Abstract
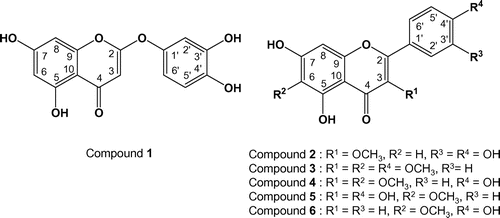
A novel 2-phenoxychromone (1) and five known flavones (2-6) were isolated from northeastern Brazilian propolis in the state of Bahia. The chemical structures of these six compounds were determined by spectroscopic investigations and single-crystal X-ray analysis. The isolated compounds showed growth-inhibitory activities, in varying degrees, against human tumor cell lines. This is the first report on the discovery of a novel 2-phenoxychromone from propolis.
A novel 2-phenoxychromone (1) and five known flavones (2–6) were isolated from Brazilian propolis in the state of Bahia.
Propolis is a solid substance that consists of resin, wax and several active ingredients. This substance is made by honeybees (Apis melifera) from several plant exudates. The main role of propolis is not only to protect beehives from invaders, rain and wind but also to maintain hygiene. Propolis has been used in traditional folk medicine. There are many reports on the biological activities of propolis, including its anti-bacterial [Citation1–3], anti-viral [Citation2–4], anti-inflammatory [Citation5] and anti-cancer properties [Citation6–12]. However, the chemical composition of propolis differs depending on the habitat of the bees and plant sources.
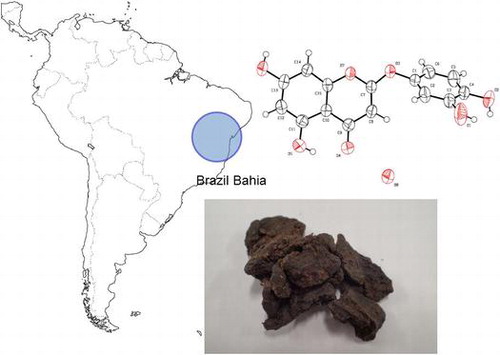
In the course of investigating the constituents of several varieties of propolis, we have discovered a novel diterpenoid unique to brown Brazilian propolis from Parana which has potent anti-proliferative activity [Citation13] in several cancer cell lines. In this study, we investigated northeastern Brazilian propolis from the Atlantic forest of Bahia, commercially called “Special propolis” (Nordeste Co., Ltd., Tokyo). Brazilian propolis has been grouped into 12 chemical types according to their botanical origin [Citation14], and this Brazilian propolis from Bahia state (BA propolis) is thought to be categorized as type 7. However, there are limited reports on the chemical components found in BA propolis and their bioactivities. In preliminary studies, we found that the ethanol extract of BA propolis has an anti-proliferative effect on cancer cells as well as anti-bacterial activity and is slightly more potent than ethanol extracts of Brazilian green propolis, which is a popular propolis in many countries. We also found that BA propolis does not include artepilin C, which is a major compound found in Brazilian green propolis [Citation15,16]. In this study, we describe the identification of the key compounds isolated from ethanol extracts of BA propolis.
Materials and methods
Extraction and isolation
Crude of BA propolis were distributed in the Atlantic forest region of Bahia state, northeastern Brazil and is collected by Nordeste Co., Ltd. BA propolis was extracted with 95% ethanol at room temperature for a day. This extraction process was repeated twice (three times in total). Each filtrate were concentrated under reduced pressure and combined to give a crude residue (1.92 g), which was chromatographed in a silica gel column, using a stepwise gradient of
CHCl3-Methanol = 100:0–1:1 to yield ten fractions (Fr. 1-10) according to their TLC profiles. Fr. 10 (140.79 mg) was purified by preparative HPLC using by liner gradient mixture of Acetonitrile-H2O = 40:60–100:0 (60 min.), to yield compound 1 (19.8 mg) and 2 (16.1 mg). Fr. 6 (277.25 mg) was fractionated by preparative HPLC using liner gradient mixture of Acetonitrile-H2O = 40:60–100:0 (60 min.) to give compound 3 (6.7 mg). Fr. 7 (423.16 mg) was separated by preparative HPLC elution with liner gradient mixture of Acetonitrile-H2O = 40:60–100:0 (60 min.) to give four fractions (Fr. 7a-7d). Further purification of Fr. 7b (63.3 mg) was accomplished by preparative HPLC using liner gradient mixture of Acetonitrile-H2O = 30:70–60:40 (60 min.) to yield five fractions (Fr. 7b1-7b5). Among these fractions, Fr. 7b3 and Fr. 7b5 were purified compound 4 (7.2 mg) and 5 (23.0 mg), respectively. Fr. 7b1 (10.8 mg) was achieved with preparative HPLC using liner gradient mixture of Acetonitrile-H2O = 30:70–60:40 (60 min.) to yield compound 6 (5.9 mg).
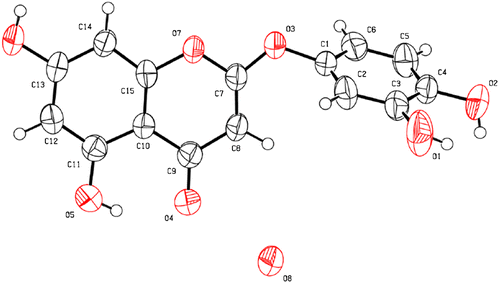
Compound 1 (5,7-dihydroxy-2-(3,4-dihydroxyphenoxy)-4H-chromen-4-one) was isolated a pale yellow crystal (m.p. 137 °C); IR (KBr) cm−1: 3321, 1658, 1613, 1503, 1427, 1357, 1219, 1142, UV λmax (MeOH) nm (log ε) : 231.5 (4.09), 289.0 (3.83), 1H-NMR (300 MHz, CD3OD): δH 6.19 (1H, d, J = 1.8 Hz; H-6), 6.30 (1H, d, J = 1.8 Hz; H-8), 6.58 (1H, dd, J = 8.4, 2.7 Hz; H-6′), 6.68 (1H, d, J = 2.7 Hz), 6.84 (1H, d, J = 8.4 Hz; H-5′), 5.14 (1H, s), 13C-NMR (75 MHz, CD3OD): δC 86.6 (C-3), 93.8 (C-8), 99.3 (C-6), 102.3 (C-10), 108.0 (C-2′), 111.3 (C-6′), 115.5 (C-5′), 144.0 (C-1′), 144.5 (C-4′), 146.6 (C-3′), 155.8 (C-9), 161.9 (C-5), 164.5 (C-7), 169.5 (C-2), 184.3 (C-4), and HR-ESI-MS: m/z 303.0509 [M+H]+ (calcd. for C15H11O7; 303.0505).
Compound 2 (3-O-methylquercetin) was obtained as a pale yellow powder; 1H-NMR (300 MHz, CD3OD): δH 3.78 (3H, s; 3-OCH3), 6.19 (1H, d, J = 1.8 Hz; H-6), 6.38 (1H, d, J = 1.8 Hz; H-8), 6.90 (1H, d, J = 8.4 Hz; H-5′), 7.52 (1H, dd, J = 8.4, 2.1 Hz; H-6′), 7.62 (1H, d, J = 2.1 Hz; H-2′) and 13C-NMR (75 MHz, CD3OD): δC 60.6 (3-OCH3), 93.5 (C-8), 98.6 (C-6), 104.7 (C-10), 115.2 (C-2′), 115.3 (C-3′), 121.1 (C-6′), 121.7 (C-1′), 138.4 (C-3), 146.6 (C-3′), 148.8 (C-4′), 156.8 (C-9), 157.2 (C-2), 161.9 (C-5), 164.7 (C-7), 178.8 (C-4).
Compound 3 (3,6,4′-trimethoxychrysin) was obtained as yellow powder; 1H-NMR (300 MHz, DMSO-d6): δH 3.75 (3H,s; 3-OCH3), 3.79 (3H, s; 6-OCH3), 3.86 (3H, s; 4′-OCH3), 6.57 (1H, s; H-8), 7.14 (1H, d, J = 9.0 Hz; H-3′, 5′), 8.03 (1H, d, J = 9.3 Hz; H-2′,6′), 12.74 (1H,s; 5-OH) and 13C-NMR (75 MHz, DMSO-d6): δC 55.4 (4′-OCH3), 59.7 (3-OCH3), 59.9 (6-OCH3), 94.0 (C-8), 104.6 (C-10), 114.2 (C-3′, 5′), 122.1 (C-1′), 129.9 (C-2′, 6′), 131.1 (C-6), 137.5 (C-3), 151.5 (C-9), 152.3 (C-5), 155.2 (C-2), 157.4 (C-7), 161.3 (C-4′), 178.2 (C-4).
Compound 4 (3,6-dimethoxyapigenin) was obtained as a yellow oil; 1H-NMR (300 MHz, Acetone-d6): δH 3.87 (3H,s; 3-OCH3), 3.87 (3H, s; 6-OCH3), 6.58 (1H, s; H-8), 7.01 (2H, d, J = 9.0 Hz; H-3′), 8.03 (2H, d, J = 9.0 Hz; H-2′) and 13C-NMR (75 MHz, Acetone-d6): δC 59.5 (3-OCH3), 60.0 (6-OCH3), 93.8 (C-8), 105.6 (C-10), 115.8 (C-3′, 5′), 122.0 (C-1′), 130.6 (C-2′, 6′), 131.2 (C-6), 138.1 (C-3), 152.4 (C-7), 153.0 (C-5), 156.3 (C-9), 157.1 (C-2), 160.3 (C-4′), 179.2 (C-4).
Compound 5 (6-methoxykaempferol) was obtained a pale yellow powder; 1H-NMR (300 MHz, CD3OD): δH 3.88 (3H, s; 6-OCH3), 6.49 (1H, s; H-8), 6.90 (2H, d, J = 9.0 Hz), 8.08 (2H, d, J = 9.0 Hz) and 13C-NMR (75 MHz, CD3OD): δC 59.8 (6-OCH3), 93.6 (C-8), 103.7 (C-10), 115.1 (C-3′, 5′), 122.5 (C-1′), 129.5 (C-2′, 6′), 131.0 (C-6), 135.7 (C-3), 147.1 (C-2), 151.8 (C-9), 152.5 (C-5), 157.5 (C-7), 159.4 (C-4′), 176.4 (C-4).
Compound 6 (6-methoxyapigenin) was obtained as a pale yellow powder; 1H-NMR (300 MHz, DMSO-d6): δH 3.75 (3H, s; 6-OCH3), 6.60 (1H, s, H-8), 6.78 (1H, s; H-3), 6.92 (2H, d, J = 8.2 Hz; H-3′), 7.92 (2H, d, J = 8.2 Hz), 13.08 (1H, s; 5-OH) and 13C-NMR (75 MHz, DMSO-d6): δC 60.0 (6-OCH3), 94.4 (C-8), 102.5 (C-3), 104.1 (C-10), 116.1 (C-3′, 5′), 121.3 (C-1′), 128.6 (C-2′, 6′), 131.5 (C-6), 152.5 (C-9), 152.9 (C-5), 157.5 (C-7), 161.3 (C-4′), 163.9 (C-2), 182.2 (C-4).
Cytotoxicity assays
In vitro cytotoxic activities of isolated compounds (1-6) were evaluated in DLD-1 (human colon cancer), MCF-7 (human breast cancer) and A549 (human lung cancer) cancer cell lines. The cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and were cultured at a density of 1 × 104 cells or 5 × 103 cells / 100 μL medium / well in 96-well microtiter plates at 37 °C in a 5% -CO2 incubator. After 24 h of culture, the cells were treated with various concentrations of the propolis extract (4.7–28 μg/mL) or the isolated compounds (6.25–200 μM) for 24 h. The test agents were dissolved in DMSO solution, and were tested in triplicate. Growth inhibition in the cancer cells was evaluated by quantifying living cells using the Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan). Briefly, after treatment with the test agents, the cells were incubated in medium containing 10% CCK-8 for 1 h at 37 °C in 5% CO2, and the absorbance of formazan, which is produced by living cells from the chromogenic substrate tetrazolium salt (WST-8), was spectrophotometrically measured at 450 nm. The IC50 values of the test agents were estimated by nonlinear regression analysis for growth inhibition of each cancer cell line using the GraphPad Prism program (Graphpad software Inc., La Jolla, CA).
Results and discussion
Compound 1 was obtained as pale yellow crystals (m.p. 137 °C), and its high-resolution (HR-) ESIMS exhibited a pseudo-molecular ion peak at m/z 303.0509 [M+H]+, corresponding to a molecular formula of C15H11O7 (Δ + 0.4 mmu) and indicating eleven degrees of unsaturation. The IR spectrum showed absorption bands for a hydroxyl group (3321 cm−1), an α, β-unsaturated carbonyl group (1658 cm−1) and an aromatic sp2 stretch (1613 cm−1) [Citation17]. The 1H-NMR spectrum of compound 1 displayed signals from an olefinic proton (H-3), three protons typical of an ABX system at H-2′, 5′ and 6′ and a m-coupled aromatic doublet at H-6 and 8. The 13C-NMR spectrum showed fifteen carbon resonances, including two olefinic carbons (C-2, 3), twelve aromatic carbons (A- and B-ring) and an α, β-unsaturated carbonyl group (C-4) which was shifted upfield. These NMR spectroscopy data were very similar to those of a typical flavone.
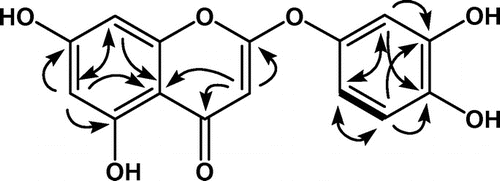
The structure of compound 1 was elucidated primarily based on the heteronuclear multiple bond connectivity (HMBC) spectrum. Long-range 13C-1H correlations (H-3/2, 4, 10, H-6/C-5, 7, 8, 10 and H-8/C-6, 10) indicated the presence of A- and C-rings (Figure ). The A- and C-rings were also confirmed by the positive ion ESI-MS spectrum, which exhibited peaks at m/z 303 [M+H]+, 195 [M+H-108]+ and 153 [M+H-150]+. The fragmentation at m/z 153 resulted from a Retro Diels-Alder (RDA) cleavage of compound 1 at the C-ring. However, there were no HMBC correlations from a tri-substituted benzene ring (B-ring). Moreover, the chemical shift of C2 (169.5 ppm) and C1′ (144.0 ppm) were downfield to those of flavones (luteolin) [Citation18]. These chemical shifts are close to capillarisin, a 2-phenoxychromone [Citation19]. This evidence suggested that the B-ring and C-ring are linked by the ether bridge C1′-O-C2.
Figure 1. 2D-NMR correlations of compound 1 (bold line shows 1H-1H COSY correlation, solid arrows show HMBC correlations).
Furthermore, compound 1 formed a crystal structure suitable for single-crystal X-ray analysis. The connectivity of the B- and C-ring was confirmed by single-crystal X-ray analysis. The ORTEP drawing of the monohydrate of compound 1 is shown in Figure . Based on these results, it was revealed that compound 1 was identified as a 5, 7-dihydroxy-2-(3, 4-dihydroxyphenoxy)-4H-chromen-4-one, an unknown compound, and has the chemical structure shown in Figure . The crystallographic information files of compound 1 have been deposited in the Cambridge Crystallographic Data Centre (CCDC deposition number 1532831).
The structures of known compounds 2 to 6 were determined by a combination of spectroscopic analysis and comparison with reported data. Compounds 2 [Citation20], 32 [Citation21,22], 4 [Citation23], 5 [Citation24] and 6 [Citation25] were identified as 3-O-methylquercetin, 3, 6, 4′-trimethoxychrysin, 3, 6-dimethoxyapigenin, 6-methoxykaempferol and 6-methoxyapigenin, respectively. To the best of our knowledge, there are few reports that the compounds (2-6) were found in other types of propolis from geographically diverse areas. Especially, this is the first report describing the discovery of 2-phenoxychromone from propolis. For these reasons, the isolated compounds must be derived from plant sources but not honey bee metabolites. In addition, 2-phenoxychromones have mainly been isolated from Asteraceae (Artemisia [Citation26], Achillea [Citation27] etc.), Fabaceae (Piliostigma [Citation28], Peltophprum [Citation29] etc.), suggesting that they are possibly one of the plant sources of BA propolis.
The growth-inhibitory activities of the propolis extract and the isolated compounds (1-6) against the three human cancer cell lines DLD-1 (human colon cancer), MCF-7 (human breast cancer) and A549 (human lung cancer) were evaluated with the CCK-8 assay in vitro. As shown in Table , the extract of BA propolis showed obvious anti-proliferation activities against each the cancer cell line. Compounds 2, 3 and 4 also showed inhibition of cell growth with nearly equal activity in all the cell lines tested (Table ). These compounds have a methoxy substituent at the 3-position in the chemical structure. There are several reports that 3-methoxy flavones inhibit cancer-cell growth [Citation30–32]. These data suggest that the presence of the methoxy substituent at position 3 of the C-ring might play an important role in the growth inhibition of cancer cells by flavone compounds. Interestingly, it was demonstrated that compound 1, a novel 2-phenoxychromone derivative, has an anti-proliferative effect on cancer cells, even though its activity seems to be lower than that of the 3-methoxy flavones.
Table 1. The growth-inhibitory activity of constituents isolated from the ethanol extract of BA propolis against human cancer cell lines.
Conclusion
We identified six main compounds in ethanol extracts of BA propolis, the northeastern Brazilian propolis in the state of Bahia. One of isolated compounds was a novel 2-phenoxychromone, while the others were flavones. In vitro studies demonstrated that three 3-methoxy flavones and 2-phenoxychromone have potent growth-inhibitory activity against human colon, breast and lung cancer cell lines. This is the first report on the discovery of a novel 2-phenoxychromone from propolis.
Author contributions
K. Ichihara conducted this study, S. Tazawa prepared experimental materials, T. Mitsui and S. Hotta performed analytical and biological experiments, respectively, and T. Mitsui, Y. Arai and K. Kato analyzed the analytical data. All authors have read and approved the manuscript to be submitted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
The supplemental data for this article can be accessed at https://doi.org/10.1080/09168451.2018.1427550
20180109_AcceptSupplementaryData_PDF.pdf
Download PDF (3.1 MB)Acknowledgement
We appreciate Mr. Kazushige Abe, Nordeste Co. Ltd. (Tokyo, Japan) for supplying clump of the raw BA propolis (Special propolis). We also thank Prof. Masahiro Ebihara, Ph.D., Gifu University (Gifu, Japan) for X-ray structure determination of compound 1.
References
- Aga H, Shibuya T, Sugimoto T, et al. Isolation and identification of antimicrobial compounds in Brazilian Propolis. Biosci Biotechnol Biochem. 1994;58(5):945–946.10.1271/bbb.58.945
- Kujumgieva A, Tsvetkovaa I, Serkedjievaa Y, et al. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64(3):235–240.10.1016/S0378-8741(98)00131-7
- Isla MI, Dantur Y, Salas A, et al. Effect of seasonality on chemical composition and antibacterial and anticandida activities of Argentine propolis. Design of a topical formulation. Nat Prod Commun. 2012;7(10):1315–1318.
- Takemura T, Urushisaki T, Fukuoka M, et al. 3,4-Dicaffeoylquinic acid, a major constituent of Brazilian Propolis, increases TRAIL expression and extends the lifetimes of mice infected with the influenza a virus. Evid Based Complement Alternat Med. 2012;2012:946867.
- Paulino N, Abreu SR, Uto Y, et al. Anti-inflammatory effects of a bioavailable compound, Artepillin C Brazilian propolis. Eur J Pharmacol. 2008;587(1–3):296–301.10.1016/j.ejphar.2008.02.067
- Scheller S, Krol W, Swiacik J, et al. Antitumoral property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin. Z Naturforsch C. 1989;44(11–12):1063–1065.
- Kimoto T, Aga M, Hino K, et al. Apoptosis of human leukemia cells induced by Artepillin C, an active ingredient of Brazilian propolis. Anticancer Res. 2001;21(1a):221–228.
- Mishima S, Ono Y, Araki Y, et al. Two related cinnamic acid derivatives from Brazilian honey bee propolis, baccharin and drupanin, induce growth inhibition in allografted sarcoma S-180 in mice. Biol Pharm Bull. 2005;28(6):1025–1030.10.1248/bpb.28.1025
- Akao Y, Maruyama H, Matsumoto K, et al. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol Pharm Bull. 2003;26(7):1057–1059.10.1248/bpb.26.1057
- Popolo A, Piccinelli LA, Morello S, et al. Antiproliferative activity of brown Cuban propolis extract on human breast cancer cells. Nat Prod Commun. 2009;4(12):1711–1716.
- Li F, Awale S, Tezuka Y, et al. Cytotoxicity of constituents from Mexican propolis against a panel of six different cancer cell lines. Nat Prod Commun. 2010;5(10):1601–1606.
- Park SI, Ohta T, Kumazawa S, et al. Korean propolis suppresses angiogenesis through inhibition of tube formation and endothelial cell proliferation. Nat Prod Commun. 2014;9(4):555–560.
- Tazawa S, Arai Y, Hotta S, et al. Discovery of a novel Diterpene in Brown Propolis from the State of Parana, Brazil. Nat Prod Commun. 2016;11(2):201–205.
- Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian Propolis. J Agric Food Chem. 2002;50(9):2502–2506.10.1021/jf011432b
- Hata T, Tazawa S, Ohta S, et al. Artepillin C, a major ingredient of Brazilian Propolis, induces a pungent taste by activating TRPA1 channels. PLoS One. 2012;7(11):e48072.10.1371/journal.pone.0048072
- Naramoto K, Kato M, Ichihara K. Effects of an ethanol extract of Brazilian green propolis on human cytochrome P450 enzyme activities in vitro. J Agric Food Chem. 2014;62(46):11296–11302.10.1021/jf504034u
- Intekhab J, Aslam M. Isolation of a flavone glucoside from Glycosmis mauritiana (Rutaceae). Arab J Chem. 2011;4(1):79–81.10.1016/j.arabjc.2010.06.023
- Lin LC, Pai YF, Tsai TH. Isolation of Luteolin and Luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and Their Pharmacokinetics in Rats. J Agric Food Chem. 2015;63(35):7700–7706.10.1021/jf505848z
- Sharon A, Ghirlando R, Gressel J. Isolation, purification, and identification of 2-(p-Hydroxyphenoxy)-5, 7-Dihydroxychromone: a fungal-induced Phytoalexin from Cassia obtusifolia. Plant Physiol. 1992;98(1):303–308.10.1104/pp.98.1.303
- Hong SS, Choi Y-H, Suh H-J, et al. Flavonoid constituents of Acacia catechu. J Appl Biol Chem. 2015;58(2):189–194.10.3839/jabc.2015.030
- Williams CA, Harborne JB, Geiger H, et al. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry. 1999;51(3):417–423.10.1016/S0031-9422(99)00021-7
- Hòrie T, Ohtsuru Y, Shibata K, et al. 13C NMR spectral assignment of the A-ring of polyoxygenated flavones. Phytochemistry. 1998;47(5):865–874.10.1016/S0031-9422(97)00629-8
- van Heerden FR, Viljoen AM, van Wyk BE. The major flavonoid of Dodonaea angustifolia. Fitoterapia. 2000;71(5):602–604.10.1016/S0367-326X(00)00201-X
- Hajime A, Shigeyuki A, Shigeharu F, et al. Physiologically active extract obtained from indigo plant polygonum tinctorium. United States patent US 6,524,625 B2. 2003 Feb. 25.
- Xia H, Qiu F, Zhu S, et al. Isolation and identification of ten metabolites of breviscapine in rat urine. Biol Pharm Bull. 2007;30(7):1308–1316.10.1248/bpb.30.1308
- Komiya T, Tsukui M, Oshio H. Studies on “Inchinko”. I. Capillarisin, a new choleretic substance (author’s transl). Yakugaku Zasshi. 1976;96(7):841–854.10.1248/yakushi1947.96.7_841
- Kijjoa A, Vieira LM, Pereira JA, et al. Further constituents of Achillea ageratum. Phytochemistry. 1999;51(4):555–558.10.1016/S0031-9422(99)00054-0
- Babajide OJ, Babajide OO, Daramola AO, et al. Flavonols and an oxychromonol from Piliostigma reticulatum. Phytochemistry. 2008;69(11):2245–2250.10.1016/j.phytochem.2008.05.003
- Polasek J, Queiroz EF, Marcourt L, et al. Peltogynoids and 2-phenoxychromones from Peltophorum pterocarpum and evaluation of their estrogenic activity. Planta Med. 2013;79(6):480–486.
- Rubio S, Quintana J, Eiroa JL, et al. Betuletol 3-methyl ether induces G(2)-M phase arrest and activates the sphingomyelin and MAPK pathways in human leukemia cells. Mol Carcinog. 2010;49(1):32–43.
- Rubio S, Quintana J, López M, et al. Phenylbenzopyrones structure-activity studies identify betuletol derivatives as potential antitumoral agents. Eur J Pharmacol. 2006;548(1–3):9–20.10.1016/j.ejphar.2006.07.020
- Li J, Zhu F, Lubet RA, et al. Quercetin-3-methyl ether inhibits lapatinib-sensitive and -resistant breast cancer cell growth by inducing G2/M arrest and apoptosis. Mol Carcinog. 2013;52(2):134–143.10.1002/mc.v52.2