Abstract
Several dietary flavonoids exhibit anti-oxidative, anti-inflammatory, and anti-osteoporotic activities relevant to prevention of chronic diseases, including lifestyle-related diseases. Dietary flavonoids (glycoside forms) are enzymatically hydrolyzed and absorbed in the intestine, and are conjugated to their glucuronide/sulfate forms by phase II enzymes in epithelial cells and the liver. The intestinal microbiota plays an important role in the metabolism of flavonoids found in foods. Some specific products of bacterial transformation, such as ring-fission products and reduced metabolites, exhibit enhanced properties. Studies on the metabolism of flavonoids by the intestinal microbiota are crucial for understanding the role of these compounds and their impact on our health. This review focused on the metabolic pathways, bioavailability, and physiological role of flavonoids, especially metabolites of quercetin and isoflavone produced by the intestinal microbiota.
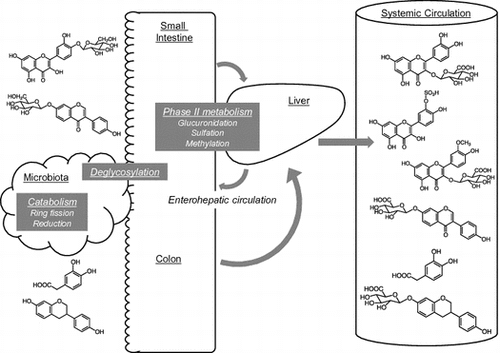
The intestinal microbiota plays important roles in the metabolism of flavonoids. Various chemical forms of flavonoids are present in the systemic circulation.
Abbreviations:
- AhR: aryl hydrocarbon receptor
- ALDH: aldehyde dehydrogenase
- CBG: cytosolic β-glucosidase
- COMT: catechol-O-methyltransferases
- DHD: dihydrodaidzein
- l-DDRC: DHD racemase
- l-DHDR: DHD reductase
- O-DMA: O-desmethylangolensin
- DOPAC: 3,4-dihydroxyphenylacetic acid
- l-DZNR, NADP(H)-dependent daidzein reductase
- FOS: fructooligosaccharides
- Keap1: Kelch-like erythroid cell-derived protein 1
- LPH: lactase-phlorizin hydrolase
- MCT: monocarboxylic acid transporter
- Nrf2: NFE2-related factor 2
- OPAC: 3-hydroxyphenylacetic acid
- OVX: ovariectomized
- PCA: 3,4-dihydroxybenzoic (protocatechuic) acid
- Q3G: quercetin 3-O-glucoside
- Q4ʹG: quercetin 4ʹ-O-glucoside
- SGLT1: sodium-glucose co-transporter type 1
- SULT: sulfotransferases, UGT: uridine-5ʹ-diphosphate-glucuronosyltransferases
- THD: tetrahydrodaidzein
- l-THDR: THD reductase
Dietary polyphenols are considered to play an important role in human health, especially for the prevention of lifestyle-related diseases including metabolic syndrome [Citation1,2], atherosclerosis and coronary heart disease [Citation3–5], and osteoporosis [Citation6]. Flavonoids are major dietary polyphenols that are found in a wide variety of plant foods and beverages [Citation7]. Most dietary flavonoids are present in their glycosidic forms, in which one or more sugar moieties are bound to phenolic groups or a hydroxyl group at C-3 position [Citation8]. The basic structure of flavonoids, meaning the structure of the aglycon form, and which type of sugar moiety is attached strongly affect their bioavailability [Citation9]. Bioavailability is a crucial factor determining their biological activity in vivo. Therefore, information on the absorption and metabolism of dietary flavonoids in the digestive tract is important for determining their physiological functions.
To understand the mechanism of action of dietary flavonoids in the body, it is necessary to determine which chemical forms of the various metabolites are found in systemic circulation, as these would be the physiologically active forms. In the present review, we focus on the bioavailability and physiological role of metabolites converted from dietary flavonoids, especially quercetin and isoflavones, as the major dietary flavonoids in Japan.
General metabolism of flavonoids: phase II conjugation and physiological function of the conjugates
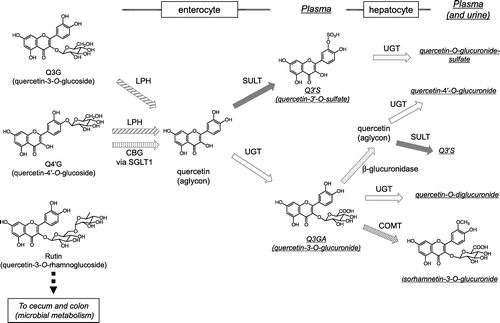
Dietary flavonoids are mostly present in their glycoside forms. However, this is not the case in the plasma, where glycosides are scarce [Citation10–12]. Deglycosylation occurs both in the small intestine and in the large intestine, depending on the type of sugar moiety. In the small intestine, two enzymes have been reported to act as β-glucosidases against flavonoid monoglucosides [Citation13]. Lactase-phlorizin hydrolase (LPH) is a brush border–associated enzyme that hydrolyzes lactose to glucose and galactose. It was first reported by Day et al. [Citation14] that LPH hydrolyzes quercetin 3-O-glucoside (Q3G) and quercetin 4ʹ-O-glucoside (Q4ʹG), as well as the monoglucosides of genistein and daidzein, to produce the corresponding aglycons in vitro. The other enzyme, cytosolic β-glucosidase (CBG), is located in enterocytes. CBG is also known to show broad specificity and is reported to hydrolyze Q4ʹG, genistein 7-O-glucoside (genistin), and daidzein 7-O-glucoside (daidzin), but not quercetin 3,4ʹ-O-diglucoside, Q3G, or quercetin 3-O-rhamnoglucoside (rutin) using cell-free extracts from human intestine and liver [Citation15]. Prior to hydrolysis by CBG, the glucosides are taken up into the cell, and sodium-glucose co-transporter type 1 (SGLT1) is reported to act as the membrane transporter for Q4ʹG, which was found using human Caco-2 cells and SGLT1 transfected rodent G6D3 cells [Citation16]. Thus, Q3G should be hydrolyzed by LPH in the intestinal lumen, whereas Q4ʹG can be absorbed via both pathways (Figure ). Human intestinal Caco-2 cells are widely used for the estimation of flavonoid bioavailability. We previously reported that Q4ʹG showed significantly higher bioavailability in Caco-2 cells than Q3G or quercetin 3,4ʹ-diglucoside [Citation17], since the expression of LPH in Caco-2 cells is much lower than that of human tissue [Citation14]. On the other hand, Q3G and Q4ʹG are reported to show similar bioavailability in humans [Citation18]. In the case of isoflavones, LPH effectively hydrolyzes both genistin and daidzin, and thus the aglycons produced in the intestinal lumen are easily absorbed. One study investigated the contribution of the two absorption pathways to the bioavailability of isoflavones in humans [Citation19]. In individuals with lactase deficiency, the plasma levels of isoflavone metabolites are suppressed only in the early phase of absorption and over time become similar to those of lactase-producing individuals, probably because the intestinal microbiota compensates for the reduced hydrolysis. Hydrolysis of isoflavone glucosides is mainly carried out by CBG in the cell-free extract of human intestine and liver, with a lesser contribution of LPH than in rats [Citation20]. Recently, calycosin-7-O-glucoside, a methylated isoflavone, was reported to be a novel substrate for SGLT1 but not for LPH in rats [Citation21]. Thus, deglycosylation in the small intestine may be important to increase the bioavailability of flavonoid monoglucosides, although performance of the subsequent step by the microbiota may be sufficient to compensate for the absence of this process.
Figure 1. Putative metabolism of major quercetin glycosides in the small intestine and the liver in humans. CBG, cytosolic β-glucosidase; COMT, catechol-O-methyltransferase; LPH, lactase-phlorizin hydrolase; SGLT1, sodium glucose co-transporter 1; SULT, sulfotransferase; UGT, uridine-5ʹ-diphosphate glucuronosyltransferase.
In the case of non-monoglucosidic glycosides, such as rutin and hyperoside (quercetin-3-O-glactoside), intestinal β-glucosidases cannot hydrolyze the sugar moiety. Thus, the intestinal microbiota acts to yield absorbable aglycon in the cecum and in the large intestine. The aglycon produced by the microbiota is absorbed via the large intestine and transported into the circulation (Figure ) [Citation22]. The microbiota also produces fission components and reduced metabolites, such as equol from daidzein [Citation23–25] (see section II, III).
Once flavonoid aglycons enter the intestinal epithelial cells, phase II enzymes produce the corresponding conjugated metabolites. There are three types of phase II enzymes reported to metabolize flavonoids, uridine-5ʹ-diphosphate-glucuronosyltransferases (UGT), sulfotransferases (SULT), and catechol-O-methyltransferases (COMT) [Citation26]. Another report determined the glutathione conjugate of quercetin [Citation27]. In the rat small intestine, UGT is considered to be a major enzyme that conjugates flavonoids with glucuronic acid [Citation26,28]. In humans, both UGT and SULT are considered to contribute to the production of monoglucuronides and sulfates (Figure ) [Citation26,29].
The liver is another important site of phase II metabolism. Following intestinal conjugation, absorbed flavonoids are transported to either the portal vein or the lacteals. In the liver, further conjugation occurs, including sulfation and methylation, and multiple conjugates are produced (Figure ). In human hepatoma HepG2 cells, quercetin glucuronides, which could be produced in the enterocytes, were partly methylated to isorhamnetin glucuronides [Citation30]. Endogenous β-glucuronidase is another enzyme to play a role in producing various metabolites Quercetin glucuronides were also hydrolyzed by β-glucuronidase and then sulfated to quercetin-3’-O-sulfate in HepG2 cells [Citation30]. Thus, although the major in vivo hepatic metabolic pathway needs further investigation, various chemical forms of flavonoids are present in the systemic circulation and in the urine (Figure ), and most are hydrophilic [Citation29,31–33]; aglycons are rarely detected in human plasma. Some plasma metabolites are excreted into the intestine via the bile [Citation34]. Excreted metabolites are then deconjugated by the microbiota and reabsorbed. Such enterohepatic circulation contributes to increasing the level and half-life of flavonoids in the plasma in humans [Citation35] and in rats [Citation36].
Murota et al. reported that some absorbed flavonoids are transported in the body via the lymph, in a study of unanesthetized lymph-cannulated rats [Citation37,38]. In general, the intestinal lymph transports lipophilic food components, as components of chylomicrons. Interestingly, when quercetin was administered into the rat duodenum, lymphatic quercetin was detected in hydrophilic conjugated forms, and not incorporated into chylomicrons [Citation38]. Although the physiological role of the lymphatic transport of flavonoids remains to be clarified, lymphatic transport is often considered the important alternative pathway for various medicines, as it bypasses the first-pass metabolism in the liver. Lymphatic components merge into the blood flow via the thoracic duct, and the lymph fluid continues to the left chest. In rats, the lung has been reported to accumulate quercetin [Citation39], suggesting that quercetin metabolites are transported via the lymph.
Many flavonoids show strong antioxidant activity and are thought to act as anti-atherosclerosis factors [Citation3,4,40,41], preventing oxidation of LDL [Citation42]. Murota et al. reported that following intake quercetin is only slightly detected in LDL and HDL, and that the majority is found in the lipoprotein-removed plasma, which is rich in albumin [Citation43]. The quercetin-rich albumin fraction did not show increased anti-oxidant activity against LDL compared with the control [Citation43]. In addition, human LDL isolated from the plasma after onion consumption did not show improved antioxidant capacity [Citation44]. The antioxidant capacity of quercetin depends on the catechol structure in the B-ring and the flavonol structure in the C-ring [Citation42,45]. Thus, antioxidant activity is markedly reduced by physiological conjugation. Some conjugated metabolites may exert physiological functions other than antioxidant activity, for example, acting as enzymatic inhibitors and ligands for receptors [Citation46–49]. On the other hand, Shimoi et al. first reported that luteolin metabolites are deconjugated at inflammatory sites [Citation50]. Later, Kawai et al. found that quercetin glucuronide is detectable in atherosclerotic lesions, in macrophages [Citation51]. Macrophages showed deglucuronidation activity against quercetin glucuronides and produced more active aglycon than its conjugated metabolites [Citation52–54]. Such deglucuronidation systems may control the physiological activity of dietary flavonoids [Citation55,56]. To estimate the physiological activity of flavonoid metabolites, the use of authentic pure molecules is critical. Recently, Ikushiro et al. established a system for biosynthesis of various phase II conjugates in budding yeast culture, making it possible to obtain isolated conjugates on a massive scale [Citation57]. These novel methods to produce various individual metabolites would be the key to further investigation.
Ring-fission products of polyphenols
Global interest in catabolic products formed by the intestinal microbiota and their role in the physiological effects of their parent flavonoids has recently increased. Only approximately 10% of the flavonoid glycosides ingested are absorbed in the upper gastrointestinal tract. Flavonoids are thought to reach the colon as non-absorbed and non-metabolized flavonoid glycosides after passing through the small intestine or as flavonoids that were metabolized as conjugates in the bile. Flavonoids have also been confirmed to influence the quantity and quality of the intestinal microbiota, and hence to indirectly influence their own metabolism and bioavailability [Citation25]. The gut microbiota contains large quantities of various types of enzymes that modify food compounds before they enter the colon. In addition to several hydroxylases, including glycosidases, glucuronidases, sulfatases, amidases, and esterases, microbial enzymes catalyze various types of reactions, including oxidation, reduction, decarboxylation, demethylation, isomerization, and ring cleavage [Citation58]. This diversity of enzymatic capability allows production of various kinds of catabolites of dietary polyphenols. In the colon, microbial enzymes can eliminate glycosides, glucuronides, and sulfates and produce flavonoid aglycons, which are further metabolized into a variety of ring-fission products. These lower molecular weight catabolites are believed to precisely reflect the physiological effects of their parent compounds. In other words, the beneficial effects of flavonoids cannot be fully realized in the absence of species that can catabolize flavonoids in the gut microbiota, even though they have been consumed.
One of the most extensively studied flavonoids in terms of catabolism is isoflavones, such as daidzein and its glucoside, daidzin. Several species of gut bacteria are able to metabolize daidzein to equol or a ring-fission product, O-desmethylangolensin (O-DMA) (Figure ), both of which also act as nonsteroidal estrogens [Citation25]. The gut bacteria strains responsible for these metabolites are shown in section III. Similar to the bacterial metabolism of daidzein, sesame lignans and ellagitannins are converted into enterolactone / enterodiol and urolithin A, respectively, all of which can bind to estrogen receptors [Citation59]. Ring-fission catabolism has been observed in the case of tea catechins, the primary class of flavonoids in green tea; γ-valerolactones have been identified in human urine after green tea ingestion (Figure ) [Citation60]. Takagaki et al. identified Adlercreutzia equolifaciens and Flavonifractor plautii as rat enteric bacteria with catechin C ring cleavage ability and phloroglucinol moiety-decomposing ability, respectively [Citation61]. Procyanidins, oligomers of flavan-3-ols, can undergo intestinal bacterial catabolism into 3,4-dihydroxyphenylvaleric acid, which is further degraded into phenolic acids such as 3,4-dihydroxyphenylpropionic (dihydrocaffeic) acid and 3,4-dihydroxybenzoic (protocatechuic) acid (PCA) [Citation62]. Various anthocyanins are mainly catabolized into PCA, p-coumaric acid, and vanillic acid in the intestine [Citation63] . PCA, one of the most abundant catabolites in the large intestine, which is derived from various polyphenols, exerts several kinds of biological effects, such as antioxidant, anti-inflammatory, anticarcinogenic, and neuroprotective activities and may thus have a protective role in various diseases [Citation25]. The phenolic acid catabolites from polyphenols are believed to be mainly transported by monocarboxylic acid transporters (MCTs) [Citation64].
Figure 2. Chemical structures of typical flavonoids and their ring-fission products generated by gut microbiota. (R)-O-DMA, (R)-O-desmethylangolensin; (R)-THPV, (R)-5-(3ʹ,4ʹ,5ʹ-trihydroxyphenyl)-γ-valerolactone; DOPAC, 3,4-dihydroxyphenylacetic acid; OPAC, 3-hydroxyphenylacetic acid; PCA, protocatechuic acid.
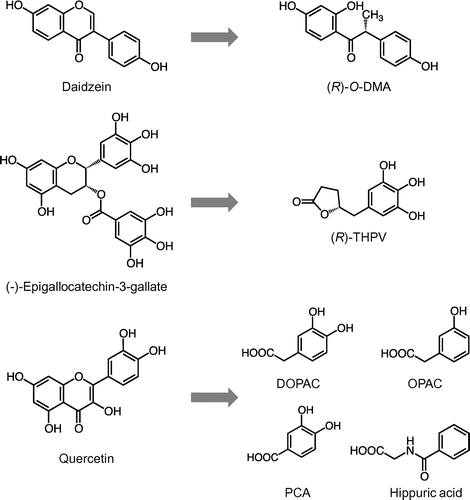
Quercetin glycosides can also be catabolized by intestinal bacteria into ring-fission products. Q4ʹG, one of the major glycosides in onions [Citation65], is superior to Q3G in the prevention of lipid peroxidation in the rat intestinal mucosa, even though Q4ʹG has lower antioxidative activity than Q3G [Citation45]. These data indicate that the antioxidative potential of Q4ʹG might corroborate effective bacterial metabolism in the intestinal mucosa. Experiments using radiolabeled Q4ʹG revealed that Q4ʹG passes through the gastrointestinal tract of rats and that almost all of Q4ʹG is converted into phenolic acids [Citation66]. The main phenolic acids produced from Q4′G detected in the colorectum are 3,4-dihydroxyphenylacetic acid (DOPAC) and 3-hydroxyphenylacetic acid (OPAC), with a small amount of PCA. In this study, 69% of [2–14C] Q4′G radioactivity was recovered in the form of phenolic acid derivatives, such as OPAC and hippuric acid, in urine. It is thus plausible that the first ring-fission product is DOPAC, which is subsequently subjected to dehydroxylation to form OPAC, followed by further catabolism into hippuric or benzoic acids, all of which can be absorbed by enterocytes (Figure ). Urinary excretion of DOPAC as well as other phenolic acid catabolites is increased in human subjects after consumption of polyphenols in chocolate [Citation67]. DOPAC has also been identified as a major catabolite of other quercetin glycosides, rutin [Citation68], and hyperoside [Citation69], as well as procyanidins [Citation62]. Some human fecal bacteria, such as Clostridium perfringens and Bacteroides fragilis, but not Escherichia coli or Lactobacillus acidophilus, have the ability to produce DOPAC from quercetin [Citation70]. These findings strongly support the idea that DOPAC is a predominant catabolite from quercetin glycosides not only in rodents, but also in humans. In addition, DOPAC is known to be a metabolite of the neurotransmitter dopamine, suggesting the existence of a metabolic pathway for DOPAC in humans [Citation71].
DOPAC has recently been identified as the most active phenolic acid derived from quercetin glycosides, in terms of free radical scavenging and induction of drug metabolic enzymes [Citation72]. In addition to its free radical scavenging activity, DOPAC inhibits the secretion of pro-inflammatory cytokines in mononuclear cells [Citation73] and the expression of P-selectin in platelets [Citation74]. DOPAC, as well as OPAC and 3′-methoxy-4′-hydroxyphenylacetic acid, inhibit the formation of advanced glycation end-products and oxidative stress-induced neuronal cell death [Citation75]. Furthermore, DOPAC significantly inhibits hydrogen peroxide-induced cytotoxicity in rat normal hepatocytes, dependent on the expression of phase 2 cytoprotective enzymes, such as hemeoxynegese-1 and glutathione S-transferase, and independent of its free radical scavenging potential [Citation72]. DOPAC also enhances not only total aldehyde dehydrogenase (ALDH) activity, but also gene and protein expression of ALDH isozymes in mouse hepatoma Hepa1c1c7 cells [Citation76]. Pretreatment with DOPAC completely protects cells from acetaldehyde-induced cytotoxicity in vitro. DOPAC simultaneously stimulates nuclear translocation of NFE2-related factor 2 (Nrf2) and aryl hydrocarbon receptor (AhR), both of which are responsible for the expression not only of phase I and II drug-metabolizing enzymes, but also of ALDHs. A previous study using a DOPAC click chemistry probe and a pull-down assay identified Kelch-like erythroid cell-derived protein 1 (Keap1), which inhibits Nrf2 activation, and AhR as targets of covalent modification by DOPAC in mouse hepatoma cells [Citation77]. Thus, even though DOPAC shows relatively weaker biological activities than quercetin aglycon [Citation72,76], DOPAC is quite plausibly a main component that facilitates the health-promoting potential of quercetin glycosides. DOPAC also has advantages for application as a food chemical because it has lower cytotoxicity than quercetin. Future efforts will be concerned with the relevance of the antioxidant effect of DOPAC in human preclinical studies as well as individual variance in DOPAC production and its attribution to certain intestinal bacteria strains.
Metabolic pathways of isoflavone metabolites
Recent studies suggest that the clinical effectiveness of isoflavones might be due to their ability to be converted into metabolites such as dihydrodaidzein (DHD), tetrahydrodaidzein (THD), equol, and O-DMA in the gut [Citation78]. In particular, equol (7-hydroxy-3-(4ʹ-hydroxyphenyl)-chroman), a metabolite of daidzein, has recently received considerable attention, because its biological activities differ from those of its precursor [Citation79–81]. Metabolites of genistein and glycitein are also primarily found in human urine (genistein: dihydrogenistein, 6ʹ-OH-O-DMA [Citation82], 4-hydroxyphenyl-2-propionic acid and phloroglucinol; glycitein: dihydroglycitein, 5ʹ-methoxy-DMA and 6-methoxy-equol) [Citation83,84]. The physiological activities of these metabolites remain unclear.
In comparison to daidzein, equol has higher estrogenicity, stronger anti-oxidative efficacy, and exhibits anti-androgenic properties [Citation79,80]. Interindividual variability in equol production may be unique to humans; all animals tested to date, including rats, mice, and chimpanzees, systematically excrete equol [Citation80,85–89]. Although O-DMA is found in 80–90% of the human population, equol is found in only 30–50% of the population [Citation90–92]. Interindividual variation in the ability to produce equol is a consequence of differences in gut microbial community. The intestinal microbiota responsible for equol production might differ across individuals [Citation89–93]. Some reports have indicated a lower disease risk for equol producers than for non-producers [Citation79–81], and several candidate bacteria responsible for daidzein metabolism have been suggested. It has been reported that bacteria such as Escherichia coli, Bacteroides ovatus, Ruminococcus productus, Streptococcus intermedius [Citation93], Eggerthella sp. strain YY7918 [Citation94], and Adlercreutzia equolifaciens [Citation95] are involved in daidzein metabolism. Some equol-producing bacteria can produce equol from either daidzein or daidzin, but some cannot produce equol unless several other species of bacteria are also present. Equol is known to be produced by strain TM-40, a Clostridium-like bacterium that can produce the intermediate metabolite DHD, but this bacterium cannot produce equol from both daidzein and daidzin [Citation96]. Wang et al. [Citation97] reported two bacterial strains that can synthesize equol, namely, Eggerthella sp. Julong 732 (from DHD to equol) and Lactobacillus sp. Niu-16 (from daidzein to DHD). From a human fecal sample, Decroos et al. [Citation98] have isolated a stable, mixed microbial culture comprising 4 species (Lactobacillus mucosae, Enterococcus faecium, Finegoldia magna, and Veillonella sp.) that are capable of transforming daidzein into equol. Regarding O-DMA-producing bacteria, Eubacterium ramulus, Clostridium sp. HGH 136, Clostridium cluster XIVa, and the named strain SY8519 metabolize daidzein to O-DMA in vitro [Citation94,99–101]. A small number of studies have evaluated disease risk factors in relation to the O-DMA–producer phenotype, with mixed results. In our previous study, the effects of O-DMA on bone and lipid metabolism in ovariectomized (OVX) mice and osteoclast cell cultures were found to be weaker than those of equol [Citation102]. However, the O-DMA–producer phenotype acts as a marker of bacteria capable of C-ring cleavage reactions. Other phytochemicals also undergo C-ring cleavage reactions. For example, E. ramulus metabolizes both daidzein and quercetin. Frankenfeld hypothesized that O-DMA–producing bacteria metabolize other phytochemicals that may influence disease risk [Citation103].
To date, several lactic acid bacteria have been identified that can transform equol directly from daidzein without producing O-DMA [Citation104]. The strain Lactococcus (Lc.) 20–92, homologous to Lc. garvieae, can also cleave the glycosidic bonds of daidzin. Uchiyama et al. detected Lc. garvieae in the Italian cheese Toma Piemontese [Citation105].
Equol is a chiral molecule that exists as the enantiomers (R)(+)-equol and (S)(-)-equol [Citation106]. Setchell et al. [Citation85] established (S)-equol as the exclusive product of human intestinal microbial synthesis using daidzein and showed that both enantiomers are bioavailable. The two chiral forms have been shown to differ in binding affinities and preferences for estrogen receptor α (ERα) and ERβ; (S)-equol has a high binding affinity for and preferentially binds to ERβ, whereas (R)-equol binds preferentially to ERα [Citation107]. In humans, metabolism of daidzein to equol results in the production of only (S)-equol [Citation85].
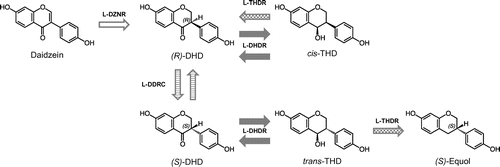
Niu-O16 can transform DHD to (S)-equol and daidzein to DHD. In Eggerthella sp. Julong 732, DHD is converted to equol via the production of cis/trans THD as an intermediate metabolite. These studies have therefore suggested that daidzein is converted to equol via DHD and cis/trans THD. Furthermore, other bacterial strains that are capable of transforming daidzein to DHD or equol have been isolated [Citation108–113]. Shimada et al. [Citation114] purified a novel NADP(H)-dependent daidzein reductase (l-DZNR) from Lc. 20–92 and clarified that recombinant histidine-tagged l-DZNR converts daidzein to (S)-DHD with enantioselectivity. The same authors identified three other enzymes, DHD reductase (l-DHDR), THD reductase (l-THDR), and DHD racemase (l-DDRC), and reported that daidzein is first converted into (R)-DHD by l-DZNR and then into (S)-DHD by l-DDRC (Figure ).[Citation115] Furthermore, the authors demonstrated that the final product, (S)-equol, is generated from (S)-trans-THD by l-THDR following the conversion of (S)-DHD into (S)-trans-THD (Figure ) [Citation116]. Tsuji et al. also identified Slackia sp. strain NATTS from healthy human feces, which has potent daidzein-to-equol conversion ability [Citation117], and two enzymes (ORF-1 and ORF-2) that catalyze conversion of cis/trans-THD to equol and DHD-to cis/trans-THD, as well as the relevant genes [Citation118]. Recently, Kawada et al. proposed that THDR is not a reductase but a new type of dismutase, because THDR converts THD in the absence of the cofactors NAD(P)H [Citation119]. Interestingly, Abiru et al. isolated six novel (S)-equol-producing bacteria, in the family Coriobacteriaceae, from the brine of stinky tofu, a traditional fermented soy food in Taiwan. This was the first report of isolation of equol-producing bacteria from food [Citation120].
Figure 3. Model of the equol biosynthetic pathway starting from daidzein in Lactococcus (Lc.) strain 20–92 (reference 116 with modification). l-DZNR, NADP(H)-dependent daidzein reductase from Lc. 20–92; l-DHDR, dihydrodaidzein reductase from Lc. 20–92; l-THDR, tetrahydrodaidzein reductase from Lc. 20–92; l-DDRC: dihydrodaidzein rasemase from Lc. 20–92.
Decroos et al. investigated the influence of various environmental conditions in the colon on equol production [Citation98]. They suggest that equol production is largely stimulated by hydrogen gas, which might act as an electron donor in the biotransformation reaction from daidzein to equol. Moreover, increased equol production is also found in the presence of short-chain fatty acids such as propionate and butyrate, suggesting that a carbohydrate-rich diet stimulates equol production. They indicated that short-chain fatty acids are also related to the production of hydrogen gas [Citation98].
Indigestible and fermentable sugars such as fructooligosaccharides (FOS) are known to be prebiotics, which enhance production of short-chain fatty acids and hydrogen gas. In OVX mice, we reported that post-OVX bone loss can be most effectively prevented with a diet containing both FOS and isoflavone glycoside conjugates, thus correlating with increased equol production [Citation88]. Non-digestible sugars such as FOS, cello-oligosaccharides, polydextrose, and raffinose [Citation121,122] as well as resistant starch (non-digestible starch) enhance equol production and inhibit bone loss in OVX mice [Citation121,123]. In an in vitro study, however, FOS inhibited equol production [Citation98]. Thus, results of in vivo and in vitro studies are discordant. Fermentation of non-digestible saccharides and starches in the colon appears to facilitate equol production. The hydrogen gas produced during fermentation has been proposed to encourage equol production. In French postmenopausal women, FOS did not increase urinary equol levels [Citation124]. In our previous pilot study as well, FOS supplementation did not affect the serum equol concentration or the urinary equol-to-daidzein concentration ratios in either equol producers or non-producers after two weeks of intervention. Efficient transformation from daidzein to equol might be unlikely to occur with hydrogen gas produced in a short-term by FOS interventions. Further human studies involving Asian subjects will be required. Several factors such as intervention term, animal species, racial background, sex, age, and genetic background, including individual variation in intestinal microbiota and diet, should be considered with regard to isoflavone metabolism and metabolite production.
Conclusions
Early in the study of flavonoids, it was the mainstream to examine the effectiveness of the aglycon form, but as research on bioavailability progressed, the physiological function of metabolic products has drawn increasing attention. In the blood and tissues, flavonoids are present in the form of conjugated metabolites, which are considered to show lower activity than the aglycon form. However, conversion to the aglycon by the deconjugating enzyme at the target site of inflammation has also been reported, and the balance of conjugation-deconjugation may control the physiological function of flavonoid metabolites. It has also been reported that some metabolites have stronger physiological activities than their precursors. In addition, since intestinal bacteria are deeply involved in the production of metabolites, it is necessary to study the interaction between metabolites and the intestinal microbiota in detail for further development of flavonoid research.
Disclosure statement
The authors declare no potential conflict of interest.
References
- Galleano M, Calabro V, Prince PD, et al. Flavonoids and metabolic syndrome. Ann N Y Acad Sci. 2012;1259:87–94.10.1111/nyas.2012.1259.issue-1
- Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17(7):573–586.10.1111/obr.v17.7
- Hertog MG, Feskens EJ, Hollman PC, et al. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342(8878):1007–1011.10.1016/0140-6736(93)92876-U
- Knekt P, Jarvinen R, Reunanen A, et al. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312(7029):478–481.10.1136/bmj.312.7029.478
- Siasos G, Tousoulis D, Tsigkou V, et al. Flavonoids in atherosclerosis: an overview of their mechanisms of action. Curr Med Chem. 2013;20(21):2641–2660.10.2174/0929867311320210003
- Sacco SM, Horcajada MN, Offord E. Phytonutrients for bone health during ageing. Br J Clin Pharmacol. 2013;75(3):697–707.10.1111/bcp.12033
- Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061–1080.10.1016/0278-6915(95)00077-1
- Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747.10.1093/ajcn/79.5.727
- Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–242S.10.1093/ajcn/81.1.230S
- Morand C, Manach C, Crespy V, et al. Quercetin 3-O-β-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic Res. 2000;33(5):667–676.10.1080/10715760000301181
- Day AJ, Mellon F, Barron D, et al. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35(6):941–952.10.1080/10715760100301441
- Sesink AL, O’Leary KA, Hollman PC. Quercetin glucuronides but not glucosides are present in human plasma after consumption of quercetin-3-glucoside or quercetin-4′-glucoside. J Nutr. 2001;131(7):1938–1941.10.1093/jn/131.7.1938
- Day AJ, Gee JM, DuPont MS, et al. Absorption of quercetin-3-glucoside and quercetin-4’-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem Pharmacol. 2003;65(7):1199–1206.10.1016/S0006-2952(03)00039-X
- Day AJ, Canada FJ, Diaz JC, et al. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468(2–3):166–170.10.1016/S0014-5793(00)01211-4
- Day AJ, DuPont MS, Ridley S, et al. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436(1):71–75.10.1016/S0014-5793(98)01101-6
- Walgren RA, Lin JT, Kinne RK, et al. Cellular uptake of dietary flavonoid quercetin 4’-β-glucoside by sodium-dependent glucose transporter SGLT1. J Pharmacol Exp Ther. 2000;294(3):837–843.
- Murota K, Shimizu S, Chujo H, et al. Efficiency of absorption and metabolic conversion of quercetin and its glucosides in human intestinal cell line caco-2. Arch Biochem Biophys. 2000;384(2):391–397.10.1006/abbi.2000.2123
- Olthof MR, Hollman PC, Vree TB, et al. Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J Nutr. 2000;130(5):1200–1203.10.1093/jn/130.5.1200
- Tamura A, Shiomi T, Hachiya S, et al. Low activities of intestinal lactase suppress the early phase absorption of soy isoflavones in Japanese adults. Clin Nutr. 2008;27(2):248–253.10.1016/j.clnu.2007.12.001
- Islam MA, Punt A, Spenkelink B, et al. Conversion of major soy isoflavone glucosides and aglycones in in vitro intestinal models. Mol Nutr Food Res. 2014;58(3):503–515.10.1002/mnfr.201300390
- Shi J, Zheng H, Yu J, et al. SGLT-1 transport and deglycosylation inside intestinal cells are key steps in the absorption and disposition of calycosin-7-O-β-d-glucoside in rats. Drug Metab Dispos. 2016;44(3):283–296.10.1124/dmd.115.067009
- Erlund I, Kosonen T, Alfthan G, et al. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur J Clin Pharmacol. 2000;56(8):545–553.10.1007/s002280000197
- Jaganath IB, Mullen W, Lean ME, et al. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic Biol Med. 2009;47(8):1180–1189.10.1016/j.freeradbiomed.2009.07.031
- Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites. 2015;5(1):56–73.10.3390/metabo5010056
- Duda-Chodak A, Tarko T, Satora P, et al. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. 2015;54(3):325–341.10.1007/s00394-015-0852-y
- van der Woude H, Boersma MG, Vervoort J, et al. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem Res Toxicol. 2004;17(11):1520–1530.10.1021/tx049826v
- Hong YJ, Mitchell AE. Identification of glutathione-related quercetin metabolites in humans. Chem Res Toxicol. 2006;19(11):1525–1532.10.1021/tx0601758
- Piskula MK, Terao J. Accumulation of (−)-epicatechin metabolites in rat plasma after oral administration and distribution of conjugation enzymes in rat tissues. J Nutr. 1998;128(7):1172–1178.10.1093/jn/128.7.1172
- Mullen W, Edwards CA, Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br J Nutr. 2006;96(1):107–116.10.1079/BJN20061809
- O’Leary KA, Day AJ, Needs PW, et al. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human β-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65(3):479–491.10.1016/S0006-2952(02)01510-1
- Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med. 2010;31(6):446–467.10.1016/j.mam.2010.09.007
- Clarke DB, Lloyd AS, Botting NP, et al. Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C3]isoflavone internal standards. Anal Biochem. 2002;309(1):158–172.10.1016/S0003-2697(02)00275-0
- Nakamura T, Murota K, Kumamoto S, et al. Plasma metabolites of dietary flavonoids after combination meal consumption with onion and tofu in humans. Mol Nutr Food Res. 2014;58(2):310–317.10.1002/mnfr.v58.2
- Arts IC, Sesink AL, Faassen-Peters M, et al. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br J Nutr. 2004;91(6):841–847.10.1079/BJN20041123
- Adlercreutz H, Hockerstedt K, Bannwart C, et al. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG). J Steroid Biochem. 1987;27(4–6):1135–1144.10.1016/0022-4731(87)90200-7
- Chen J, Wang S, Jia X, et al. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33(12):1777–1784.
- Murota K, Terao J. Quercetin appears in the lymph of unanesthetized rats as its phase II metabolites after administered into the stomach. FEBS Lett. 2005;579(24):5343–5346.10.1016/j.febslet.2005.08.060
- Murota K, Cermak R, Terao J, et al. Influence of fatty acid patterns on the intestinal absorption pathway of quercetin in thoracic lymph duct-cannulated rats. Br J Nutr. 2013;109(12):2147–2153.10.1017/S0007114512004564
- de Boer VC, Dihal AA, van der Woude H, et al. Tissue distribution of quercetin in rats and pigs. J Nutr. 2005;135(7):1718–1725.10.1093/jn/135.7.1718
- Hertog MG, Kromhout D, Aravanis C, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155(4):381–386.10.1001/archinte.1995.00430040053006
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526.10.1016/0140-6736(92)91277-F
- Yamamoto N, Moon JH, Tsushida T, et al. Inhibitory effect of quercetin metabolites and their related derivatives on copper ion-induced lipid peroxidation in human low-density lipoprotein. Arch Biochem Biophys. 1999;372(2):347–354.10.1006/abbi.1999.1516
- Murota K, Hotta A, Ido H, et al. Antioxidant capacity of albumin-bound quercetin metabolites after onion consumption in humans. J Med Invest. 2007;54(3–4):370–374.10.2152/jmi.54.370
- Moon JH, Nakata R, Oshima S, et al. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am J Physiol Regul Integr Comp Physiol. 2000;279(2):R461–467.10.1152/ajpregu.2000.279.2.R461
- Murota K, Mitsukuni Y, Ichikawa M, et al. Quercetin-4′-glucoside is more potent than quercetin-3-glucoside in protection of rat intestinal mucosa homogenates against iron ion-induced lipid peroxidation. J Agric Food Chem. 2004;52(7):1907–1912.10.1021/jf035151a
- Day AJ, Bao Y, Morgan MRA, et al. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med. 2000;29(12):1234–1243.10.1016/S0891-5849(00)00416-0
- Gimenez-Bastida JA, Gonzalez-Sarrias A, Vallejo F, et al. Hesperetin and its sulfate and glucuronide metabolites inhibit TNF-α induced human aortic endothelial cell migration and decrease plasminogen activator inhibitor-1 (PAI-1) levels. Food Func. 2016;7(1):118–126.10.1039/C5FO00771B
- Kinjo J, Tsuchihashi R, Morito K, et al. Interactions of phytoestrogens with estrogen receptors α and β (III). Estrogenic activities of soy isoflavone aglycones and their metabolites isolated from human urine. Biol Pharm Bull. 2004;27(2):185–188.10.1248/bpb.27.185
- Pugazhendhi D, Watson KA, Mills S, et al. Effect of sulphation on the oestrogen agonist activity of the phytoestrogens genistein and daidzein in MCF-7 human breast cancer cells. J Endocrinol. 2008;197(3):503–515.10.1677/JOE-07-0384
- Shimoi K, Saka N, Nozawa R, et al. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos. 2001;29(12):1521–1524.
- Kawai Y, Nishikawa T, Shiba Y, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283(14):9424–9434.10.1074/jbc.M706571200
- Kawai Y. beta-Glucuronidase activity and mitochondrial dysfunction: the sites where flavonoid glucuronides act as anti-inflammatory agents. J Clin Biochem Nutr. 2014;54(3):145–150.10.3164/jcbn.14-9
- Ishisaka A, Kawabata K, Miki S, et al. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS One. 2013;8(11):e80843.10.1371/journal.pone.0080843
- Lodi F, Jimenez R, Moreno L, et al. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis. 2009;204(1):34–39.10.1016/j.atherosclerosis.2008.08.007
- Kaneko A, Matsumoto T, Matsubara Y, et al. Glucuronides of phytoestrogen flavonoid enhance macrophage function via conversion to aglycones by β-glucuronidase in macrophages. Immun Inflamm Dis. 2017;5(3):265–279.10.1002/iid3.163
- Menendez C, Duenas M, Galindo P, et al. Vascular deconjugation of quercetin glucuronide: the flavonoid paradox revealed? Mol Nutr Food Res. 2011;55(12):1780–1790.10.1002/mnfr.v55.12
- Ikushiro S, Nishikawa M, Masuyama Y, et al. Biosynthesis of drug glucuronide metabolites in the budding Yeast Saccharomyces cerevisiae. Mol Pharm. 2016;13(7):2274–2282.10.1021/acs.molpharmaceut.5b00954
- Hervert-Hernández D, Goñi I. Dietary polyphenols and human gut microbiota: a review. Food Rev Int. 2011;27(2):154–169.10.1080/87559129.2010.535233
- Högger P. Nutrition-derived bioactive metabolites produced by gut microbiota and their potential impact on human health. Nutr Med. 2013;1(1):1.
- Meng X, Sang S, Zhu N, et al. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem Res Toxicol. 2002;15(8):1042–1050.10.1021/tx010184a
- Takagaki A, Kato Y, Nanjo F. Isolation and characterization of rat intestinal bacteria involved in biotransformation of (−)-epigallocatechin. Arch Microbiol. 2014;196(10):681–695.10.1007/s00203-014-1006-y
- Appeldoorn MM, Vincken J-P, Aura A-M, et al. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J Agric Food Chem. 2009;57(3):1084–1092.10.1021/jf803059z
- Faria A, Fernandes I, Norberto S, et al. Interplay between anthocyanins and gut microbiota. J Agric Food Chem. 2014;62(29):6898–6902.10.1021/jf501808a
- Konishi Y, Kobayashi S. Microbial metabolites of ingested caffeic acid are absorbed by the monocarboxylic acid transporter (MCT) in intestinal caco-2 cell monolayers. J Agric Food Chem. 2004;52(21):6418–6424.10.1021/jf049560y
- Tsushida T, Suzuki M. Isolation of flavonoid-glycosides in onion and identification by chemical synthesis of the glycosides (flavonoids in fruits and vegetables part 1) (in Japanese). J Jpn Soc Food Sci Technol. 1995;42(2):100–108.10.3136/nskkk.42.100
- Mullen W, Rouanet J-M, Auger C, et al. Bioavailability of [2-14C]Quercetin-4ʹ-glucoside in Rats. J Agric Food Chem. 2008;56(24):12127–12137.10.1021/jf802754s
- Rios LY, Gonthier M-P, Rémésy C, et al. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr. 2003;77(4):912–918.10.1093/ajcn/77.4.912
- Aura AM, O’Leary KA, Williamson G, et al. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J Agric Food Chem. 2002;50(6):1725–1730.10.1021/jf0108056
- Yang J, Qian D, Guo J, et al. Identification of the major metabolites of hyperoside produced by the human intestinal bacteria using the ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. J Ethnopharmacol. 2013;147(1):174–179.10.1016/j.jep.2013.02.029
- Peng X, Zhang Z, Zhang N, et al. In vitro catabolism of quercetin by human fecal bacteria and the antioxidant capacity of its catabolites. Food Nutr Res. 2014;58(1):23406.10.3402/fnr.v58.23406
- Gesi M, Santinami A, Ruffoli R, et al. Novel aspects of dopamine oxidative metabolism (confounding outcomes take place of certainties). Pharmacol Toxicol. 2001;89(5):217–224.10.1034/j.1600-0773.2001.d01-151.x
- Tang Y, Nakashima S, Saiki S, et al. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res Int. 2016;89(Pt 1):716–723.10.1016/j.foodres.2016.09.034
- Monagas M, Khan N, Andrés-Lacueva C, et al. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br J Nutr. 2009;102(2):201–206.10.1017/S0007114508162110
- Rechner AR, Kroner C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb Res. 2005;116(4):327–334.10.1016/j.thromres.2005.01.002
- Verzelloni E, Pellacani C, Tagliazucchi D, et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol Nutr Food Res. 2011;55(Suppl 1):S35–43.10.1002/mnfr.v55.5s
- Liu Y, Kurita A, Nakashima S, et al. 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells. Biosci Biotechnol Biochem. 2017;81(10):1978–1983.10.1080/09168451.2017.1361809
- Nakashima S, Liu Z, Yamaguchi Y, et al. A novel tag-free probe for targeting molecules interacting with a flavonoid catabolite. Biochem Biophys Rep. 2016;7:240–245.
- Heinonen S, Wähälä K, Adlercreutz H. Identification of isoflavone metabolites dihydrodaidzein, dihydrogenistein, 6ʹ-OH-O-DMA, and cis-4-OH-equol in human urine by gas chromatography–mass spectroscopy using authentic reference compounds. Anal Biochem. 1999;274(2):211–219.10.1006/abio.1999.4279
- Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584.10.1093/jn/132.12.3577
- Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med. 2005;230(3):155–170.10.1177/153537020523000302
- Ingram D, Sanders K, Kolybaba M, et al. Case-control study of phyto-oestrogens and breast cancer. Lancet. 1997;350(9083):990–994.10.1016/S0140-6736(97)01339-1
- Heinonen S-M, Hoikkala A, Wähälä K, et al. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects: identification of new metabolites having an intact isoflavonoid skeleton. J Steroid Biochem Mol Biol. 2003;87(4):285–299.10.1016/j.jsbmb.2003.09.003
- Coldham NG, Darby C, Hows M, et al. Comparative metabolism of genistin by human and rat gut microflora: detection and identification of the end-products of metabolism. Xenobiotica. 2002;32(1):45–62.10.1080/00498250110085809
- Hur H-G, Rafii F. Biotransformation of the isoflavonoids biochanin A, formononetin, and glycitein by Eubacterium limosum. FEMS Microbiol Lett. 2000;192(1):21–25.10.1111/fml.2000.192.issue-1
- Setchell KD, Clerici C, Lephart ED, et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81(5):1072–1079.10.1093/ajcn/81.5.1072
- Lundh T. Metabolism of estrogenic isoflavones in domestic animals. Proc Soc Exp Biol Med. 1995;208(1):33–39.10.3181/00379727-208-43828
- Adlercreutz H, Musey PI, Fotsis T, et al. Identification of lignans and phytoestrogens in urine of chimpanzees. Clin Chim Acta. 1986;158(2):147–154.10.1016/0009-8981(86)90230-5
- Ohta A, Uehara M, Sakai K, et al. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J Nutr. 2002;132(7):2048–2054.10.1093/jn/132.7.2048
- Kelly GE, Joannou GE, Reeder AY, et al. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208(1):40–43.10.3181/00379727-208-43829
- Lampe JW, Karr SC, Hutchins AM, et al. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217(3):335–339.10.3181/00379727-217-44241
- Arai Y, Uehara M, Sato Y, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10(2):127–135.10.2188/jea.10.127
- Frankenfeld CL, McTiernan A, Tworoger SS, et al. Serum steroid hormones, sex hormone-binding globulin concentrations, and urinary hydroxylated estrogen metabolites in post-menopausal women in relation to daidzein-metabolizing phenotypes. J Steroid Biochem Mol Biol. 2004;88(4):399–408.10.1016/j.jsbmb.2004.01.006
- Ueno T, Uchiyama S. Identification of the specific intestinal bacteria capable of metabolising soy isoflavone to equol. Ann Nutr Metab. 2001;45(Suppl):114 (abs).
- Yokoyama S-i, Oshima K, Nomura I, et al. Complete genomic sequence of the equol-producing bacterium Eggerthella sp. strain YY7918, isolated from adult human intestine. J Bacteriol. 2011;193(19):5570–5571.10.1128/JB.05626-11
- Maruo T, Sakamoto M, Ito C, et al. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol. 2008;58(Pt 5):1221–1227.10.1099/ijs.0.65404-0
- Tamura M, Tsushida T, Shinohara K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe. 2007;13(1):32–35.10.1016/j.anaerobe.2006.10.001
- Wang XL, Kim HJ, Kang SI, et al. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch Microbiol. 2007;187(2):155–160.10.1007/s00203-006-0183-8
- Decroos K, Vanhemmens S, Cattoir S, et al. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol. 2005;183(1):45–55.10.1007/s00203-004-0747-4
- Schoefer L, Mohan R, Braune A, et al. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol Lett. 2002;208(2):197–202.10.1111/fml.2002.208.issue-2
- Blaut Schoefer. Braune. Transformation of flavonoids by intestinal microorganisms. Int J Vitamin Nutr Res. 2003;73(2):79–87.
- Hur H-G, Beger RD, Heinze TM, et al. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch Microbiol. 2002;178(1):8–12.10.1007/s00203-002-0414-6
- Ohtomo T, Uehara M, Peñalvo JL, et al. Comparative activities of daidzein metabolites, equol and O-desmethylangolensin, on bone mineral density and lipid metabolism in ovariectomized mice and in osteoclast cell cultures. Eur J Nutr. 2008;47(5):273–279.10.1007/s00394-008-0723-x
- Frankenfeld CL. O-Desmethylangolensin: the importance of equol’s lesser known cousin to human health. Adv Nutr. 2011;2(4):317–324.10.3945/an.111.000539
- Uchiyama S, Ueno T, Suzuki T. Identification of a newly isolated equol-producing lactic acid bacterium from the human feces. J Intest Microbiol. 2007;21(3):217–220.
- Uchiyama S, Kimura H, Ueno T, et al. Detection of Lactococcus garvieae in foods and its existence in the human intestine (in Japanese). J Intest Microbiol. 2007;21(3):221–225.
- Wang X-L, Hur H-G, Lee JH, et al. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol. 2005;71(1):214–219.10.1128/AEM.71.1.214-219.2005
- Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors α and β. Bioorg Med Chem. 2004;12(6):1559–1567.10.1016/j.bmc.2003.11.035
- Rowland I, Wiseman H, Sanders T, et al. Metabolism of oestrogens and phytoestrogens: role of the gut microflora. Biochem Soc Trans. 1999;27(2):304.10.1042/bst0270304
- Minamida K, Tanaka M, Abe A, et al. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J Biosci Bioeng. 2006;102(3):247–250.10.1263/jbb.102.247
- Minamida K, Ota K, Nishimukai M, et al. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int J Syst Evol Microbiol. 2008;58(Pt 5):1238–1240.10.1099/ijs.0.64894-0
- Yokoyama S-i. Suzuki, T. Isolation and characterization of a novel equol-producing cacterium from human feces. Biosci Biotechnol Biochem. 2008;72(10):2660–2666.10.1271/bbb.80329
- Yu Z-T, Yao W, Zhu W-Y. Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiol Lett. 2008;282(1):73–80.10.1111/fml.2008.282.issue-1
- Tamura M, Hori S, Nakagawa H. Lactobacillus rhamnosus JCM 2771: impact on metabolism of isoflavonoids in the fecal flora from a male equol producer. Curr Microbiol. 2011;62(5):1632–1637.10.1007/s00284-011-9904-6
- Shimada Y, Yasuda S, Takahashi M, et al. Cloning and expression of a novel NADP(H)-Ddependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus Strain 20-92. Appl Environ Microbiol. 2010;76(17):5892–5901.10.1128/AEM.01101-10
- Shimada Y, Takahashi M, Miyazawa N, et al. Identification of two novel reductases involved in equol biosynthesis in lactococcus strain 20–92. J Mol Microbiol Biotechnol. 2011;21(3–4):160–172.10.1159/000335049
- Shimada Y, Takahashi M, Miyazawa N, et al. Identification of a novel dihydrodaidzein racemase essential for biosynthesis of equol from daidzein in Lactococcus sp. strain 20-92. Appl Environ Microbiol. 2012;78(14):4902–4907.10.1128/AEM.00410-12
- Tsuji H, Moriyama K, Nomoto K, et al. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch Microbiol. 2010;192(4):279–287.10.1007/s00203-010-0546-z
- Tsuji H, Moriyama K, Nomoto K, et al. Identification of an enzyme system for daidzein-to-equol conversion in Slackia sp. Strain NATTS. Appl Environ Microbiol. 2012;78(4):1228–1236.10.1128/AEM.06779-11
- Kawada Y, Yokoyama S, Yanase E, et al. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci Microbiota Food Health. 2016;35(3):113–121.
- Abiru Y, Kumemura M, Ueno T, et al. Discovery of an S-equol rich food stinky tofu, a traditional fermented soy product in Taiwan. Int J Food Sci Nutr. 2012;63(8):964–970.10.3109/09637486.2012.687369
- Tousen Y, Uehara M, Kruger MC, et al. Effects of dietary fibre and tea catechin, ingredients of the Japanese diet, on equol production and bone mineral density in isoflavone-treated ovariectomised mice. J Nutr Sci. 2012;1:e13.10.1017/jns.2012.14
- Fujii S, Takahashi N, Inoue H, et al. A combination of soy isoflavones and cello-oligosaccharides changes equol/O-desmethylangolensin production ratio and attenuates bone fragility in ovariectomized mice. Biosci Biotechnol Biochem. 2016;80(8):1632–1635.10.1080/09168451.2016.1184559
- Tousen Y, Abe F, Ishida T, et al. Resistant starch promotes equol production and inhibits tibial bone loss in ovariectomized mice treated with daidzein. Metabolism. 2011;60(10):1425–1432.10.1016/j.metabol.2011.02.009
- Clavel T, Fallani M, Lepage P, et al. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. J Nutr. 2005;135(12):2786–2792.10.1093/jn/135.12.2786
