Abstract
Immunoglobulin E (IgE) is involved in the onset of allergic reaction, and the suppression of IgE production leads to alleviation of allergic symptoms. We found that mango peel ethanol extract (MPE) significantly suppresses IgE production by human myeloma cell line U266 cells, suggesting that MPE has an anti-allergic effect by inhibiting the production of IgE. Although mangiferin is contained in mango, which suppresses IgE production by U266 cells, it was not contained in MPE. We investigated the suppressive effect of MPE in 2,4-dinitrofluorobenzene (DNFB)-induced allergic contact dermatitis model mice. The elevation of serum IgE level was significantly suppressed by oral administration of MPE. Intake of MPE also suppressed the expression level of IL-4 in the DNFB-challenged ears, suggesting that MPE suppresses the IL-4-mediated maturation into IgE-producing cells. Our findings indicate that MPE has a potential to alleviate the increase in serum IgE level that is feature of type I allergy.
Graphical abstract
Oral administration of MPE suppressed the elevation of IgE level in serum and the expression of IL-4 gene in ear of DNFB-induced allergic contact dermatitis model mice.
Allergy is a hypersensitivity reaction due to immunological responses that occur in response to inherently harmless substances called as allergens such as pollen, foods, and drugs. Allergy is mainly classified into five types. Type I, II, and III responses are involved in humoral immunity mediated by the different classes of antibody secreted by plasma cells. Type IV responses, known as delayed allergic reactions, are involved in cellular immunity mediated by activated antigen-specific cytotoxic T lymphocytes releasing various cytokines in response to the antigen. Type V responses are irritant reactions that are often included in the type II responses and caused by an autoantibody binding to cell surface receptors as a ligand. The number of patients with allergic diseases has been increasing, and particular type I responses referred to as immediate allergic reactions are the most common allergic reactions such as hay fever and asthma [Citation1]. When an allergen enters the body, dendritic cells capture it and are activated. The activated dendritic cells present peptides derived from the allergen through the major histocompatibility complex (MHC) class II molecules to naïve T cells. In addition, the naïve T cells are stimulated by interleukin (IL)-4 derived from wide range of cells including basophils, mast cells, eosinophils, and natural killer T cells, thereby inducing the differentiation of the naïve T cells into T helper 2 (Th2) cells. The Th2 cells produce Th2 cytokines such as IL-4, IL-5, IL-9, and IL-13. These cytokines and the ligation of suitable co-stimulatory molecules (CD40 with CD40 ligand and CD80 or CD86 with CD28) amplify the Th2 response and stimulate the class switching of B cells [Citation2]. As a result, the B cells differentiate into plasma cells and produce the specific IgE in the presence of antigen. Therefore, in patients with allergic diseases, total and allergen-specific IgE levels in blood are high compared with healthy individuals. The produced IgE binds to the high-affinity IgE receptor (FcεRI) on the surface of mast cells and basophils, and then the chemical mediators such as histamine, eicosanoids, and inflammatory cytokines are released from intracellular granules of their cells, which is called degranulation [Citation3–5] Released histamine induces acute allergic responses such as contraction of smooth muscle, vasodilatation, and increased vascular permeability [Citation6]. So far, various food components have been reported to exhibit anti-allergic effects that inhibit the effector phase of allergic responses such as degranulation [Citation7–10]. However, their effects are not a fundamental treatment of allergy symptoms because IgE levels in blood of patients with allergic disease are still rising. Omalizumab, known as a humanized recombinant monoclonal anti-IgE antibody that binds circulating IgE and prevents interaction with FcεRI on mast cells and basophils, has established the efficacy in patients with moderate-to-severe allergic asthma [Citation11,12]. In addition, omalizumab has also been shown to reduce production of IgE [Citation13]. These reports indicate that the reduction in IgE levels in blood of allergic patients leads to a fundamental solution of allergic symptoms.
Mango (Mangifera indica L.) is a representative tropical fruit native to India to Southeast Asia. In Japan, it is mainly produced in Okinawa, Miyazaki, and Kagoshima prefectures. There are over 500 cultivars and the size, shape, and color of fruit or peel varies depending on cultivars. Mango distributed in Japan is mostly Irwin cultivar with a red peel, but Keitt cultivar with a green peel is also cultivated in Okinawa prefecture. Mango belongs to the Anacardiaceae family and is known to develop a rash because it contains urushiol that causes contact dermatitis. On the other hand, the stem bark aqueous extract of mango and its major component mangiferin, a natural C-glucoside xanthone contained in mango, have been reported to exhibit anti-allergic effects in an ovalbumin (OVA)-induced asthmatic mouse model by controlling Th1/Th2 cytokine balance [Citation14–17]. These studies indicate that the extract and mangiferin suppress the serum IgE level in the OVA-induced allergic asthma model mice. Although mango includes many substances showing a positive or negative effect on allergic responses, the suppressive effect of mango peel components on IgE production has not been reported yet. Therefore, we examined the inhibitory effect of mango peel extract on IgE production using human myeloma cell line U266 cells in vitro and allergic contact dermatitis model mice in vivo.
Materials and methods
Reagents
Roswell Park Memorial Institute 1640 (RPMI 1640) medium, penicillin, insulin, transferrin, ethanolamine, sodium selenite, streptomycin, fetal bovine serum (FBS), bovine serum albumin (BSA), and mangiferin were products of Sigma-Aldrich (St. Louis, MO, USA). Anti-human IgE antibody and biotin-conjugated anti-human IgE antibody were purchased from Calbiochem (San Diego, CA, USA) and Biosource International (Camarillo, CA, USA), respectively. Rat anti-mouse IgE and biotin-conjugated rat anti-mouse IgE were from BD Pharmingen (San Diego, CA, USA). Goat anti-mouse IgA antibody, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgA antibody, rabbit anti-mouse IgM antibody, HRP-conjugated goat anti-mouse IgM antibody, and HRP-conjugated streptavidin were purchased from Invitrogen (Carlsbad, CA, USA). Goat anti-mouse IgG antibody was purchased from MP Biomedicals (Santa Ana, CA, USA). HRP-conjugated goat anti-mouse IgG antibody was purchased from Thermo Fisher Scientific (Waltham, MA, USA). 1-Fluoro-2,4-dinitrobenzene (dinitrofluorobenzene; DNFB) was obtained from Nacalai Tesque (Kyoto, Japan). All other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan) or Nacalai Tesque unless otherwise noted.
Sample preparation
Keitt mango provided by Okinawa Miyako-ichiba (Okinawa, Japan) was used as a sample. Mango was divided into fruit and peel. Freeze-dried mango peel powder was suspended in 100% ethanol at 0.2 g/mL at room temperature for 24 h. After the suspension was centrifuged at 20,000×g at 4 °C for 20 min, the supernatant was collected and used as a mango peel ethanol extract (MPE). The weight of MPE was measured by weighing a portion of an evaporated sample. Mangiferin was dissolved in dimethyl sulfoxide (DMSO; Wako Pure Chemical Industries). When each sample was added to the culture media, the final concentration of ethanol or DMSO was adjusted to be 0.5%.
Mice
BALB/c mice were purchased from Japan SLC (Shizuoka, Japan) and kept in an animal room under a 12 h light/dark cycle at a temperature of 24 ± 1 °C. Animals were received a pelleted basal diet and water ad libitum. All animal experiments described in this study were carried out in accordance with the protocol approved by the Laboratory Animal Care Committee of Ehime University. Mice were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Ehime University.
Cells and cell culture
IgE-producing human myeloma cell line U266 cells were obtained from American Type Culture Collection (Rockville, MD, USA). U266 cells were cultured in RPMI 1640 medium supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 15% FBS at 37 °C under humidified 5% CO2.
Assay of IgE production-suppressing activity
U266 cells were suspended in RPMI 1640 medium supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, 10 μg/mL of insulin, 20 μg/mL of transferrin, 20 μM ethanolamine, and 25 nM sodium selenite (ITES-RPMI 1640 medium) containing various concentrations of MPE or 0.5% ethanol alone (vehicle) as control and seeded into a 96-well culture plate (Corning, Corning, NY, USA) at 1.0 × 104 cells/well. When the effect of mangiferin on IgE production was examined, 100 and 25 μM mangiferin or 0.5% DMSO (vehicle) was used. After incubation for 24 h at 37 °C, human IgE concentration in culture media was determined by an in-house-developed enzyme-linked immunosorbent assay (ELISA). Cell viability was measured by WST-8 assay after collecting the culture media from each well for ELISA.
ELISA
The concentration of human IgE in culture media was measured by an in-house-developed ELISA as shown previously [Citation18,19]. Each well of a 96-well microtiter plate (Nunc, Roskilde, Denmark) was coated with 20 ng of anti-human IgE diluted in 50 mM carbonate buffer (pH 9.6) at 4 °C overnight. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) 3 times, each well was blocked with PBS containing 5% (w/v) skim milk for 2 h at 37 °C. After washing with PBS-T, 50 μL of culture media were added to each well and incubated for 1 h at 37 °C. After washing with PBS-T, wells were treated with 10 ng of biotin-conjugated anti-human IgE diluted in PBS containing 5% (w/v) skim milk for 1 h at 37 °C. After washing with PBS-T, the wells were treated with 31.3 ng of HRP-labeled streptavidin diluted in PBS containing 1% BSA for 1 h at 37 °C. After washing with PBS-T, 0.6 mg/mL of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid ammonium salt) (Wako Pure Chemical Industries) dissolved in 0.03% H2O2–0.05 M citrate buffer (pH 4.0) was added to wells at 100 μL, and the absorbance at 415 nm was measured using a Model 550 microplate reader (Bio-Rad Laboratories; Hercules, CA, USA) after the addition of 1.5% oxalic acid at 100 μL/well to terminate the coloring reaction.
Real-time reverse transcription-polymerase chain reaction (Real-time RT-PCR)
U266 cells were suspended in ITES-RPMI 1640 medium containing 0.5% ethanol and 3.2 μg/mL of MPE or 0.5% ethanol alone as control and seeded into a 6-well culture plate (Nippon Genetics, Tokyo, Japan) at 1.0 × 106 cells/well. After incubation for 24 h at 37 °C, the cells were collected and centrifuged at 160×g for 5 min. The cell pellet was washed with PBS and centrifuged again. Total RNA was then isolated from the cells using Sepasol-RNA I Super G (Nacalai Tesque) according to the manufacturer’s instructions and used as a template for cDNA synthesis with MMLV-reverse transcriptase (Wako Pure Chemical Industries) and an oligo-(dT)20 primer. A real-time PCR mixture, with a final volume of 20 μL, consisted of Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan), 10 pmol of a forward primer, 10 pmol of a reverse primer, and 0.1 μg of a cDNA sample. Thermal cycling conditions were 20 s at 95 °C, followed by 40 cycles of 3 s at 95 °C and 30 s at 60 °C. PCR products were measured on a StepOnePlus Real-time PCR System (Applied Biosystems, Foster City, CA, USA), and relative gene expression was calculated based on the comparative CT method using StepOne Software v2.1 (Applied Biosystems). Expression of GAPDH gene was used as an endogenous control. Specific oligonucleotide sequences for each gene are as follows. Human GAPDH: sense, 5-TCCATGACAACTTTGGCATCGTGG-3 and anti-sense, 5-GTTGCTGTTGAAGTCACAGGAGAC-3; human IgE: sense, 5-ATGACCTTACCAGCCACCAC-3 and anti-sense, 5-GGTTTTGTTGTCGACCCAGT-3; mouse GAPDH: sense, 5-CCTGGAGAAACCTGCCAAGTATG-3 and anti-sense, 5-AGAGTGGGAGTTGCTGTTGAAGTC-3; mouse IL-4: sense, 5-ACAGGAGAAGGGACGCCAT-3 and anti-sense, 5-GAAGCCCTACAGACGAGCTCA-3.
Ultra performance liquid chromatography (UPLC) analysis
UPLC analysis was performed using an ACQUITY UPLC system (Waters, Milford, MA, USA). ACQUITY UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm, Waters) was used with the column oven temperature at 35 °C. Acetonitrile containing 0.1% formic acid (ACN-FA) was used as the elute solvent. Elution program was applied as follows; 0–1 min, 1% ACN-FA; 1–6 min, linear gradient from 1 to 100% ACN-FA; 6–7 min, 100% ACN-FA; 7–8 min, linear gradient from 100 to 1% ACN-FA. An injection volume was set up in 10 μL containing 25 μg of MPE or 20 μM mangiferin, and the chromatogram was monitored at a wavelength of 258 nm.
DNFB-induced allergic contact dermatitis model mice
A mouse model of DNFB-induced allergic contact dermatitis was developed as previously described [Citation20] and treatment of mice with DNFB was conducted as scheduled in Figure . Briefly, 5-week-old female BALB/c mice were divided into 4 groups (7 or 8 mice per group) and the abdominal hair was shaved on day −1 after 1 week of acclimation. They were orally administered with 20 μL of distilled water containing 2% ethanol for intact and control groups, 20 μL of distilled water containing 2% ethanol and 20 μg of MPE for high dose group, or 20 μL of distilled water containing 2% ethanol and 4 μg of MPE for low dose group for 15 consecutive days from day 0 to day 14. DNFB was dissolved in a solution of acetone:olive oil = 4:1 (A/O). As shown in Figure (A), the mice in control and MPE-administered groups were sensitized on day 0 by applying 50 μL of 0.5% DNFB diluted in A/O to the shaved abdominal skin. On day 4, the dorsal hair of all mice were shaved. The mice in control and MPE-administered groups were then challenged by applying 80 μL of 0.2% DNFB diluted in A/O to the shaved dorsal skin on days 5, 8, 11, and 14. At the same time, both ears of the mice were applied with 10 μL of 0.2% DNFB. The mice in intact group were treated with A/O alone as shown in Figure (B). On days 0, 6, 9, 12, and 15, the thickness of ears of all mice was measured to evaluate allergic symptom. On day 15, the blood and both ears were collected from all mice. Serum levels of IgE, IgG, IgA, and IgM were measured by in-house-developed ELISA as shown previously [Citation20]. The collected ears were immediately stored in liquid nitrogen and homogenized by SK-200 (Tokken, Chiba, Japan). Total RNA was then isolated from ears using Sepasol-RNA I Super G according to the manufacturer’s instructions, and the gene expression level of IL-4 was evaluated by real-time RT-PCR
Statistical analysis
Data obtained were expressed as mean ± standard deviation (SD). One way ANOVA followed by Dunnett’s test or Tukey-Kramer test was used to assess the statistical significance of the difference. Values with *p < 0.05 or **p < 0.01 were considered statistically significant.
Results and discussion
MPE suppresses IgE production by U266 cells without affecting the cell viability
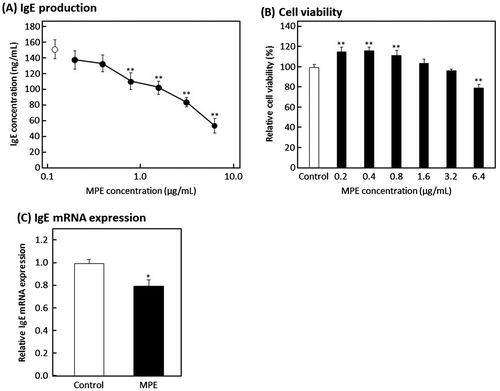
At first, we examined the effect of MPE on IgE production by U266 cells. U266 cells were cultured for 24 h in ITES-RPMI 1640 medium containing various concentrations of MPE, and the concentration of IgE in the culture media was measured using ELISA. As shown in Figure (A), MPE significantly suppressed the production of IgE by U266 cells in a dose-dependent manner. Next, we examined whether the suppressive effect of MPE on IgE production was due to cytotoxicity. According to the result of WST-8 assay, MPE showed no cytotoxicity to U266 cells up to 3.2 μg/mL, although the cell viability significantly decreased at 6.4 μg/mL of MPE as shown in Figure (B). These results suggested that MPE suppresses IgE production by U266 cells without cytotoxicity at 3.2 μg/mL. To clarify that, the effect of MPE on gene expression level of IgE in U266 cells was evaluated by real-time RT-PCR. Total RNA was extracted from U266 cells treated with 3.2 μg/mL of MPE for 24 h. As shown in Figure (C), MPE suppressed the mRNA expression level of IgE in U266 cells. These result indicated that MPE suppresses the production of IgE by down-regulating the transcription of IgE gene.
Figure 2. Effect of MPE on IgE production by U266 cells. (A) U266 cells were suspended in ITES-RPMI 1640 medium containing various concentrations of MPE or 0.5% ethanol alone (vehicle) as control and incubated for 24 h at 37 °C. Human IgE concentration in culture media was determined by an in-house-developed enzyme-linked immunosorbent assay. Open circle, control; closed circles, MPE. (B) Cell viability was measured by WST-8 assay. (C) The mRNA expression levels of IgE were evaluated by real-time RT-PCR. U266 cells were suspended in ITES-RPMI 1640 medium containing 3.2 μg/mL of MPE or 0.5% ethanol alone as control and incubated for 24 h at 37 °C. Data were represented as the mean ± standard deviation (n = 3). *p < 0.05, **p < 0.01 against control by Dunnett’s test.
Mangiferin is not an active substance in MPE
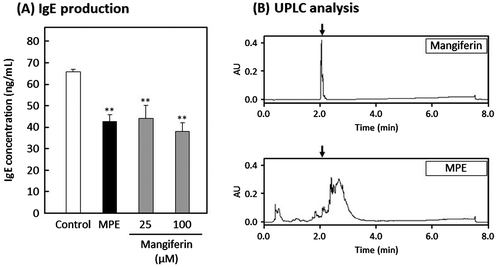
Mangiferin is contained in stem bark and leaves of mango. It has been reported that mangiferin reduces the level of serum antigen-specific IgE in OVA-induced allergic asthma model mice [Citation16,17] Therefore, we confirmed whether the effect of MPE is due to mangiferin. As a result, mangiferin suppressed IgE production by U266 cells (Figure (A)), suggesting that mangiferin has the suppressive effect on IgE production. Then, we analyzed the content of mangiferin in MPE by UPLC. A peak with a maximum absorption wavelength at 258 nm was observed at the retention time of 2.05 min in the chromatogram of mangiferin (Figure (B); upper). However, the peak with the same retention time as mangiferin was not confirmed in the chromatogram of MPE (Figure (B); lower), suggesting that MPE does not contain mangiferin. These results indicated that the active substance in MPE is not mangiferin. The active substance in MPE is under investigation.
Figure 3. Effect of mangiferin on IgE production by U266 cells. U266 cells were suspended in ITES-RPMI 1640 medium containing each sample and incubated for 24 h at 37 °C. (A) Human IgE concentration in culture media was determined by an in-house-developed enzyme-linked immunosorbent assay (ELISA). Each sample was prepared as follows; control, 0.5% ethanol and 0.5% dimethyl sulfoxide (DMSO); MPE, 3.2 μg/mL of MPE; Mangiferin, 100 or 25 μM mangiferin. Data were represented as the mean ± standard deviation (n = 3). **p < 0.01 against control by Dunnett’s test. (B) UPLC analysis was performed using an ACQUITY UPLC system. ACQUITY UPLC BEH C18 column was used with the column oven temperature at 35 °C, and acetonitrile containing 0.1% formic acid (ACN-FA) was used as an elute solvent. Elution program was applied as follows; 0–1 min, 1% ACN-FA; 1–6 min, linear gradient from 1 to 100% ACN-FA; 6–7 min, 100% ACN-FA; 7–8 min, linear gradient from 100 to 1% ACN-FA. An injection volume was set up in 10 μL containing 25 μg of MPE or 20 μM mangiferin, and the chromatogram was monitored at a wavelength of 258 nm.
Ingestion of MPE alleviates the allergic symptoms of DNFB-induced allergic contact dermatitis model mice
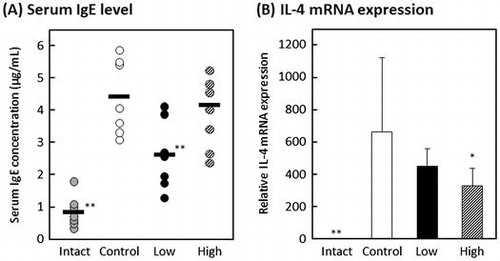
The effect of oral administration of MPE on DNFB-induced allergic contact dermatitis model mice was investigated. Contact dermatitis is generally classified into type IV hypersensitivity. However, it has been reported that repeated elicitation of contact hypersensitivity induces a shift in antigen-specific hypersensitivity responses from a delayed-type to an early-type, which is Th2-dominated response [Citation21,22]. The mouse model of DNFB-induced allergic contact dermatitis was developed according to the schedule shown in Figure . Mice were orally administered with 20 μL of distilled water containing 2% ethanol for intact and control groups, 20 μL of distilled water containing 2% ethanol and 20 μg of MPE for high-dose group, or 20 μL of distilled water containing 2% ethanol and 4 μg of MPE for low-dose group for 15 consecutive days. On day 15, all mice were collected the blood and both ears. As shown in Figure , the serum levels of IgE and IgG significantly decreased in low-dose group compared with control group (p < 0.01 and p < 0.05, respectively), and the serum IgM level significantly increased in low-dose group (p < 0.05). On the other hand, there was no difference in serum IgA level between control group and MPE-administrated groups. These results suggested that intake of MPE regulates the amount of IgE in blood among other antibodies. On days 0, 6, 9, 12, and 15, the thickness of ears of all mice were measured to evaluate allergic symptom. As shown in Figure (A), although a slight suppression tendency was observed in low-dose group compared to control group, there was no significant difference on day 15 (p = 0.1). Since the difference between low-dose group and control group was observed with each passing day, the relieving effect of allergic symptoms may have been obtained by prolonging the test period. In addition, when we evaluated the gene expression in the DNFB-challenged ears, the mRNA expression level of IL-4 was suppressed in MPE-administrated groups (Figure (B)). IL-4 plays a crucial role in allergy that induces the differentiation of B cells and a class switching from IgM into IgE or IgG1. As a result of Figure , the serum levels of IgE and IgG decreased, whereas that of IgM increased. It is known that there are two main pathways of immunoglobulin class switching for IgE; a direct pathway from IgM to IgE isotype and a sequential pathway from IgM to an IgG1 intermediate and then to IgE [Citation23]. These results suggested that MPE suppresses the elevation of serum IgE level by interfering the IL-4-mediated class switching from IgM to IgE via direct or sequential pathways.
Figure 4. Effect of oral administration of MPE on a mouse model of allergic contact dermatitis. Mice in intact and control groups were orally administered with 20 μL of distilled water containing 2% ethanol for intact and control groups, 20 μL of distilled water containing 2% ethanol and 20 μg of MPE for high-dose group, or 20 μL of distilled water containing 2% ethanol and 4 μg of MPE for low-dose group for 15 consecutive days. Serum levels of (A) IgE, (B) IgG, (C) IgA, and (D) IgM were measured by in-house-developed enzyme-linked immunosorbent assay (ELISA). Bars represent the average of each group (n = 7 or 8). *p < 0.05, **p < 0.01 against control group by Dunnett’s test.
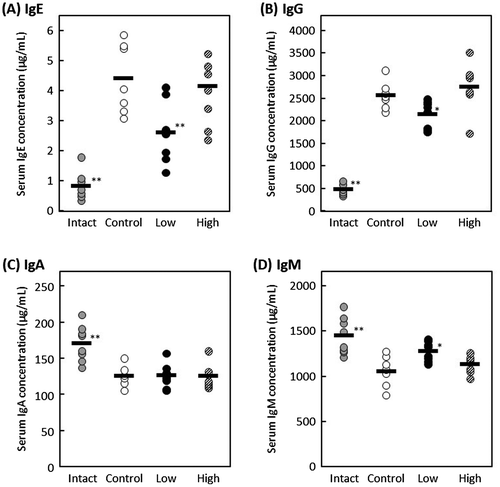
Figure 5. Effect of oral administration of MPE on an allergic symptom of a mouse model of allergic contact dermatitis. (A) On days 0, 6, 9, 12, and 15, the thickness of ears of all mice were measured to evaluate allergic symptom. Shaded circle, intact group; open circle, control; closed circle, low-dose group; hatched circle, high-dose group. (B) After measuring the thickness of ears on day 15, both ears were collected and the gene expression level of IL-4 was evaluated by real-time RT-PCR. *p < 0.05, **p < 0.01 against control group by Dunnett’s test.
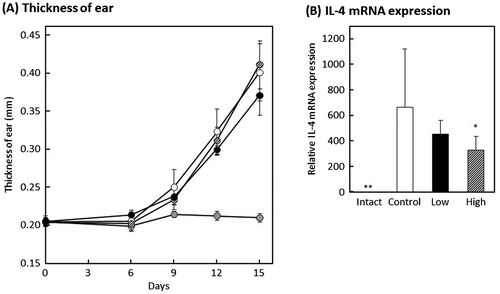
In this experiment, the elevation of serum IgE level was not suppressed in high-dose group but significantly suppressed in low-dose group. However, the mRNA expression level of IL-4 in the DNFB-challenged ears was significantly suppressed in high-dose group but not suppressed in low-dose group. From these results, we assume that MPE contains substances that act positive or negative in allergic reaction. We are considering as follows; the positive substances suppress the production of Th2 cytokine which induce differentiation into IgE-producing cells or the production of IgE by IgE-producing cells, resulting in suppression of the elevation of serum IgE. On the other hand, the negative substances induce an increase in serum IgE level and severity of allergic symptoms without affecting the expression of IL-4 gene. In high-dose group, although the positive substances suppressed the mRNA expression of IL-4, the negative substances may dominate over positive substances and cause severe allergic reaction. In addition, we estimate that the suppression of the increase in serum IgE level was observed in low-dose group because the effect of the positive substances is sufficiently exhibited even at a low concentration and exceeded that of the negative substances.
Conclusion
Our experiments using a cell line showed that MPE suppresses the production of IgE in plasma cells because MPE suppressed the gene expression of IgE in U266 cells. In addition, experiments in vivo suggested that MPE interferes to the class switching to IgE mediated by IL-4. Therefore, MPE may show anti-allergic effect by two approaches; one is to suppress the production of IgE by downregulating the transcription of IgE gene in IgE-producing cells, and the other is to suppress the IL-4-mediated maturation into IgE-producing cells. Our findings indicate that MPE may have a potential to alleviate the increase in serum IgE level that is feature of type I allergy.
Author contributions
M. Ishida, T. Sasaki, K. Nishi, and T. Sugahara conceived and designed the experiments. T. Tamamoto provided mango samples. M. Ishida and T. Sasaki performed the experiments and analyzed the data. M. Ishida, T. Sasaki, K. Nishi and T. Sugahara wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Pawankar R, Baena-Cagnani CE, Bousquet J, et al. State of world allergy report 2008: allergy and chronic respiratory diseases. WAO J. 2008;1(S1):S4–S17.
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454.10.1038/nature07204
- Turner H, Kinet J-P. Signalling through the high-affinity IgE receptor FcεRI. Nature. 1999;402:24–30.10.1038/35037021
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225.10.1016/j.jaci.2006.04.015
- Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646.10.1016/j.jaci.2009.08.035
- White M. Mediators of inflammation and the inflammatory process. J Allergy Clin Immunol. 1999;103:S378–S381.10.1016/S0091-6749(99)70215-0
- Sakai S, Sugawara T, Matsubara K, et al. Inhibitory effect of carotenoids on the degranulation of mast cells via suppression of antigen-induced aggregation of high affinity IgE receptors. J Biol Chem. 2009;284:28172–28179.10.1074/jbc.M109.001099
- Itoh T, Tsukane M, Koike M, et al. Inhibitory effects of whisky congeners on IgE-mediated degranulation in rat basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J Agric Food Chem. 2010;58:7149–7157.10.1021/jf100998c
- Ishida M, Nishi K, Watanabe H, et al. Inhibitory effect of aqueous spinach extract on degranulation of RBL-2H3 cells. Food Chem. 2013;136:322–327.10.1016/j.foodchem.2012.08.079
- Kondo M, Nishi K, Sugahara T. Ishizuchi dark tea suppresses IgE-mediated degranulation of RBL-2H3 cells and nasal rubbing behavior of pollinosis in mice. J Funct Foods. 2015;14:659–669.10.1016/j.jff.2015.02.045
- Holgate ST, Djukanovic R, Casale T, et al. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35:408–416.10.1111/cea.2005.35.issue-4
- Wu LC, Scheerens H. Targeting IgE production in mice and humans. Curr Opin Immunol. 2014;31:8–15.10.1016/j.coi.2014.08.001
- Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–320.10.1111/j.1365-2125.2011.03962.x
- García D, Escalante M, Delgado R, et al. Anthelminthic and antiallergic activities of Mangifera indica L. stem bark components Vimang and mangiferin. Phytother Res. 2003;17:1203–1208.10.1002/ptr.1343
- Rivera DG, Balmaseda IH, León AA, et al. Anti-allergic properties of Mangifera indica L. extract (Vimang) and contribution of its glucosylxanthone mangiferin. J Pharm Pharmacol. 2006;58:385–392.10.1211/jpp.58.3.0014
- Rivera DG, Hernández I, Merino N, et al. Mangifera indica L. extract (Vimang) and mangiferin reduce the airway inflammation and Th2 cytokines in murine model of allergic asthma. J Pharm Pharmacol. 2011;63:1336–1345.10.1111/j.2042-7158.2011.01328.x
- Guo H-W, Yun C–X, Hou G–H, et al. Mangiferin attenuates Th1/Th2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS ONE. 2014;9:e100394.10.1371/journal.pone.0100394
- Sugahara T, Nishimoto S, Morioka Y, et al. White sorghum (Sorghum bicolor (L.) Moench) bran extracts suppressed IgE production by U266 cells. Biosci Biotech Bioch. 2009;73:2043–2047.
- Ohno F, Sugahara T, Kanda K, et al. Proteose peptone fraction of bovine milk depressed IgE production in Vitro and in Vivo. Biosci Biotech Bioch. 2010;74:1332–1337.10.1271/bbb.90809
- Mizusaki A, Nishi K, Nishiwaki H, et al. Suppressive effect of ethanol extract from passion fruit seeds on IgE production. J Funct Foods. 2017;32:176–184.10.1016/j.jff.2017.02.030
- Kitagaki H, Ono N, Hayakawa K, et al. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997;159:2484–2491.
- Matsumoto K, Mizukoshi K, Oyobikawa M, et al. Establishment of an atopic dermatitis-like skin model in a hairless mouse by repeated elicitation of contact hypersensitivity that enables to conduct functional analyses of the stratum corneum with various non-invasive biophysical instruments. Skin Res Technol. 2004;10:122–129.10.1111/srt.2004.10.issue-2
- Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. 2014;14:247–259.10.1038/nri3632