Abstract
In April 2015, Consumer Affairs Agency of Japan launched a new food labeling system known as “Foods with Function Claims (FFC).” Under this system, the food industry independently evaluates scientific evidence on foods and describes their functional properties. As of May 23, 2017, 1023 FFC containing 8 fresh foods have been launched. Meanwhile, to clarify the health-promoting effects of agricultural products, National Agriculture and Food Research Organization (NARO) implemented the “Research Project on Development of Agricultural Products” and demonstrated the risk reduction of osteoporosis of β-cryptoxanthin rich Satsuma mandarins and the anti-allergic effect of the O-methylated catechin rich tea cultivar Benifuuki. These foods were subsequently released as FFC. Moreover, NARO elucidated the health-promoting effects of various functional agricultural products (β-glucan rich barley, β-conglycinin rich soybean, quercetin rich onion, etc.) and a healthy boxed lunch. This review focuses on new food labeling system or research examining functional aspects of agricultural products.
Abbreviations:
- CAA: Consumer Affairs Agency
- FOSDU: Foods for Special Dietary Uses
- FHCs: Foods with Health Claims
- FOSHU: Foods for Specified Health Uses
- FNFC: Foods with Nutrient Function
- FFC: Foods with Function Claims
- NARO: National Agriculture and Food Research Organization
- EGCG: epigallocatechin-3-O-gallate
- EGCG3′′Me: epigallocatechin-3-O-(3-O-methyl) gallate
- TG: triglyceride
- HDL: high-density lipoprotein
- LDL: low-density lipoprotein
- DHA: docosahexaenoic acid
- EPA: eicosapentaenoic acid
- VFA: visceral fat area
- AVF: abdominal visceral fat
- BMI: body mass index
- TBARS: thiobarbituric acid reactive substances
- MMSE: Mini-Mental State Examination
- OGTT: oral glucose tolerance test
- LAB: low-density lipoprotein receptor-1 containing apolipoprotein B
- ALT: serum alanine aminotransferase
- 1,5-AG, 1,5-anhydroglucitol
Recently, Japan has been experiencing a decline in population and a rapid acceleration towards a “super aging” society [Citation1,2]. Thus, the number of patients suffering from lifestyle-related diseases is increasing [Citation2–5], and the national medical expenditure increased to 41,500 billion yen in 2015 [Citation6]. On the other hand, food plays an important role in health maintenance/enhancement and disease prevention, as described by the familiar expression “Medicine and Food Have the Same Origin (‘Ishoku-Dougen’ in Japanese)”. The study of functional foods to prevent lifestyle-related diseases has been conducted actively since 1984 in Japan [Citation7–9].
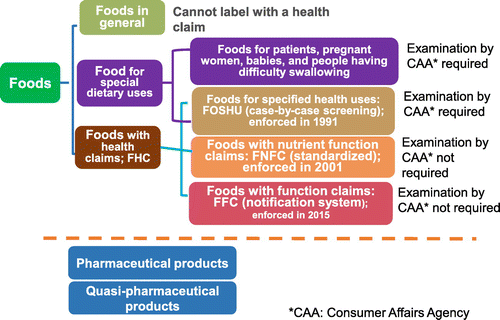
Figure shows the classification of orally ingested products in Japan. Prior to April 2015, the Japanese Consumer Affairs Agency (CAA) regulated “foods for special dietary uses (FOSDU) (enforced in 2008)” and “foods with health claims (FHCs)” [Citation1] as “foods for specified health uses (FOSHU) (enforced in 1991)” and “foods with nutrient function claims (FNFC) (enforced in 2001)” (Figure ) [Citation10]. The CAA permitted FOSDU labeling of foods that contribute to health maintenance and/or recovery from disease under the following four categories: (1) medical uses for diseases (low-sodium foods, low-calorie foods, low-protein foods, no/low-protein and high-calorie foods, high-protein foods, allergen-free foods, lactose-free foods, sodium-reduced meals, meals for diabetes, meals for liver disease, and meals for adults with obesity); (2) formulas for pregnant and lactating women; (3) infant formulas; and (4) foods for elderly individuals with chewing or swallowing difficulties. The CAA grants permission for FOSDU labeling according to 1. Standard approval processes ((2), (3), and (4)) and 2. Individual approval processes (1), in which foods are reviewed without established standards.
FOSHU are scientifically recognized for their health maintenance and health promoting effects and may be labeled with claims such as “slows cholesterol absorption”. The government evaluates these claims, as well as product safety, and the Secretary-General of the CAA approves the labeling of food products that satisfy all of the following requirements. Each FOSHU is approved only after detailed review of the scientific evidence. Conditions necessary for FOSHU designation are (1) Improvements in dietary habits, health maintenance, and enhancement can be expected by those who consume the product, (2) Scientific evidence is available to support the product’s health claims, (3) Clinical and nutritional intake level of the product and/or its functional component is established, (4) The product and/or functional ingredient are safe for human consumption, (5) The functional ingredients are defined in terms of: (a) Physical, chemical, and biological characteristics, (b) Methods of qualitative and quantitative analytical determination, (c) No significant variations in nutrient constituents of the food, (d) The food is intended to be consumed daily and not on rare occasions, (e) The product or its functional ingredient is not considered to be a pharmaceutical.
Categories of FOSHU include (1) Standardized FOSHU: (a) No detailed review process for food products meeting established standards and specifications, (b) Must be accompanied by sufficient accumulation of scientific evidence, and (c) Fast-track approval for products with previously approved safety; (2) Reduction of disease risk FOSHU: (a) Detailed review process requiring scientific evidence, and (b) Products with clinically and nutritionally established ingredients that reduce the risk of certain diseases (i.e. calcium for osteoporosis and folic acid for neural tube defects); and (3) Qualified FOSHU: (a) Detailed review process requiring scientific evidence, (b) Products containing ingredients with demonstrated health effects that do not reach established standards for FOSHU approval, and (c) Labeled as “Qualified Food for Specified Health Uses”. However, all three categories of FOSHU can be sold with a FOSHU seal of approval.
Examples of approved “foods with specified uses” products are as follows (1099 items as of July 29, 2017): Special health uses of foods are: (1) for modifying gastrointestinal conditions; principle ingredients (ingredients exhibiting health functions): oligosaccharides, lactose, bifidobacteria, lactic acid bacteria, and dietary fiber (ingestible dextrin, polydextrose, guar gum, and psyllium seed coat), (2) for acting on blood cholesterol levels: chitosan, soybean protein, and degraded sodium alginate, (3) for acting on blood glucose levels: indigestible dextrin, wheat albumin, guava tea polyphenol, and L-arabinose, (4) for acting on blood pressure: lactotripeptide, casein dodecapeptide, tochu leaf glycoside (geniposidic acid), and sardine peptide, (5) for promoting dental hygiene: palatinose, maltitol, and erythritol, (6) for lowering cholesterol and improve gastrointestinal conditions: degraded sodium alginate, and dietary fiber from psyllium seed husk, (7) for affecting mineral absorption: calcium citrate malate, casein phosphopeptide, heme iron, and fructooligosaccharide, (8) for promoting osteogenesis: soybean isoflavone and milk basic protein, (9) for mitigating triacylglycerol and body fat: Medium chain fatty acids, tea catechin, chlorogenic acid, EPA, DHA, quercetin glycoside, apple procyanidin, mannooligosaccharide, oolong tea polyphenol, (10) for reducing the risk of specific diseases: osteoporosis; principle ingredient: calcium, (11) for preventing neural tube defects (spondyloschisis): folic acid.
FNFC can be used to supplement daily nutritional intake. Because these foods contain known concentrations of nutrients with scientifically substantiated functions, claims can be applied to products according to standards without submitting a notification to the government. FNFC labeling indicates specific nutrient activities; thus, consumers can supplement their diets in accordance with the criteria specified by the CAA. Accordingly, FNFC refers to all foods that are labeled with nutritional claims specified by the CAA, which include nutrient and calorie contents, recommended daily consumption, consumption methods, notes for consumption, recommendations for a well-balanced diet, and language indicating that the food has not been subjected to case-by-case review by the CAA. Standards and specifications for nutritional labeling are established for 13 vitamins (niacin, pantothenic acid, biotin, vitamins A, B1, B2, B6, B12, C, D, E, and K, and folic acid), n-3 polyunsaturated fatty acids and six minerals (zinc, calcium, iron, copper, magnesium, and potassium) [Citation11]. These foods may be freely manufactured and distributed without permission from, or notification to, the national government, provided they meet established standards and specifications. In regards to labeling, nutritional ingredient quantity must be within a specified range (and meet the recommended daily allowance) and nutritional claims must be displayed with warning indications against excess intake or use in infants and young children.
Most of the world’s functional claim labeling systems can be classified as either the individual approval type (such as FOSHU), or the standards and specifications type (such as FNFC). The existing notification systems are limited to the American dietary supplement system and the Japanese functional labeling system. In this review, I focus on a new functional labeling system for “Foods with Function Claims (FFC)”, the development of functional agricultural products utilizing the labeling system, research of functional agricultural products by National Agriculture and Food Research Organization (NARO), and technical problems encountered in the development of agricultural products.
Regulation of health claims and new foods using the health claim labeling system in Japan
In June 2013, Japanese Prime Minister Abe published new economic policies and growth strategies, commonly referred to as “Abenomics”. These policies precipitated review of the labeling system for health foods from the viewpoint of convenience for consumers and manufacturers. As described above, before this system was in place, nutritional claims on food labels were only allowed for government-approved FOSHUs and FNFCs that complied with government-designated specifications and standards. Although these systems remain in place, an additional category for new types of FHCs, known as “foods with function claims” (FFC) [Citation12,13], was enforced in April 2015 (Figure ) to make more products available with clearly labeled nutritional or health benefits, and to enable consumers to make more informed choices. Notably, these food products can be labeled with the food function and its specified health effects. To ensure safety, food products are labeled with nutritional claims based on scientific Figure evidence, and labeling is the responsibility of the food business operator (including food importers, manufacturers, producers, and retailers).
The CAA launched a new food labeling system for FFC in April 2015 [Citation12,13]. Under this system, companies and agricultural producers can independently evaluate and describe scientific evidence of health food benefits and functional properties to promote informed consumption. This labeling system differs from the FOSHU and FNFC criteria, and FFC are presented to the Secretary-General of the CAA as products labeled with health claims based on scientific evidence, as assessed by food business operators. However, it is advisable to carefully check product label warnings and the information disclosed on the website of the CAA before purchasing and consuming FFC. As of 23 August 2017, 1034 FFC had been registered (Table and ). Companies and agricultural producers have high expectations of this labeling system and anticipate continued development of foods with labeled nutritional and health-related claims.
Table 1a. Foods with function claims (1034 items).
Table 1b. Foods with function claims (1034 items).
Characteristics of the FFC system are as follows: (1) FFCs are aimed at people who are not suffering from disease (excluding minors, women who are pregnant or planning to become pregnant, and lactating women), (2) All food products including fresh produce are subject to oversight, (3) Prior to market entry, food business operators are required to submit food safety and effectiveness information and demonstrate to the Secretary-General of the CAA that a system is in place to collect information concerning adverse health effects, (4) Unlike FOSHU, the government does not evaluate the safety or effectiveness of the submitted product, (5) The submitted information is disclosed on the website of the CAA.
Requirements of this labeling system are as follows: (1) The distributor is responsible for providing scientific evidence of food safety (prior notification system), (2) The functional ingredient has been identified using a validated method, (3) The mechanisms of action have been characterized using in vitro and in vivo testing, and in human clinical trials, (4) Scientific evidence has been acquired from human clinical trials or research reviews of functional ingredients, (5) Nutritional components contribute to maintenance and/or improvement of health, (6) Sufficient history of consumption and safety in Japan, (7) Notice is given to the CAA within 60 days of sale and is displayed on the packaging, (8) The recommended daily intake for health benefits is an appropriate volume to eat.
In December 2016, the CAA announced the results of committee investigation on nutritional information. The committee suggested that carbohydrates and saccharides, which were removed from the original functional food labeling guidelines, be recognized as functional ingredients. However, carbohydrates and saccharides, such as glucose, fructose, galactose, sucrose, lactose, maltose, starch, and glycogen, are not considered to be sources of nutrients. Moreover, the committee suggested that plant-derived “extracts and secretions” (abbreviated as “extract”) should be recognized as functional ingredients, and that “extract” should be included as a specific functional ingredient, which would be clearly shown as part of the functionality.
The “extract” labeled as functional must be equivalent to the “extract” for which scientific evidence has been provided. In addition, the CAA required that all methods for qualitative and quantitative analyses be disclosed on the CAA website.
Agricultural products registered in the FFC
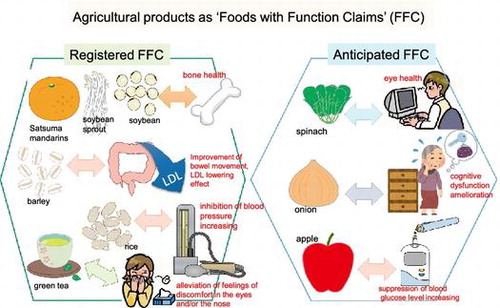
As of August 4, 2017, the following agricultural products had been registered in the FFC: Satsuma mandarin “Unshu” [Citation14] (notification numbers A79, B189, B467, B604), soybean sprouts [Citation15,16] (A80, A206, B201, B519), green tea cultivar “Benifuuki” (A67, B45, B46, B47, B121, B145), barley [Citation17,18] (A49, A100, A239, A302, B22, B483), rice [Citation19] (A114, B223), steamed soybean [Citation20,21] (A123, A283, A304, B66, B67), tomato juice [Citation22] (A106), and Kazunoko (herring roe) [Citation23,24] (C2) [Citation25].
Registered health claims of agricultural products for FFC, such as soybean sprouts and steamed soybean, barley, rice, tomato juice, and Kazunoko (herring roe), are “This food contains isoflavone, which reportedly maintains bone health”, “This food contains β-glucan, which reportedly decreases serum LDL cholesterol levels and improves bowel movement”, “This food contains γ (gamma)-aminobutyric acid (GABA), which reportedly maintains blood pressure at normal levels in people with relative hypertension”, “This food contains lycopene, which reportedly increases HDL cholesterol levels”, and “This food contains DHA/EPA, which reportedly decreases serum triglyceride levels”, respectively. Agricultural products for which FFC classification is anticipated, following clinical studies of end products or systematic reviews of functional ingredients [Citation46], are listed in Table . Some business operators are preparing to send notifications for lutein-rich spinach, and polyphenol-rich apple to CAA by utilizing the systematic reviews.
Table 2. Agricultural products with anticipated FFC status.
Study of agricultural products as FFC by NARO
Satsuma mandarin oranges were found to have high levels of β-cryptoxanthin, potentially reducing the risk of osteoporosis in postmenopausal women [Citation26], non-alcoholic steatohepatitis [Citation27], type-2 diabetes [Citation28], arteriosclerosis [Citation29], and metabolic syndrome [Citation30].
In addition, NARO developed the Japanese tea cultivar Benifuuki, which is rich in O-methylated catechin [Citation31–37], a compound that is present at low levels in common green tea and has anti-allergic effects [Citation38–42]. O-methylated catechins relieve allergies by inhibiting the release of histamine from mast cells and basophils [Citation31,33,37]. Specifically, O-methylated catechins inhibit mast cell activation by preventing tyrosine phosphorylation (Lyn, Syk, and Btk) in cellular proteins [Citation43], leading to decreased FcεRI expression [Citation44] and phosphorylation of myosin light chains [Citation45].
These data suggest that mast cell degranulation, histamine and leukotriene release, and interleukin secretion after FcεRI cross-linking are inhibited by O-methylated catechins. In a randomized placebo controlled clinical trial, symptoms such as rhinitis and itchy eyes were reduced in subjects with symptoms of cedar pollinosis who drank Benifuuki containing 1.5–2.5% O-methylated catechin (dry weight) [Citation40,41].
This was equivalent to a daily total O-methylated catechin intake of >34 mg [Citation38]. Furthermore, subjects who began ingesting Benifuuki 1.5 months before pollen dispersal showed reduced frequencies of nose-blowing, tearing, and sore throat compared with those who started drinking Benifuuki after pollen dispersal [Citation42]. As previously mentioned, the efficacy and safety of Benifuuki green tea in subjects with mild perennial allergic rhinitis were evaluated in a double-blind, randomized, parallel-group study.
In this study, seventy-five subjects with mild perennial allergic rhinitis that met predetermined criteria were assigned to either the Benifuuki (containing 34 mg of EGCG3′′Me) or Yabukita (not containing EGCG3′′Me) green tea consumption group. Subjects consumed 700 mL of tea and recorded nasal and ocular symptoms every day for 12 weeks, and visited the hospital every six weeks for consultation and blood collection. In subsequent data analyses, the scores for nasal and ocular symptoms in the Benifuuki group were lower than those in the Yabukita group, and significant differences in nasal and ocular scores were observed at weeks 7–12 and 4–12, respectively [Citation38].
These data indicate that Benifuuki green tea significantly inhibits symptoms of allergic rhinitis. Moreover, no adverse effects were observed in physiological, hematological, or biochemical parameters, with normal immune responses of peripheral blood leukocytes and no subjective symptoms throughout the experiment. In an additional study of nine healthy subjects without any allergic symptoms, subjects were given 700 mL of Benifuuki green tea daily for 12 weeks and no adverse effects were noted throughout the study [Citation39].
In light of above results, NARO initiated research collaborations with private companies to develop Satsuma mandarin oranges and Benifuuki green tea as functional agricultural products, and “Mikkabi mikan” (notification number: A79), “Ashitanokarada” (A105), “Benifuuki green teabag” (A67), and “Memehanacha” (A69) were released as FFCs in 2015 (1). Registered health claims of Satsuma mandarin Unshu and “Benifuuki” green tea are “This food contains β-cryptoxanthin, which reportedly maintains bone health” and “This food contains O-methylated catechin. O-methylated catechin is reported to alleviate the eye and nose discomfort caused by exposure to house dust and cedar pollen”, respectively. “Memehanacha” is a green tea that contains about 34 mg of O-methylated catechin in two bottles (700 mL). It is classified as an FFC because it contains O-methylated catechin and alleviates the eye and nose discomfort caused by exposure to house dust and cedar pollen. “Ashitanokarada” is a fruit drink made from Japanese mandarin oranges that facilitates efficient processing of by-products. As a result of technological advances in recovering large amounts of β-cryptoxanthin from waste (3 mg), essential for the maintenance of bone metabolism, each pack contains the caloric equivalent of one piece of fruit and the β-cryptoxanthin content of three pieces of fruit.
Study of health-promoting effects of agricultural products by NARO
The Ministry of Agriculture, Forestry and Fisheries in Japan has been investigating functional foods since 1989 and continues to fund functional food research. NARO assesses agricultural products that contribute to health maintenance and improvement of the elderly. To clarify the effects of functional agricultural products on human health, NARO implemented the “Research Project on Development of Agricultural Products and Foods with Health-Promoting Benefits” (study duration, 2012–2015; total budget, 2 billion yen; number of projects, 18 studies). NARO developed agricultural products expected to improve health, assessed in human clinical trials, and established production and distribution technologies in collaboration with a prefectural agricultural experimental station, a university, and private companies. In addition, NARO aimed to build a database of functional agricultural products and create a functional food delivery system that address personal health conditions, establish a nutrition care station, and produce a functional boxed lunch (O-Bento) that contributes to the maintenance and improvement of personal health. As the results of this project, NARO contributed assessments of the health benefits of β-glucan rich barley [Citation47], β-conglycinin rich soybean [Citation48], rutin rich tartary buckwheat [Citation49], quercetin rich onion [Citation50], β-cryptoxanthin rich satsuma mandarins [Citation27–30], procyanidin rich apple [Citation51], green tea rich in O-methylated catechin (epigallocatechin-3-O-(3-O-methyl) gallate (EGCG3′′Me)) [Citation52], various other catechins [Citation53], resistant starch rich rice [Citation54], and zinc-rich oyster [Citation55]. The most recent scientific findings obtained through this project are outlined below.
The intake of high-β-glucan barley (cultivar, Kirarimochi, 2 g of β-glucan per day intake) led to significant and safe reductions in visceral fat area (VFA), body weight, BMI, and waist circumference in obese subjects with VFA ≥100 cm2 compared with the placebo group (cultivar, bgl, 0 g per day intake). Barley high in β-glucan may help prevent visceral fat obesity [Citation47].
Serum triglyceride (TG) levels were significantly decreased in the enriched-β-conglycinin soybean (cultivar, Nanahomare) intake subject group (5.5 g of β-conglycinin per day) compared with the placebo group (low-β-conglycinin soybean) (cultivar, Nagomimaru) group (0.5 g of β-conglycinin per day) at weeks 4 and 12. In addition, in subjects with baseline TG levels ≥100 mg/dL, the levels significantly improved in the enriched-β-conglycinin soybean group at weeks 4 and 12. These results suggest that the ingestion of enriched-β-conglycinin soybean improves serum TG levels [Citation48].
Thiobarbituric acid reactive substances (TBARS) levels, body weight, and BMI in the rutin-rich Tartary buckwheat (cultivar, Mantenkirari, 320 mg of rutin per day) intake group were significantly lower than those in the placebo group at week 8. Body fat percentage in the Mantenkirari group at week 4 was significantly lower than that in the placebo group. Thus, it was suggested that rutin-rich Tartary buckwheat intake might be effective for body weight reduction due to its antioxidant properties [Citation49].
The 24-week consecutive administration of onion (cultivar, Quergold) quercetin aglycone (60 mg per day) did not significantly improve the Mini-Mental State Examination (MMSE) and cognitive impairment rating scale scores compared to the placebo group. However, in younger subjects (under 72 years old), the MMSE scores were significantly higher in the Quergold intake group than in the placebo food group at week 24 (p = 0.019) [Citation50].
The 12-week consecutive administration of apple polyphenol (AP; 600 mg per day) significantly reduced the increase in glucose at 30-min post-75 g OGTT (OGTT30-min glucose) value, compared to the placebo regimen in high-normal blood glucose and borderline human subjects. It was suggested that chronic AP administration significantly improved impaired glucose tolerance [Citation51].
Serum LDL cholesterol levels in subjects who consumed O-methylated catechin (EGCG3′′Me) rich green tea (cultivar, Benifuuki) were significantly lower than those who received barley infusion (not containing catechin) without green tea. Furthermore, the lectin-like oxidized low-density lipoprotein receptor-1 containing apolipoprotein B (LAB) levels in “Benifuuki” drinkers were significantly lower than those in the barley infusion group and the “Benifuuki” baseline LAB level. In subjects whose green tea intake was irregular, “Benifuuki” significantly reduced serum LDL cholesterol and LAB levels compared to those following barley infusion consumption [Citation52].
A cohort study was conducted to longitudinally investigate whether serum carotenoids at baseline are associated with the risk of developing elevated serum alanine aminotransferase (ALT) among Japanese subjects. A cohort of 213 males and 574 females free of elevated serum ALT (>30 IU/ml) at baseline was studied. Over a mean follow-up period of 7.4 (SD 3.1) years, thirty-one males and forty-nine females developed new elevated serum ALT. After adjustments for confounders, the hazard ratios for elevated serum ALT in the highest tertiles of basal serum β-carotene, β-cryptoxanthin and total provitamin A carotenoids against the lowest tertiles were 0.43 (95% CI 0.22, 0.81), 0.51 (CI 0.27, 0.94), and 0.52 (CI 0.28, 0.97), respectively. These results support the hypothesis that antioxidant carotenoids, especially provitamin A carotenoids, might help prevent earlier pathogenesis of non-alcoholic liver disease in Japanese subjects [Citation27].
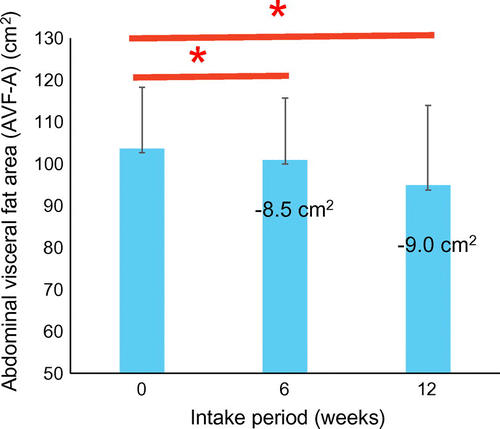
NARO also investigated the abdominal visceral fat (AVF)-lowering effects of a functional boxed lunch (O-bento), with the aim of developing foods with combinations of functional agricultural products that prevent metabolic syndrome. Subsequently, we performed a randomized placebo-controlled trial of 159 healthy adults with BMIs of 25–30 and over 100 cm2 AVF areas (AVF-A). Test foods were 50% barley rice and brown rice, Benifuuki green tea, and side dishes of functional agricultural products such as lycopene-rich tomato and quercetin-rich onion. Placebo controls consumed foods that did not contain functional agricultural products.
Three test groups were administered only one test food each, and one test group was given all three test foods (rice, tea, and side dishes). All subjects consumed the study foods at lunchtime on all weekdays for 12 weeks, and the primary endpoint was AVF-A. Secondary endpoints included HbA1c and 1,5-anhydroglucitol (AG) levels. In this study, the intervention reduced AVF-A significantly (Figure ) and subgroup analyses of AVF-A among subjects with a median baseline AVF-A of less than 127 cm2 showed a mean reduction of 7.9 cm2. Moreover, among women in the rice group, AVF-A was significantly decreased by 14.9 cm2 (p = 0.012). Similarly, Benifuuki tea consumption led to significantly lower serum levels of 1,5-AG and no side effects were observed, suggesting that the changes in eating habits during daily intake of a functional boxed lunch can reduce AVF. This study showed that continuous intake of a functional box lunch might decrease visceral fat area [Citation56].
Technical problems in developing functional agricultural products
As with fresh products such as vegetables and fruit, functional ingredient contents vary greatly between cultivars, production centers (farms), cultivation times, and cultivation methods, and may deteriorate during the retail period. Accordingly, the Ministry of Agriculture, Forestry and Fisheries summarized the corresponding countermeasure as “Technical Measures for Functional Labeling” and released it online 24 August 2015 [Citation57]. This document describes the data collection methods before FFC notification; methods for determining recommended intake values of functional ingredients before notification; monitoring of functional ingredient contents after notification; and labeling nutritional claims on fresh and processed foods such as green tea, barley, rice, dried mushrooms, and freshly squeezed juice. These claims assert that the products only contain raw materials from agriculture, forestry, and fishery. Accordingly, companies and agricultural producers may add package statements such as
We manage cultivation and shipment closely so that functional ingredient contents are present within a defined range. However, actual functional ingredient contents may be lower than the lower labeled limit value due to the cultivation conditions of raw fresh food.
In clinical trials of fresh food effects, the selection and production of placebo controls is difficult, as the placebo food needs to have a similar appearance to the functional food, yet be completely free of the functional ingredient. Thus, future protocols for clinical food trials require careful adaptation.
Conclusion
Consumers can acquire more accurate information and make product choices with increased precision. Consequently, consumers must develop the ability to choose functional foods for themselves amidst a deluge of information available on the CAA website and elsewhere. Enhancing consumer nutritional literacy will likely facilitate the beneficial use of agricultural products as FFCs.
In the context of functional foods as components of balanced meals, it is desirable to avoid being reliant on a limited number of agricultural products. Therefore, continued increases in functional ingredients and new function claims for FNFC will ensure that consumers can acquire specific functional ingredients from a variety of agricultural products. An ongoing focus on diversifying product choice is expected. Moreover, increased availability of fresh food items containing raw materials from a variety of sources is needed to promote public health.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by a Research Project on Development of Agricultural Products and Foods with Health-promoting Benefits [grant number NARO-A11-C3], Japan.
Acknowledgments
We would like to express our sincere appreciation to Dr. T. Hirosawa and Dr. S. Kawamoto.
References
- Arai H, Ouchi Y, Toba K, et al. Japan as the front-runner of super-aged societies: perspectives from medicine and medical care in Japan. Geriatr Gerontol Int. 2015;15:673–687.10.1111/ggi.2015.15.issue-6
- Overview of the system and the basic statics [Internet]. Tokyo: Ministry of Health, Labour and Welfare; [cited 2017 Aug 25]. Available from: http://www.mhlw.go.jp/english/wp/wp-hw9/dl/01e.pdf
- Nakanishi N, Nishina K, Okamoto M, et al. Clustering of components of the metabolic syndrome and risk for development of type 2 diabetes in Japanese male office workers. Diabetes Res Clin Pract. 2004;63:185–194.10.1016/j.diabres.2003.08.007
- Yamamoto A, Temba H, Horibe H, et al; Research group on serum lipid survey 1990 in Japan. Life style and cardiovascular risk factors in the Japanese population–from an epidemiological survey on serum lipid levels in Japan. Part 2: association of lipid parameters with hypertension. J Atheroscler Thromb. 1990;2003(10):176–185.
- Kanauchi M, Kanauchi K, Hashimoto T, et al. Metabolic syndrome and new category ‘pre-hypertension’ in a Japanese population. Curr Med Res Opin. 2004;20:1365–1370.10.1185/030079904X2042
- Trends in national medical care expenditure [Internet]. Tokyo: Ministry of Health, Labour and Welfare; [cited 2017 Aug 25]. Available from: http://www.mhlw.go.jp/file/04-Houdouhappyou-12401000-Hokenkyoku-Soumuka/0000136497.pdf. Japanese.
- Chiba E, editor. Shokuhin no Seitaichosetsukinou [Physiological function of foods]. Tokyo: Gakkai Shuppan Center; 1992. Japanese.
- Asai S, editor. Kinouseishokuhin no Kenkyu [The studies of food function]. Tokyo: Gakkai Shuppan Center; 1995. Japanese.
- Fujimaki M, editor. Shokuhin no Kinousei [Functionality of foods]. Tokyo: Gakkai Shuppan Center; 1998. Japanese.
- Regulatory systems of health claims in Japan [Internet]. Tokyo: Consumer Affairs Agency; [cited 2017 Aug 25]. Available from: http://www.caa.go.jp/en/pdf/syokuhin338.pdf
- Foods with nutrient function claims [Internet]. Tokyo: Consumer Affairs Agency; [updated 2016 Oct 4; cited 2017 Aug 25]. Available from: http://www.caa.go.jp/foods/pdf/foods_161004_0004.pdf. Japanese.
- The system of “Foods with Function Claims” has been launched [Internet]. Tokyo: Consumer Affairs Agency; [updated 2015 Dec; cited 2017 Aug 25]. Available from: http://www.caa.go.jp/foods/pdf/151224_2.pdf
- What are “Foods with Function Claims” [Internet]. Tokyo: Consumer Affairs Agency; [updated 2015 Dec; cited 2017 Aug 25]. Available from: http://www.caa.go.jp/foods/pdf/151224_2.pdf
- Yamaguchi M, Igarashi A, Uchiyama S, et al. Effect of β-Crytoxanthin on circulating bone metabolic markers: intake of juice (Citrus Unshiu) supplemented with β-Cryptoxanthin has an effect in menopausal women. J Health Sci. 2006;52:758–768.10.1248/jhs.52.758
- Taku K, Melby MK, Takebayashi J, et al. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: meta-analysis of randomized controlled trials. Asis Pac J Clin Nutr. 2010;19:33–42.
- Ma FD, Qin LQ, Wang PY, et al. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Eur J. Clin Nutr. 2008;62:155–161.10.1038/sj.ejcn.1602748
- Li J, Kaneko T, Qin LQ, et al. Effects of barley intake on glucose tolerance, lipid metabolism, and bowel function in women. Nutrition. 2003;19:926–929.10.1016/S0899-9007(03)00182-5
- Behall KM, Scholfield DJ, Hallfrisch J. Lipids significantly reduced by diets containing barley in moderately hypercholesterolemic men. J Am Coll Nutr. 2004;23:55–62.10.1080/07315724.2004.10719343
- Nishimura M, Yoshida S, Haramoto M, et al. Effects of white rice containing enriched gamma-aminobutyric acid on blood pressure. J Tradit Complement Med. 2015;6:66–71.
- Uesugi S, Watanabe S, Ishiwata N, et al. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. Biofactors. 2004;22:221–228.10.1002/biof.v22:1/4
- Yamori Y, Moriguchi EH, Teramoto T, et al. Soybean isoflavones reduce postmenopausal bone resorption in female Japanese immigrants in Brazil: a ten-week study. J Am Coll Nutr. 2002;21:560–563.10.1080/07315724.2002.10719255
- Blum A, Merei M, Karem A, et al. Effect of tomatoes on the lipid profile. Clin Invest Med. 2006;29:298–300.
- Kobayashi K, Hamazaki K, Fujioka S, et al. The effect of n-3 PUFA/γ-cyclodextrin complex on serum lipids in healthy volunteers: a randomized, placebo-controlled, double-blind trial. Asia Pac. J Clin. Nutr. 2007;16:429–434.
- Matsumoto M, Matsunaga H, Nishimura T, et al. Effects of purified fish oil-containing dietary supplement on serum triglycerides, blood pressure, and cognitive function in healthy Japanese middle-agers: randomized, double-blind, placebo-controlled parallel-group trial. [ Yakuri to Chiryo] Jpn Pharmacol Ther. 2016;44:235–246. Japanese.
- Retrieval of ‘Foods with Function Claims’ [Internet]. Tokyo: Consumer Affairs Agency; [updated 2017 Jul 28; cited 2017 Aug 25]. Available from: https://www.fld.caa.go.jp/caaks/cssc01/. Japanese.
- Sugiura M, Nakamura M, Ogawa K, et al. High serum carotenoids associated with lower risk for bone loss and osteoporosis in post-menopausal Japanese female subjects: prospective cohort study. PLoS ONE. 2012;7:e52643.10.1371/journal.pone.0052643
- Sugiura M, Nakamura M, Ogawa K, et al. High serum carotenoids are associated with lower risk for developing elevated serum alanine aminotransferase among Japanese subjects: the Mikkabi cohort study. Br J Nutr. 2016;115:1462–1469.10.1017/S0007114516000374
- Sugiura M, Nakamura M, Ogawa K, et al. High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res Care. 2015;3:e000147.10.1136/bmjdrc-2015-000147
- Nakamura M, Sugiura M, Ogawa K, et al. Serum β-cryptoxanthin and β-carotene derived from Satsuma mandarin and brachial-ankle pulse wave velocity: the Mikkabi cohort study. Nutr Metab Cardiovasc Dis. 2016;26:808–814.10.1016/j.numecd.2016.04.001
- Sugiura M, Nakamura M, Ogawa K, et al. High serum carotenoids associated with lower risk for the metabolic syndrome and its components among Japanese subjects: Mikkabi cohort study. Br J Nutr. 2015;114:1674–1682.10.1017/S0007114515003268
- Maeda-Yamamoto M, Kawahara H, Matsuda N, et al. Effects of tea infusions of various varieties or different manufacturing types on inhibition of mouse mast cell activation. Biosci. Biotech. Biochem. 1998;62:2277–2279.10.1271/bbb.62.2277
- Sano M, Suzuki M, Miyase T, et al. Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 1999;47:1906–1910.10.1021/jf981114 l
- Tachibana H, Sunada Y, Miyase T, et al. Identification of a methylated tea catechin as an inhibitor of degranulation in human basophilic KU812 cells. Biosci. Biotech. Biochem. 2000;64:452–454.10.1271/bbb.64.452
- Suzuki M, Yoshino K, Maeda-Yamamoto M, et al. Inhibitory effects of tea catechins and O-methylated derivatives of (-)-epigallocatechin-3-O-gallate on mouse type-IV allergy. J Agric Food Chem. 2000;48:5649–5653.10.1021/jf000313d
- Suzuki M, Sano M, Yoshida R, et al. Epimerization of tea catechins and O-methylated derivatives of (-)-epigallocatechin-3-O-gallate: relationship between epimerization and chemical structure and epimerization of tea epicatechin during the extraction with hot water. J Agric Food Chem. 2003;51:510–514.10.1021/jf0210627
- Maeda-Yamamoto M, Nagai H, Asai K, et al. Changes in epigallocatehin-3-O-(3-O-methyl) gallate and strictinin contents of tea (Camellia sinensis L.) cultivar ‘Benifuuki’ in various degree of maturity and leaf order. Food Sci Technol Res. 2004;10:186–190.10.3136/fstr.10.186
- Maeda-Yamamoto M, Ema K, Tokuda Y, et al. Epicatechin-3-O-(3-O-methyl) gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release. J Agric Food Chem. 2012;60:2165–2170.10.1021/jf204497b
- Yasue M, Nagai H, Maeda-Yamamoto M, et al. The clinical effects and the safety of the intakes of ‘Benifuuki’ green tea in patients with perennial allergic rhinitis. J Jpn Soc Clin Nutr. 2005;27:33–51. Japanese.
- Yasue M, Ohtake Y, Nagai H, et al. The efficacy and safety of ‘Benifuuki’ green tea containing O-methylated catechin: clinical study in patients with mild perennial allergic rhinitis. Nippon Shokuhin Shinsozaikenkyukaishi. 2005;8:65–80. Japanese.
- Maeda-Yamamoto M, Ema K, Shibuichi I. In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology. 2007;55:135–142.10.1007/s10616-007-9112-1
- Masuda S, Maeda-Yamamoto M, Usui S, et al. ‘Benifuuki’ green tea containing O-methylated catechin reduces symptoms of Japanese cedar pollinosis: a randomized, double-blind, placebo-controlled trial. Allergology Int. 2014;63:211–217.10.2332/allergolint.13-OA-0620
- Maeda-Yamamoto M, Ema K, Monobe M, et al. The efficacy of early treatment of seasonal allergic rhinitis with Benifuuki green tea containing O-methylated catechin before pollen exposure: an open randomized study. Allergology Int. 2009;58(3):437–444.10.2332/allergolint.08-OA-0066
- Maeda-Yamamoto M, Inagaki N, Kitaura J, et al. O-methylated catechins from tea leaves, inhibit multiple protein kinases in mast cells. J. Immunol. 2004;172:4486–4492.10.4049/jimmunol.172.7.4486
- Fujimura Y, Umeda D, Yano S, et al. The 67 kDa laminin receptor as a primary determinant of anti-allergic effects of O-methylated EGCG. Biochem Biophys Res Commun. 2007;364:79–85.10.1016/j.bbrc.2007.09.095
- Fujimura Y, Tachibana H, Maeda-Yamamoto M, et al. Antiallergic tea catechin: (-)-Epigallocatechin-3-O-(3-O-methyl)-gallate, suppresses fcepsilonRI expression in human basophilic KU812 cells. J Agric Food Chem. 2002;50:5729–5734.10.1021/jf025680z
- Research reviews of nine agricultural products [Internet]. Tsukuba: NARO; [cited 2017 Aug 25]. Available from: http://www.naro.affrc.go.jp/project/f_foodpro/2016/063236.html. Japanese.
- Aoe S, Ichinose Y, Kohyama N, et al. Effects of high β-glucan barley on visceral fat obesity in Japanese individuals: a randomized, double-blind study. Nutrition. 2017;42:1–6. 10.1016/j.nut.2017.05.002
- Nishimura M, Ohkawara T, Sato Y, et al. Improvement of triglyceride levels through the intake of enriched-β-conglycinin soybean (Nanahomare) revealed in a randomized, double-blind, placebo-controlled study. Nutrients. 2016;8:491–498.10.3390/nu8080491
- Nishimura M, Ohkawara T, Sato Y, et al. Effectiveness of rutin-rich Tartary buckwheat ( Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in body weight reduction related to its antioxidant properties: a randomised, double-blind, placebo-controlled study. J Funct Foods. 2016;26:460–469.10.1016/j.jff.2016.08.004
- Nishimura M, Ohkawara T, Nakagawa T, et al. A randomized, double-blind, placebo-controlled study evaluating the effects of quercetin-rich onions on cognitive function in elderly subjects. Funct Foods Health Dis. 2017;7:353–374.
- Shoji T, Yamada M, Miura T, et al. Chronic administration of apple polyphenols ameliorates hyperglycaemia in high-normal and borderline subjects: a randomised, placebo-controlled trial. Diabetes Res Clin Pract. 2017;129:43–51.10.1016/j.diabres.2017.03.028
- Imbe H, Sano H, Miyawaki M, et al. “Benifuuki” green tea, containing O-methylated EGCG, reduces serum low-density lipoprotein cholesterol and lectin-like oxidized low-density lipoprotein receptor-1 ligands containing apolipoprotein B: a double-blind, placebo-controlled randomized trial. J Funct Foods. 2016;25:25–37.10.1016/j.jff.2016.05.004
- Momose Y, Maeda-Yamamoto M, Nabetani H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int J Food Sci Nutr. 2016;20:1–8.
- Noro W, Akaishi R, Nakamura S, et al. Prevention of abrupt increases in postprandial blood glucose levels by rice bread made with the novel rice cultivar “Konayukinomai”. Food Sci Technol Res. 2016;22:793–799.10.3136/fstr.22.793
- Saito H, Cherasse Y, Suzuki R, et al. Zinc-rich oysters as well as zinc yeast- and astaxanthin-enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Mol Nutr Food Res. 2017;65. DOI:10.1002/mnfr.201600882
- Maeda-Yamamoto M, Hirosawa T, Mihara Y, et al. Randomized, placebo-controlled, clinical study to investigate anti-metabolic syndrome effects of functional foods in humans. Nippon Shokuhin Kagaku Kogaku Kaishi. 2017;64:23–33. Japanese.10.3136/nskkk.64.23
- Technical Measures for Functional Labeling [Internet]. Tsukuba: NARO; [updated 2015 Aug 24; cited 2017 Aug 25]. Available from: http://www.s.affrc.go.jp/docs/kinousei_pro/reference.htm. Japanese.
- Watanabe J, Ayugase J. Effect of low temperature on flavonoids, oxygen radical absorbance capacity values and major components of winter sweet spinach (Spinacia oleracea L.). J Sci Food Agric. 2015;95:2095–2104.10.1002/jsfa.2015.95.issue-10
- Schalch W, Cohn W, Barker FM, et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin – the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007;458:128–135.10.1016/j.abb.2006.09.032