 NaCl, 4.2 NaHCO3, 0.34 Na2HPO4, 5.4 KCl, 0.44 KH2PO4, 1.25 CaCl2, 0.49 MgCl2, 0.41 MgSO4, 5.6 D-glucose, 4.0 L-glutamine, mmol/L in HEPES, pH 7.4) at 37 °C, test solution containing 2%(w/v) or 4%(w/v) IMM, Lucifer yellow (100 μmol/L) and various inhibitors or anti-caveoline-1 (1/250 dilution) in HBSS was added to the apical chamber. After 40 min of incubation, the medium in the basolateral chamber was collected, and the fluorescence of Lucifer yellow was measured (Figures and ).
Calculation and statistics
All values are expressed as the mean and standard error of the mean. Statistical analysis was performed with one-way ANOVA, and the differences among groups were determined by Tukey–Kramer’s test. Differences with p < 0.05 were considered significant.
Results
Megalo-type isomaltosaccharides (IMM) reduces the permeability of tight junctions in the rat jejunum
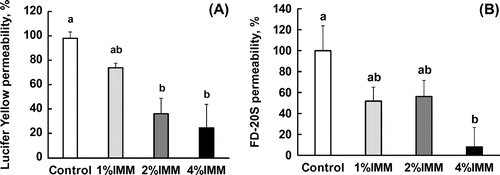
The transport of a low molecular weight (MW. 444) and water-soluble tight junction-permeable marker, Lucifer yellow, was strongly suppressed by the injection of the IMM solution into the lumen of a ligated jejunal segment after 20 min (Figure (a)). An oligo-type isomaltosaccharide (IMO) with a shorter chain length (DP = 3.3) did not significantly reduce Lucifer yellow transport. The results with a higher molecular weight permeable marker, FD-20S (average MW. 20,000), were similar to those with Lucifer yellow, but the extent of the suppression effect on FD-20S transport was higher than that on Lucifer yellow (Figure (b)). The reduction in the transport rate of the tight junction markers by IMM were dose dependent. For Lucifer yellow (Figure (a)), the values in the 2%(w/v) and 4%(w/v) IMM groups were significantly lower compared to those in the control group, and for FD-20S (Figure (b)), the values for the 4%(w/v) IMM group were lower compared to those in the control group.
Figure 1. Effects of oligo-type isomaltosaccharide (IMO) or megalo-type isomaltosaccharide (IMM) on the transport of Lucifer yellow (a) and FD-20S (b), which are permeable markers of tight junctions, in a closed loop of the anesthetized rat jejunum for 20 min. The final concentration of the test sugars was 4%(w/v) in the injected fluid. Values are shown as percentages for the amounts of the injected markers and shown as the mean ± SEM (n = 7–8). Means not sharing a common alphabetical letter differ significantly (p < 0.05).
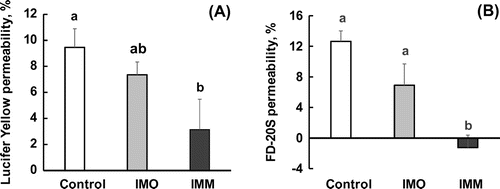
Figure 2. Dose-dependent suppression of the transport of permeable markers of tight junctions, Lucifer yellow and FD-20S, by megalo-type isomaltosaccharide (IMM) in a closed loop of the anesthetized rat jejunum for 20 min. Permeability (%) are values with the control group as 100% and shown as the mean ± SEM (n = 7–8). Means not sharing a common alphabetical letter differ significantly (p < 0.05).
Action mechanisms of IMM in Caco-2 cell monolayer
The application of IMM on Caco-2 monolayers increased TEER gradually up to 40 min in a dose-dependent manner (Figure (a)). The transport rate of Lucifer yellow tended to be suppressed by 4%(w/v) IMM (p = 0.072, Figure (b)). Figure (a) shows the changes in TEER following the application of IMO and IMM and the effects of disrupting the cell membrane microdomain by methyl-β-cyclodextrin (MBC) [Citation23]. The TEER values rapidly and gradually increased following the application of IMM, but not IOM. The increase in TEER induced by IMM was abolished by sequestration of cholesterol from Caco-2 cell monolayers with 0.6%MBC treatment. There were no significant effects of MBC treatment on control monolayer (supplemental figure). The transport rates of Lucifer yellow were not significantly different between groups despite large variations. However, the transport rates tended to be suppressed by the application of 4%(w/v) IMM, but not 4%(w/v) IMO. The suppressed transport induced by IMM was partially restored after treatment with MBC. The results in Figure demonstrate that treatment with caveolin-1 antibody completely abolished the enhancement of TEER by IMM.
Figure 3. Effects of 2%(w/v) or 4%(w/v) megalo-type isomaltosaccharide (IMM) on transepithelial electrical resistance and the transport rate of the tight junction marker Lucifer yellow (100 μmol/L in apical medium) for 40 min in Caco-2 cell monolayers. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p< 0.05).
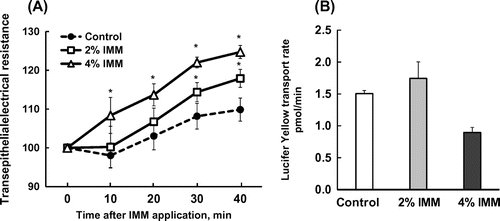
Figure 4. Changes in transepithelial electrical resistance (TEER, A) and the transport rate of Lucifer yellow (B) after application of 4%(w/v) oligo-type isomaltosaccharide (IMO) or 4%(w/v) megalo-type isomaltosaccharide (IMM) and the effects of the sequestration of cholesterol from Caco-2 cell monolayers by pre-treatment with 0.6%(w/v) methyl-β-cyclodextrin (MBC) for 40 min. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p< 0.05).
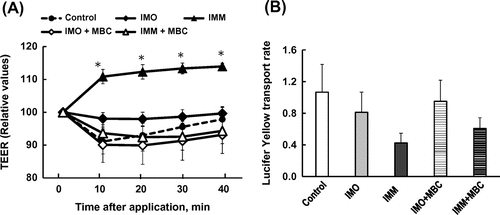
Figure 5. Inhibition of the increase in transepithelial electrical resistance (TEER) after application of megalo-type isomaltosaccharide (IMM) by treatment of Caco-2 cell monolayers with caveolin-1 antibody (1/250 dilution). Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p < 0.05).
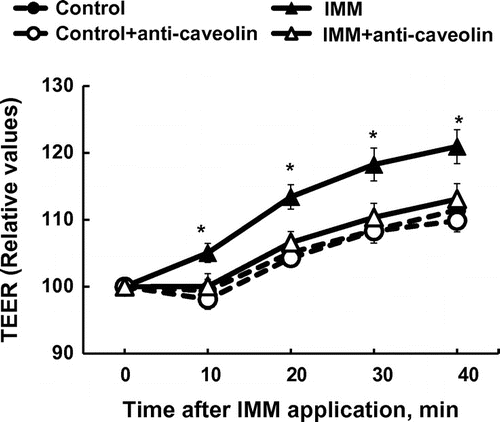
Figure shows the effects of inhibiting three endocytosis mechanisms, caveolae-mediated and clathrin-mediated endocytosis, and macropinocytosis, by nystatin, chlorpromazine, and 5-(N-ethyl-N-isopropyi)-amiloride, respectively [Citation24]. The increase in TEER in Caco-2 monolayers induced by 4%(w/v) IMM was prevented by the inhibition of the caveolae-dependent mechanism but not by the inhibition of the clathrin-dependent mechanism and macropinocytosis. Dynasore, an inhibitor of dynamin, which is an essential protein for both clathrin-dependent and caveolae-dependent coated vesicle formation [Citation25,26], did not suppress, but rather enhanced, the increase in TEER induced by IMM application, and also enhanced TEER in the control Caco-2 monolayer group (Figure ).
Figure 6. Effects of three endocytosis inhibitors on the increase in transepithelial electrical resistance (TEER) induced by 4%(w/v) megalo-type isomaltosaccharide (IMM) in Caco-2 cell monolayers. Nystatin (50 μmol/L) as a caveolae inhibitor, chlorpromazine hydrochloride (30 μmol/L) as a clathrin-mediated endocytosis inhibitor, and 5-(N-ethyl-N-isopropyi)-amiloride (20 μmol/L) as a macropinocytosis inhibitor were used. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p < 0.05).
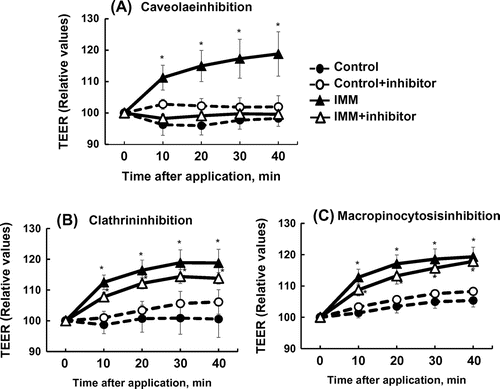
Figure 7. Effects of dynasore (80 μmol/L), an inhibitor of dynamin, an essential component for budding processes in both caveolae- and clathrin-dependent endocytosis, on the increase in transepithelial electrical resistance (TEER) induced by the application of megalo-type isomaltosaccharide (IMM) in Caco-2 cell monolayers. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p < 0.05).
Discussion
Our results demonstrate that IMM, a polymer of D-glucose with continuous α-1,6-glycoside linkages, suppressed the transport of tight junction markers in the rat intestine. The suppression was chain-length dependent, that is, a mid-chain length “megalosaccharide,” but not a short-chain length “oligosaccharide,” enhanced the epithelial barrier function of the rat intestine. This effect on tight junctions was confirmed in Caco-2 cell monolayers, a model of the human intestinal epithelium. Possible targets of IMM for the suppression effect in the rat intestine are the enteroendocrine systems, intestinal nervous systems, and gut immune system. However, we found that IMM reduced the permeability of tight junctions in Caco-2 cells in a similar fashion as in the rat intestine, which indicates a direct action of IMM on the intestinal epithelial cells, not on the intestinal systems mentioned above.
We found that the reduction of tight junction permeability was shown 20 min after IMM treatment in the rat intestine, and the enhancement in TEER was observed 10–20 min after IMM application in the Caco-2 cell monolayers. These results indicate that the effects of IMM are not adaptive responses, such as epithelial cell proliferation or differentiation. In Caco-2 monolayers, the effect of IMM is mediated by a caveolae-dependent mechanism. We showed the caveolae dependency of the IMM effect by removing cholesterol from the cell membrane using methyl-β-cyclodextrin or cholesterol sequestration by nystatin, and both caused the disruption of the cell membrane microdomain “rafts” with caveolin-1 [Citation23]. Nystatin has been shown to selectively interrupt caveola-mediated endocytosis without affecting internalization by clathrin-mediated endocytosis [Citation27]. We also confirmed the caveolae dependency by treating Caco-2 monolayers with caveolin-1 antibody. Internalization of the tight junction proteins with endocytosis has been known, and this process has a role for regulation of the tight junction permeability [Citation28]. We observed involvement of other endocytosis mechanisms, clathrin-mediated endocytosis and macropinocytosis. However, these endocytosis mechanisms were not involved in the action of IMM, as indicated by the results when using selective inhibitors for both endocytosis mechanisms. However, a dynamin inhibitor, dynasore, did not suppress the increase in TEER induced by IMM. Dynasore suppresses the release of budding vesicles in caveolae, but not destroy caveolae structure as nystatin [Citation29,30]. This result suggests that the endocytic process of caveolae is not associated with the enhancement in the epithelial barrier function induced by IMM. Figure shows that dynasore treatment increased TEER in both the control and IMM groups. The reason for the increase in TEER induced by dynasore is not known. Dynamine-dependent endocytosis process possibly be involved in the effects of IMM, and the suppression of the endocytosis process affects the properties of tight junctions controlling epithelial permeability, for example, reducing the turnover of the tight junction proteins. We should define distribution of the tight junction proteins in the intestinal epithelial cells in future, which may change by reducing turnover.
Many cell surface proteins, including GPI-anchor proteins, accumulate on caveolae [Citation31–33]. IMM may interact with unknown “receptor” proteins or some sugar chains present on the caveolae/lipid microdomain in epithelial cell membranes. IMM is a D-glucose polymer with continuous α-1,6 glycoside linkages, which is a flexible structure. The hydrophobic side of the mid-size glucose polymer in IMM possibly participates in hydrophobic interactions with caveolae components because of their flexibility. The interaction between IMM and caveolae may initiate some signals that affect regulatory components of the tight junction but not endocytosis processes. Further studies are needed to define the cell signaling pathways initiated from caveolae following the application of IMM. β-Glucan has been shown to bind dectin-1, a cell membrane protein in intestinal immune cells [Citation34] and enterocytes [Citation35,36], and it regulates the intestinal immune systems. Partially hydrolyzed guar gum, a water-soluble dietary fiber, was shown to affect tight junction permeability and suppress DSS-induced colitis with the suppression of pro-inflammatory cytokines [Citation37]. Consuming IMM would possibly have similar effects as consuming these dietary fibers. It is also necessary to examine the physiological effects on or prevention of certain diseases caused by the action of IMM on intestinal tight junctions.
In conclusion, mid-chain length D-glucose polymer, IMM, enhanced the barrier function of intestinal epithelial tight junction through caveolae in a chain-length dependent manner. IMM may suppress inflammation resulting from the permeation of luminal pro-inflammatory substances and reduce the risks of many chronic diseases.
Author contributions
Hiroshi Hara and Shunsuke Kume designed the experiments. Shunsuke Kume performed the experiments. Hiroshi Hara and Shunsuke Kume performed statistical analysis. Takahisa Iizuka, Yoshinori Fujimoto, and Atsuo Kimura prepared megalo-type isomaltosaccharides. Hiroshi Hara wrote the manuscript. Hiroshi Hara and Atsuo Kimura edited the manuscript. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Science and technology research promotion program for agriculture, forestry, fisheries and food industry [grant number 26062B].
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/09168451.2017.1398065.
Supplemental_figure-h.pptx
Download MS Power Point (83.8 KB)References
- Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111.10.1083/jcb.200110122
- Ivanov AI, McCall IC, Parkos CA, et al. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–2651.10.1091/mbc.E04-02-0163
- Shen L, Black ED, Witkowski ED, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106.10.1242/jcs.02915
- Nusrat A, Giry M, Turner JR, et al. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633.10.1073/pnas.92.23.10629
- Segain JP, Raingeard de la Blétière D, Sauzeau V, et al. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology 2003;124:1180–1187.10.1016/S0016-5085(03)00283-X
- Arnold TR, Stephenson RE, Miller AL. Rho GTPases and actomyosin: partners in regulating epithelial cell–cell junction structure and function. Exp Cell Res. 2017;358:20–30.
- Winer DA, Luck H, Tsai S, et al. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016;23:413–426.10.1016/j.cmet.2016.01.003
- Endo H, Niioka M, Kobayashi N, et al. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS ONE. 2013;8:e63388.10.1371/journal.pone.0063388
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809.10.1038/nri2653
- Brandl K, Schnabl B. Is intestinal inflammation linking dysbiosis to gut barrier dysfunction during liver disease? Expert Rev Gastroenterol Hepatol. 2015;9:1069–1076.10.1586/17474124.2015.1057122
- Lechuga S, Ivanov AI. Disruption of the epithelial barrier during intestinal inflammation: quest for new molecules and mechanisms. Biochim Biophys Acta. 2017;1864:1183–1194.10.1016/j.bbamcr.2017.03.007
- Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab. 2010;7:19.10.1186/1743-7075-7-19
- Lindmark T, Nikkila T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 1995;275:958–964.
- Ohata A, Usami M, Miyoshi M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 2005;21:838–847.10.1016/j.nut.2004.12.004
- Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305.
- Li Q, Zhang Q, Wang M, et al. n-3 polyunsaturated fatty acids prevent disruption of epithelial barrier function induced by proinflammatory cytokines. Mol Immunol. 2008;45:1356–1365.10.1016/j.molimm.2007.09.003
- Basuroy S, Sheth P, Mansbach CM, et al. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367–G375.10.1152/ajpgi.00464.2004
- Suzuki T, Hara H. Role of flavonoids in intestinal tight junction regulation. J Nutr Biochem. 2011;22:401–408.10.1016/j.jnutbio.2010.08.001
- Mercado J, Valenzano MC, Jeffers C, et al. Enhancement of tight junctional barrier function by micronutrients: compound-specific effects on permeability and claudin composition. PLoS ONE. 2013;8:e78775.10.1371/journal.pone.0078775
- Lang W, Kumagai Y, Sadahiro J, et al. Different molecular complexity of linear-isomaltomegalosaccharides and β-cyclodextrin on enhancing solubility of azo dye ethyl red: towards dye biodegradation. Bioresour Technol. 2014;169:518–524.10.1016/j.biortech.2014.07.025
- Shinoki A, Lang W, Thawornkuno C, et al. A novel mechanism for the promotion of quercetin glycoside absorption by megalo α-1,6-glucosaccharide in the rat small intestine. Food Chem. 2013;136:293–296.10.1016/j.foodchem.2012.08.028
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951.
- Matthews LC, Taggart MJ, Westwood M. Effect of cholesterol depletion on mitogenesis and survival: the role of caveolar and noncaveolar domains in insulin-like growth factor-mediated cellular function. Endocrinology 2005;146:5463–5473.10.1210/en.2005-0236
- Singh RD, Puri V, Valiyaveettil JT, et al. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell. 2003;14:3254–3265.10.1091/mbc.E02-12-0809
- Torgersen ML, Skretting G, van Deurs B, et al. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114:3737–3747.
- Macia E, Ehrlich M, Massol R, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850.10.1016/j.devcel.2006.04.002
- Chen Y, Wang S, Lu X, et al. Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways. Blood 2011;117:6392–6403.10.1182/blood-2010-12-322867
- Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta. 2008;1778:709–716.10.1016/j.bbamem.2007.07.027
- Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114.10.1083/jcb.141.1.101
- Goldberg DS, Ghandehari H, Swaan PW. Cellular entry of G3.5 poly (amido amine) dendrimers by clathrin- and dynamin-dependent endocytosis promotes tight junctional opening in intestinal epithelia. Pharm Res. 2010;27:1547–1557.10.1007/s11095-010-0153-3
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39.10.1038/35036052
- Lakhan SE, Sabharanjak S, De A. Endocytosis of glycosylphosphatidylinositol-anchored proteins. J Biomed Sci. 2009;16:93.10.1186/1423-0127-16-93
- Sangiorgio V, Pitto M, Palestini P, et al. GPI-anchored proteins and lipid rafts. Ital J Biochem. 2004;53:98–111.
- Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature 2001;413:36–37.
- Volman JJ, Mensink RP, Ramakers JD, et al. Dietary (1–>3), (1–>4)-beta-D-glucans from oat activate nuclear factor-kappaB in intestinal leukocytes and enterocytes from mice. Nutr Res. 2010;30:40–48.10.1016/j.nutres.2009.10.023
- Cohen-Kedar S, Baram L, Elad H, et al. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur J Immunol. 2014;44:3729–3740.10.1002/eji.201444876
- Hung TV, Suzuki T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J Nutr. 2016;146:1970–1979.10.3945/jn.116.232538
