ABSTRACT
Cyclooxygenases are responsible for the production of prostaglandin H2 (PGH2) from arachidonic acid. PGH2 can be converted into some bioactive prostaglandins, including prostaglandin F2α (PGF2α), a potent chemical messenger used as a biological regulator in the fields of obstetrics and gynecology. The chemical messenger PGF2α has been industrially produced by chemical synthesis. To develop a biotechnological process, in which PGF2α can be produced by a microorganism, we transformed an oleaginous fungus, Mortierella alpina 1S-4, rich in triacylglycerol consisting of arachidonic acid using a cyclooxygenase gene from a red alga, Gracilaria vermiculophylla. PGF2α was accumulated not only in the mycelia of the transformants but also in the extracellular medium. After 12 days of cultivation approximately 860 ng/g and 6421 µg/L of PGF2α were accumulated in mycelia and the extracellular medium, respectively. The results could facilitate the development of novel fermentative methods for the production of prostanoids using an oleaginous fungus.
Graphical Abstract
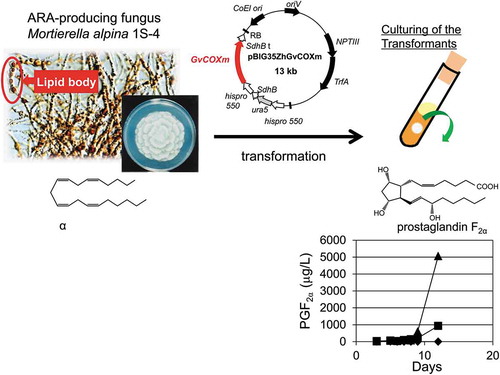
We succeeded in prostaglandin F2α production by molecular breeding of an oleaginous fungus Mortierella alpina using a cyclooxygenase gene from a red alga.
Prostaglandins (PGs) are a group of biologically active lipids derived from C20 polyunsaturated fatty acids, including arachidonic acid (ARA, 20:4ω-6), dihomo-γ-linoleic acid (DGLA, 20:3ω-6), and eicosapentaenoic acid (EPA, 20:5ω-3). They work as hormone-like chemical messengers in homeostatic functions and pathogenic reactions, including inflammatory responses [Citation1]. Broad attention has been paid to the PGs, including prostaglandin F2α (PGF2α), as therapeutic agents. Commercially available PGs, e.g. dinoprost and dinoprostone (PGF2α and prostaglandin E2, respectively), are mostly supplied by organic chemical synthesis [Citation2,Citation3]. However, chemical synthesis requires numerous steps, which results in high PG costs.
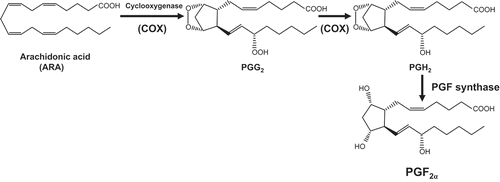
PGF2α is biosynthesized from ARA via the cyclooxygenase (COX) enzyme system in mammals (). The key enzyme, COX (EC. 1. 14. 99. 1), catalyzes two step of reactions in the pathway, in which ARA is first converted into prostaglandin G2 (PGG2) and next PGG2 is reduced to form prostaglandin H2 (PGH2) [Citation4]. PGH2 can be converted into PGF2α by the enzyme PGF synthase as well as into various types of prostaglandins or prostacyclins by specific synthases, which is responsible for various biological activities in PGs.
Figure 1. Schematic diagram of the reactions from ARA to PGF2α in the COX enzyme system.
PGG2, prostaglandin G2; PGH2, prostaglandin H2; PGF synthase, prostaglandin F synthase; PGF2α, prostaglandin F2α.
A COX gene (GvCOX gene) with high activity was recently cloned from a red alga, Gracilaria vermiculophylla [Citation5]. The GvCOX, when expressed in Escherichia coli, functions without any post-translational modification [Citation5–Citation7] and its potential as a catalyst for PG production has been evaluated in in vitro reactions. Following the introduction of the GvCOX gene, transgenic liverworts were generated, which not only catalyzed the production of PGF2α from ARA in vitro reactions but also produced prostaglandins de novo [Citation8]. However, the de novo productivity in the transgenic liverworts is not higher than that in red algae, which naturally produce PGs.
Mortierella alpina is an oleaginous fungus belonging to subphylum Mucoromycotina [Citation9]. The fungus produces lipids rich in triacylglycerols with dry weights that constitute up to 50% ARA, which is commercially used for the fermentative production of ARA [Citation10,Citation11]. We developed a method for transforming the fungus using the gene transferring system of a plant-infecting bacterium, Agrobacterium tumefaciens [Citation12].
In the present study, we introduced the GvCOX gene into M. alpina using the Agrobacterium tumefaciens-mediated transformation (ATMT) method for the fermentative production of PGs.
Materials and methods
Strains and media
M. alpina 1S-4 ura5− strain has been previously described [Citation13]. The uracil auxotroph strain was used as the host strain for the transformation. Media used to culture the host and transformed strains in the present study were SC agar with and without uracil, Czapek–Dox agar supplemented with 0.05 mg/μL of uracil, and GY broth [2% (w/v) glucose and 1% (w/v) yeast extract], which have been previously described [Citation12]. Escherichia coli DH5α was used for DNA manipulation and grown on LB agar [Citation14] containing 50 μg/mL kanamycin.
Plasmid construction
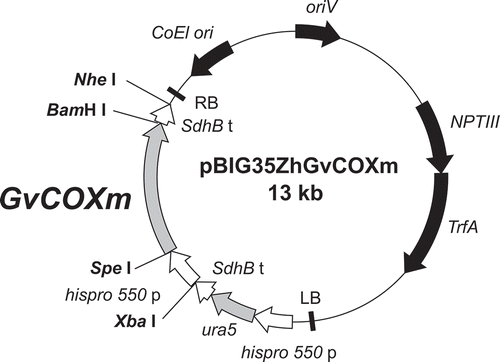
The cyclooxygenase gene of G. vermiculophylla (GvCOX) [Citation5], a red alga, with optimized codon usage to reflect the codon bias in M. alpina obtained from the Kazusa database (http://www.kazusa.or.jp/codon/) was purchased from GENEART (Regensburg, Germany), with additional Spe I and BamH I sites at the 5′ and 3′ flanking open reading frames, respectively. The gene (approx. 1.7 kb) was inserted downstream of hispro550 promoter in plasmid pBIG35ZhGUSm [Citation15] by Spe I and BamH I to give pBIG35ZhGvCOXm ().
Figure 2. Schematic diagram of the plasmid pBIG35ZhGvCOXm with the GvCOX gene downstream of the histone promoter.
GvCOXm, the codon-optimized GvCOX gene for M. alpina; hispro550 p, M. alpina histone protein promoter; SdhB t, M. alpina SdhB gene transcription terminator; ura5, the orotate phosphoribosyl transferase gene of M. alpina 1S-4; NPTIII, neomysin phosphotransferase III gene; TrfA, TrfA locus, which produces two proteins that promote the replication of the plasmid; ColE1 ori, ColE1 origin of replication; oriV, pRK2 origin of replication; RB and LB, the right and left border, respectively, from Ti-plasmid of A. tumefaciens.
Transformation of M. alpina 1S-4 ura5− strain
M. alpina was transformed using the ATMT method as previously described [Citation12]. The colonies that grew on fresh uracil-free SC agar plates were inoculated onto GY broth and then evaluated as follows.
Evaluation of the transformants
The transformants were evaluated for their capacity to catalyze the reaction, in which ARA was converted to prostaglandins in vitro and those of fermentative prostaglandin production intra- and extracellularly.
For the ARA conversion in vitro, the individual transformant was cultivated in GY medium supplemented with 50 mg/L of uracil at 12°C for 12 days. The cultivated cells were collected by filtration with No. 2 filter papers (Advantec, Toyo Roshi Co. Ltd., Tokyo, Japan) and mixed with 30.45 mg/L of ARA (Sigma, St. Louis, MI, USA) in a reaction buffer consisting of 100 mM of Tris-HCl (pH7.5), 5 mM of EDTA, and 1 µM of Hematin. The reaction mixture was incubated at 23°C for 1 hour. Lipids in the reaction mixture were extracted by mixing with ethyl acetate after the addition of 60 ng of PGF2α–d4 as an internal control. The organic phase obtained following centrifugation was transferred into a new tube, and the solvents were removed using a rotary evaporator with a diaphragm vacuum pump. The extracted lipids were resuspended in ethanol and stored at −80°C until they were analyzed using liquid chromatography-mass spectrophotometry (LC-MS) as described later.
For the evaluation of fermentative production, the produced lipids were extracted from the cultured cells and media after cultivated in 15 mL GY medium supplemented with 50 mg/L of uracil at 12ºC for 12 days. The cells and media were separated by filtration with No. 2 filter papers (Advantec, Toyo Roshi Co. Ltd.). The cultured cells were weighed and frozen at −20°C. Chloroform and methanol volumes, double and equal to the flesh weight of the cells, respectively, were added and the solutions were mixed. After extraction at −20°C for 16 hours, an equal volume of water was added, and the solutions were well mixed. The organic phase obtained by centrifugation was transferred into a new tube, and the solvents were removed using a rotary evaporator with a diaphragm vacuum pump. The extracted lipids were resuspended in ethanol and stored at −80°C. The lipids in the filtrated media were extracted by adding twice the volume of the ethylacetate after acidification with hydrochloric acid. The solutions were then mixed well and centrifuged to separate the organic and water phases. The organic phase was evaporated as described above, and the extracted lipids were resuspended in ethanol and stored at −80°C. The extracted lipids were analyzed using LC-MS (LC-MS 2020, Shimadzu, Kyoto, Japan) equipped with a 5C18-AR-II COSMOSIL packed column (4.6 mm i.d. x 150 mm, Nacalai Tesque, Kyoto, Japan). For the column using a 20%–100% acetonitrile gradient in 0.1% formic acid, in which the concentration of acetonitrile was increased to 20%–40% for 10 min, 40%–100% for 10 min, and maintained at 100% for 25 min at a flow rate of 0.2 mL/min with the column temperature set at 40°C. For the mass spectrometry conditions (negative ion mode), the electron spray ionization interface was performed at a heat block temperature of 200°C and a desolvation-line temperature at 250°C. The nebulizer gas flow rate was 1.5 L/min. Quantification was performed using standard calibration lines obtained from analyses of authentic standards. The standard calibration lines were made from the peak area in the MS chromatograms of m/z value 303, 355, 353, 357, and 351, obtained by analyzing multiple concentrations of ARA, PGF1α, PGF2α, PGF2α-d4, and PGF3α, respectively. All PG standards were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). The calculations were done from the data of the samples diluted to the level within the standard lines.
Results and discussions
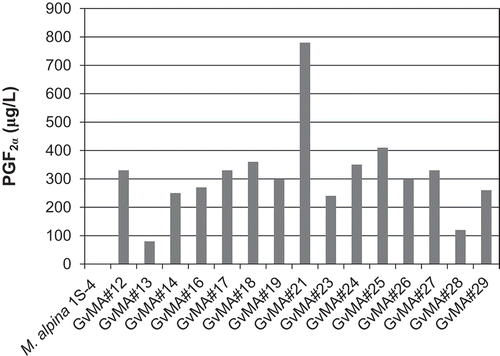
The GvCOX gene was introduced into the M. alpina 1S-4 ura5− strain using the ATMT, in which a single copy of the gene would often be introduced into the genome. A total of 30 transformants grew on the SC without uracil plates. Initially, the COX activity in the transformants was tested in vitro. The COX activity was evaluated based on the amount of PGF2α converted from ARA by the cells obtained from cultures in a similar volume (15 mL) of the medium. The transformants exhibited relatively high activity as shown in . The transformants produced higher PGF2α than the parental strain, which produces trace amounts of PGF2α de novo, indicating that the introduced GvCOX gene conferred to M. alpina the ability to produce PGF2α. The highest concentration of PGF2α was observed in GvMA#21 (approximately 800 μg/L). Notably, the transformants produced PGF2α from ARA without the need of a reducing agent for the conversion of PGH2 into PGF2α following the in vitro reactions. Because several Mortierella species can produce PGs [Citation16], the PGF2α-forming enzyme genes could also be present in M. alpina. Therefore, we evaluated the fermentative PGF2α production by the transformants.
Figure 3. Evaluation of in vitro COX activity in the transformants.
Resting cells were used to convert 30.45 mg/L of ARA to PGF2α as described in Materials and Methods.
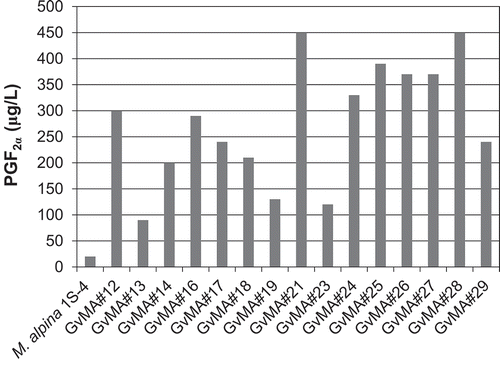
In a previous study, 90% of the total PGs produced de novo by several Mortierella species were found in the culture medium [Citation16]. Consequently, the transformants were initially tested to determine if they released PGF2α into the media during cultivation. All transformants released much higher amounts of PGF2α into the media than the host strain (). Transformant GvMA#21 produced the highest amounts of PGF2α, as predicted based on its in vitro activity. Another transformant, GvMA#28, produced PGF2α at a comparable level to GvMA#21, although GvMA#28 exhibited much lower activity in the in vitro conversion of ARA to PGF2α (). Culturing of GvMA#21 and GvMA#28 for 12 days resulted in a yield of 450 μg/L of fermentative PGF2α production (). The results demonstrate that ARA in the mycelia was converted into PGF2α and released into the media.
Figure 4. Evaluation of fermentative production of PGF2α that was released into the media during the 12-day cultivation of the transformants.
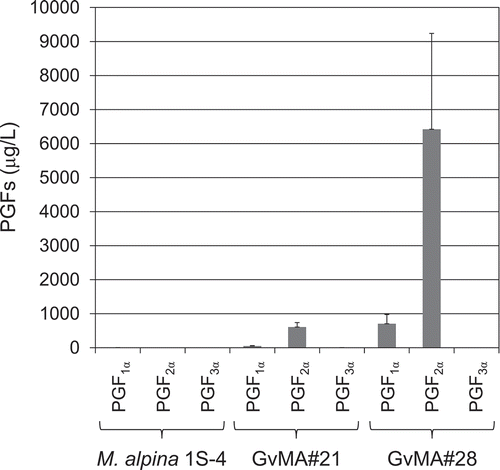
The two transformants, GvMA#21 and GvMA#28, were cultured afresh using similar methods and their fermentative production of PGs in mycelia () and in media () was investigated. A unit weight (1 g) of mycelia of the transformants GvMA#21 and GvMA#28 cultured for 12 days was estimated to contain 500 and 860 ng of PGF2α, respectively (). The mycelia also contained PGF1α and PGF3α at levels of ~10% and ~5% of PGF2α, respectively (). The two PGs were considered to be from DGLA and EPA stored in the mycelia [Citation17], respectively. Additionally, PGF2α was accumulated extracellularly in media with 609 and 6421 μg/L attributed to GvMA#21 and GvMA#28, respectively (). The media also contained PGF1α at a level ~10% of PGF2α but not PGF3α (). The values were calculated as the averages of six samples. To determine the reasons for the differences in PGF2α accumulation in the results that are presented in and , and to achieve more stable and higher production levels, we investigated the kinetics of PGF2α accumulation in the media during the cultivation.
Figure 5. Fermentative intracellular production of prostaglandins F1α, F2α, and F3α in the parental (M. alpina 1S-4) and transformant (GvMA#21 and GvMA#28) strains.
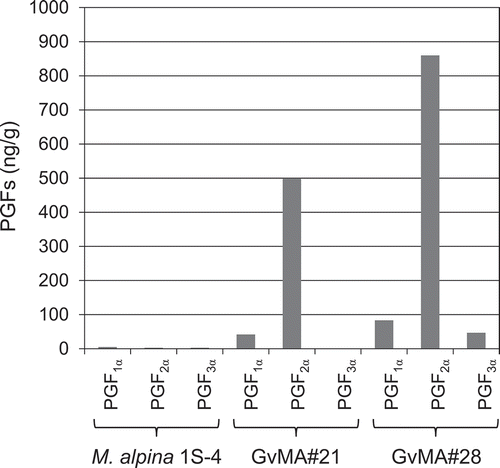
Figure 6. Fermentative extracellular production of prostaglandins F1α, F2α, and F3α in the parental (M. alpina 1S-4) and transformant (GvMA#21 and GvMA#28) strains.
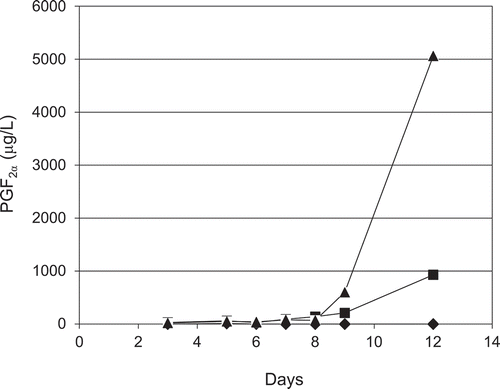
Lipids in filtered media were extracted from the GvMA#21 and GvMA#28 cultures at 3, 5, 7, 9, and 12 days after inoculation, and the kinetics of PGF2α accumulation were investigated (). PGF2α in the cultivation media of the transformants gradually increased from early cultivation days to day 7 and dramatically at day 9 until the end of cultivation, day 12. At day 12, the GvMA#28 culture media contained PGF2α at a 5-fold concentration than that in GvMA#21 (). However, no substantial PGF2α accumulation was observed in the media from the wildtype culture during the experimental period. The considerable change in the rate of PGF2α accumulation might be caused by some changes that could have influenced metabolism rates during cultivation, e.g., depletion of nutrients. Similar to other COXs, GvCOX prefers free fatty acids as a substrate [Citation6]. However, free ARA is present in trace amounts in M. alpina whereas most ARA molecules are esterified in triacylglycerol. Therefore, it is probable that the GvCOX-mediated reactions would be under the influence of a lipid-controlling mechanism in M. alpina, including the release of free ARA. Activation of such mechanisms or conferring a mechanism for the release of free ARA would enhance PGF2α production. In addition, the de novo accumulation of the PGF2α in the culturing media as well as in the mycelia strongly suggests that PGF2α could be produced from the COX product, PGG2/PGH2, by endogenous PGF2α-forming enzymes and exported to the media. Further studies will be required to elucidate such elements that influence PGF2α production in M. alpina transformants using the GvCOX gene. We demonstrated that the transformation of M. alpina by the GvCOX gene facilitated fermentative production of PGF2α. The fermentative system was considered as a high potential system that could be used to produce PGF2α, in which PGF2α could be obtained from extracellular media.
Figure 7. Time course analysis of extracellular PGF2α production in 12 days of cultivation by the parental (◆, M. alpina 1S-4) and transformant (■, GvMA#21 and ▲, GvMA#28) strains.
It is likely that the amount of GvCOX influences transformant activity as demonstrated in the transformed liverworts [Citation8]. In the present study, GvCOX expression levels were not evaluated. Because stronger promoters have been found in M. alpina, e.g., pp3p or pp6p [Citation15],, the effects of the substitution of the hispro550 promoter with stronger promoters are currently under evaluation for the enhancement of PGF2α production.
Author contribution
A. Ando and J. Ogawa designed research. Y. Shimada, H. Nagano, F.A. Mohd Fazli, and K. Munesato performed research. M. Takemura gave scientific advices. M. Takeuchi, A. Ando, and J. Ogawa analyzed data. F.A. Mohd Fazli, H. Nagano, A. Ando, and J. Ogawa wrote the manuscript. All authors discussed results and commented on the manuscript.
Acknowledgments
We are grateful to Dr. Kanamoto (Res. Ins. Biore. Biotech., Ishikawa Pref. Univ.) for the gift of a plasmid cloned the cyclooxygenase gene from Gracilaria vermiculophylla.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Samuelsson B, Goldylne M, Granstrom E, et al. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029.
- Corey EJ, Schaaf TK, Huber W, et al. Total synthesis of prostaglandins F2α and E2 as the naturally occurring forms. J Am Chem Soc. 1970;92:397–398.
- Noyori R, Suzuki M. Organic synthesis of prostaglandins: advanced biology. Science. 1993;259:44–45.
- Sigal E. The molecular biology of mammalian arachidonic acid metabolism. Am J Physiol. 1991;260:13–28.
- Kanamoto H, Takemura M, Ohyama K. Identification of a cyclooxygenase gene from the red alga Gracilaria vermicuphylla and bioconversion of arachidonic acid to PGF2α in engineered Escherichia coli. Appl Genet Mol Biotech. 2011;91:1121–1129.
- Varvas K, Kasvandik S, Hansen K, et al. Structural and catalytic insights into the algal prostaglandin H synthase reveal atypical features of the first non-animal cyclooxygenase. Biochem Biophys Acta. 2013;1831:863–871.
- Guder JC, Buchhaupt M, Huth I, et al. Biotechnological approach towards a highly efficient production of natural prostaglandins. Biotechnol Lett. 2014;36:2193–2198.
- Takemura M, Kanamoto H, Nagaya S, et al. Bioproduction of prostaglandins in a transgenic liverwort, Marchantia polymorpha. Transgenic Res. 2013;22:905–911.
- Hibbett DS, Binder M, Bischoff JF, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007;111:509–547.
- Sakuradani E, Shimizu S. Single cell oil production by Mortierella alpina. J Biotechnol. 2009;144:31–36.
- Wang L, Chen W, Feng Y, et al. Genome characterization of the oleaginous fungus Mortierella alpina. PLoS One. 2011;6:e28319.
- Ando A, Sumida Y, Negoro H, et al. Establishment of Agrobacterium tumefaciens-mediated transfomation of an oleaginous fungus, Mortierella alpina 1S-4, and its application for eicosapentaenoic acid producer breeding. Appl Environ Microbiol. 2009;75:5529–5535.
- Takeno S, Sakuradani E, Murata S, et al. Cloning and sequencing of the ura3 and ura5 genes, and isolation and characterization of uracil auxotrophs of the fungus Mortierella alpina 1S-4. Biosci Biotechnol Biochem. 2004;68:277–285.
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300.
- Okuda T, Ando A, Sakuradani E, et al. Selection and characterization of promoters based on genomic approach for the molecular breeding of oleaginous fungus Mortierella alpina 1S–4. Curr Genet. 2014;60:183–191.
- Lamačka M, Šajbidor J. The content of prostaglandins and their precursors in Mortierella and Cunninghamella species. Lett Appl Microbiol. 1998;26:224–226.
- Kikukawa H, Sakuradani E, Ando A, et al. Arachidonic acid production by the oleaginous fungus Mortierella alpina 1S-4: A review. J Adv Res. 2018;11:15–22.
