ABSTRACT
The immunosuppressive activity of myriocin (ISP-1), a lead compound of fingolimod (FTY720), is derived from its 2-amino-1,3-propandiol structure. A non-proteinogenic amino acid, (2S,6R)-diamino-(5R,7)-dihydroxy-heptanoic acid (DADH), that contains this structure, was recently identified as a biosynthetic intermediate of a dipeptide secondary metabolite, vazabitide A, in Streptmyces sp. SANK 60404; however its effect on adaptive immunity has not yet been examined. In this study, we examined whether DADH suppresses mixed lymphocyte reaction using mouse bone marrow-derived dendritic cells (BMDCs) and allogeneic splenic T cells. Although T cell proliferation induced by cross-linking CD3 and CD28 were not suppressed by DADH unlike ISP-1, the pre-incubation of BMDCs with DADH but not ISP-1 significantly decreased allogeneic CD8+ T cell expansion. Based on these results, we concluded that DADH suppresses DC-mediated T cell activation by targeting DCs.
Graphical abstract
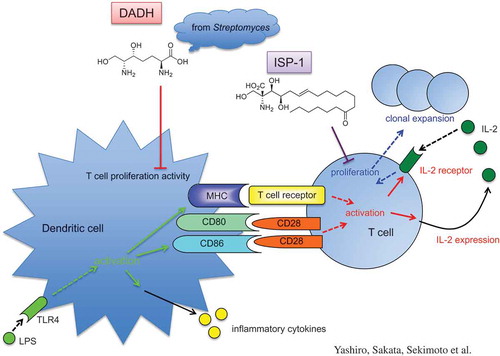
DADH suppresses T cell proliferation activity of dendritic cells, whereas ISP-1 targets T cells directly.
KEYWORDS:
Recently, non-proteinogenic amino acid (2S,6R)-diamino-(5R,7)-dihydroxy-heptanoic acid (DADH) was found to be a biosynthetic intermediate of a secondary metabolite, vazabitide A in Streptmyces sp. SANK 60404 during the research on amino-group carrier protein-mediated biosynthetic pathways [Citation1], which was originally found in the biosynthetic pathway of lysine of thermophilic prokaryotes [Citation2,Citation3]. DADH contains a 2-amino-1,3-propandiol structure that is essential for the immunosuppressive activity of myriocin (ISP-1), which was initially isolated from Isaria sinclairii (a member of Cordyceps) as an immunosuppressive compound by a screening process using mixed lymphocyte reaction [Citation4,Citation5]. ISP-1 functions as an analog of sphingosine due to its 2-amino-1,3-propandiol moiety that has a structure similar to that of the phosphorylation site in sphingosine [Citation5]. Sphingosine converts to sphingosine 1-phosphate (S1P) after phosphorylation by a sphingosine kinase, SphK [Citation6]. Moreover, fingolimod (FTY720), which is a chemical derivative of ISP-1, exhibits agonistic function for S1P1 (a receptor for S1P) after phosphorylation by SphK in vivo. S1P1 activation by FTY720 decreases the number of circulating T and B cells [Citation7–Citation10].
Prior to the finding its immunosuppressive effect, ISP-1 was identified as an antibiotic agent [Citation11,Citation12]. As for DADH, biological activity of DADH against plant, mammalian cell lines, and microbes was examined [Citation1], and it was shown that DADH inhibits hypocotyl elongation of Arabidopsis thaliana, but does not exhibit cytotoxic or antimicrobial activity against any of tested mammalian cells and microbes, respectively. The structural feature of DADH is that it contains a 2-amino-1,3-propandiiol structure essential for the immunosuppressive activity of ISP-1; however, the effect of DADH on immunoresponses has not yet been examined. Therefore, in the present study, we evaluated the effects of DADH on allogeneic activation of T cells and found that DADH may suppress the proliferation of T cells by modulating DC function.
Materials and methods
Mice and cells
BALB/c and C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan). BMDCs were prepared as previously described [Citation13]. Whole T cells were purified from splenocytes using a Pan T cell isolation kit II (mouse) and an auto MACS Pro Separator (both from Miltenyi Biotec, Tubingen, Germany). All animal experiments were performed according to the approved guidelines of the Institutional Review Board of Tokyo University of Science. This study was specifically approved by the Animal Care and Use Committee of Tokyo University of Science.
Compounds
DADH was prepared in a manner as previously described [Citation1]. ISP-1 was purchased from Cayman (#63,150).
T cell proliferation activity in mixed lymphocyte reaction
BMDCs were subjected to allogeneic T cell proliferation assays as follows. Briefly, BMDCs pre-incubated in the presence or absence of 1 µg/mL LPS (#L3024, Sigma-Aldrich) for 6 h were treated with mitomycin C (MP Biomedicals, Solon, OH) for 30 min. After washing with PBS 3 times, the harvested DCs (1 x 105 cells) were co-cultured with 1 × 105 T cells obtained from allogeneic mice spleens for 72 h. BrdU was added to the media in the last 2 h of co-culture between T cells and DCs. The uptake of BrdU in the nucleus was determined by a Cell Proliferation ELISA BrdU (colorimetric) (#11-647-229-001, Roche).
Stimulation of T cells by anti-CD3 Ab and anti-CD28 Ab
Each well of 96-well plate was coated with 200 µL PBS containing 1 µg/mL anti-CD3 Ab (#145-2C11, TONBO) and 1 µg/mL anti-CD28 Ab (#37.51, TONBO). Pan T cells (1 x 105 cells) were cultured in coated wells for 72 h.
Flow cytometric analysis
T cells were stained with APC-labeled anti-CD4 (GK1.5) Ab (#100412, BioLegend) and PE/Cy5-labeled anti-CD8α (53–6.7) Ab (#55–0081-U025, TONBO, Japan) after blocking of FcγRs with non-labeled anti-CD16/CD32 purified (2.4G2) Ab (#70–0161-U500, TONBO). The cells were subsequently analyzed by MACS Quant (Miltenyi Biotec).
Cell surface expression level of MHC class II and CD86 was determined by flow cytometry as in our previous reports [Citation14,Citation15]. FITC-labeled anti-mouse H-2 (#125507, BioLegend) was used to stain MHC class I.
Statistical analysis
We performed Tukey-Kramer test for comparing multiple samples. P values <0.05 were considered significant.
Results and discussion
DADH suppresses allogeneic T cell proliferation
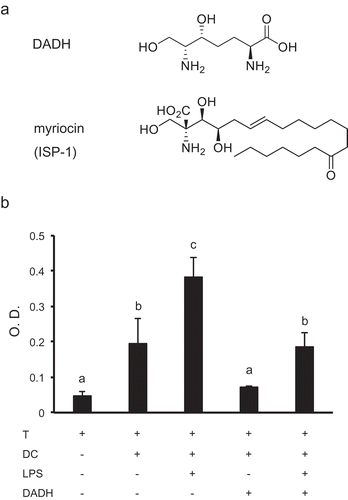
ISP-1 suppresses allogeneic T cell proliferation without reducing IL-2 production unlike immunosuppressive agents such as cyclosporin A and tacrolimus [Citation4]. DADH contains the 2-amino-1,3-propandiol structure that is essential for the immunosuppressive activity of ISP-1 (). However, the effect of DADH on immunoregulation has not so far been examined. Therefore, we examined the effect of DADH on allogeneic T cell proliferation. T cells isolated from C57BL/6 splenocytes that were co-cultured with BALB/c BMDCs had significantly higher proliferation rates, which were further accelerated by DC stimulation by LPS (). However, the proliferation was significantly decreased by DADH addition into culture media (), suggesting that DADH has suppressive effects on allogeneic T cell proliferation in mixed lymphoid reaction.
Figure 1. Effect of DADH on allogeneic T cell proliferation.
(a) Structures of DADH and myriocin (ISP-1). (b) Allogeneic DC-mediated proliferation of T cells. Pan T cells (C57BL/6) were co-cultured with BMDCs (BALB/c) in the presence or absence of 50 µg/mL (270 µM) DADH. O.D. refers to the uptake of BrdU in the nucleus of T cells. The results are shown as mean ± S.D. (n = 3). Similar results were obtained in two other independent experiments. Means indicated by different small letters are significantly different, based on Tukey-Kramer (p < 0.05).
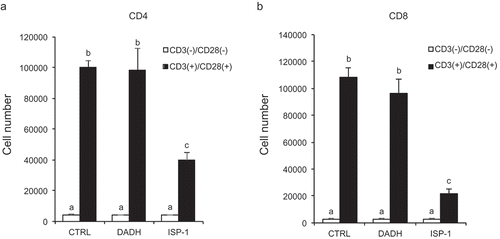
DADH does not suppress CD3- and CD28-mediated T cell proliferation
To further evaluate the effect of DADH on T cell proliferation, we stimulated T cells by cross-linking both T cell receptors and CD28 with using plate-coated anti-CD3 Ab and anti-CD28 Ab, respectively, instead of using antigen-presenting cells (APCs). Flow cytometric analysis showed that the proliferation of both CD4+ T and CD8+ T cells was induced by Ab-mediated stimulation (control in ). Proliferations of both CD4+ and CD8+ T cells decreased in the presence of ISP-1, which suppresses IL-2-dependent T cell proliferation [Citation4] and induces apoptosis in IL-2-dependent T cells [Citation16]. In contrast, DADH did not affect the Ab-mediated proliferation of T cells (). These results suggest that the mechanism of suppression of DC-mediated T cell proliferation by DADH differs from that of ISP-1, which targets T cells directly.
Figure 2. Effect of DADH and ISP-1 on T cell proliferation induced by plate-coated Abs.
Purified splenic whole T cells were stimulated by plate-coated anti-CD3 Ab and anti-CD28 Ab.Number of CD4+ (a) and CD8+ T cells (b) was determined by flow cytometric analysis. The results are shown as mean ± S.D. (n = 3). Similar results were obtained in two other independent experiments. DADH (50 µg/mL = 270 µM) and ISP-1 (10 nM) was used with the concentration that exhibits suppressive effect in mixed lymphocyte reaction.Open bars, without stimulation; closed bars, stimulated with plate-coated Abs against CD3 and CD28. Means indicated by different small letters are significantly different, based on Tukey-Kramer (p < 0.05).
Effect of DADH on T cell proliferation activity of DCs
The above results suggested that the suppressive effects of DADH and ISP-1 on allogeneic T cell proliferation occur through different pathways. To confirm whether DADH modulates the function of DCs, we co-cultured freshly isolated T cells with allogeneic BMDCs that were pre-treated with DADH. As shown in , T cell proliferation activity of DCs was accelerated by stimulation with LPS. When DADH-treated BMDCs were used as APCs, the degree of T cell proliferation induced by LPS-stimulated DCs significantly decreased compared with LPS-stimulated control BMDCs (). In this experimental condition, the expansion of T cells was preferentially observed in CD8+ T cells, which was significantly decreased when DCs pre-treated with DADH were used as APCs (). Although we cannot show the effect of DADH on CD4+ T cell expansion caused by LPS-stimulated BMDCs in this experimental condition and cannot explain the mechanism, we suggest that DADH suppresses CD8+ T cell proliferation activity of DCs.
Figure 3. Suppressive effect of DADH on T cell proliferation activity of DCs.
(a) BrdU uptake into T cells co-cultured with DADH-treated BMDCs.
(b) Number of CD4+ T cells and CD8+ T cells co-cultured with DADH-treated BMDCs.
(c) Number of CD4+ T cells and CD8+ T cells co-cultured with ISP-1-treated BMDCs.
Open bars, number of CD4+; closed bars, number of CD8+ (B and C).
BMDCs were pre-incubated with DADH (a and b) or ISP-1 (c) for 42 h and incubated for an additional 6 h in the presence or absence of LPS. Number of T cells was determined by flow cytometric analysis. The results are shown as means ± S.D.s (n = 3). Similar results were obtained in two other independent experiments. Means indicated by different small letters (a), or small letters for CD4 and capital letters for CD8 (b and c) are significantly different, based on Tukey-Kramer (p < 0.05).
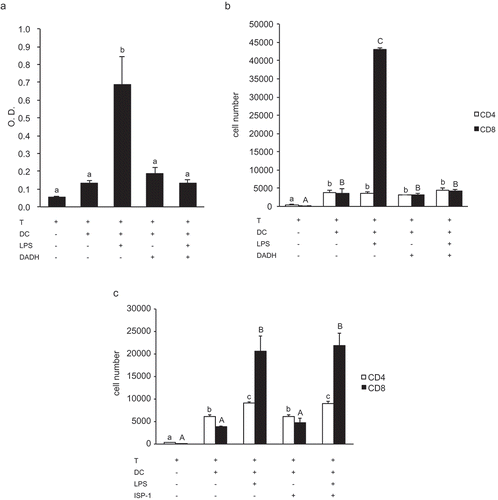
Effect of ISP-1 on T cell proliferation activity of DCs
ISP-1 is an immunosuppressive reagent with similar 2-amino-1,3-propadiol structure. This prompted us to clarify whether ISP-1 exhibits immunosuppressive activity targeting DCs in a similar manner to DADH. The DC-mediated expansion of T cells was not affected by pre-treatment of DCs with ISP-1 (). This indicates that the suppression of allogeneic T cell proliferation via modulation of DC function is a specific characteristic of DADH.
Cell surface expression levels of MHC molecules on DCs
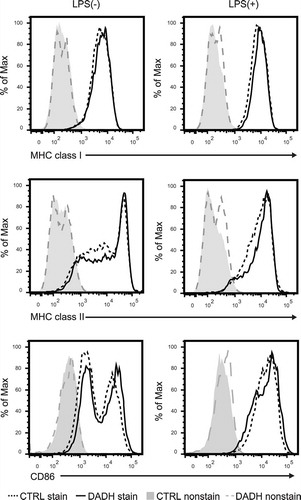
Interactions between MHC molecules on DCs and T cell receptors on T cells are required to stimulate naïve T cells. Furthermore, stimulation by LPS induces antigen-presenting activity of DCs by up-regulating the expression of MHC molecules. To clarify whether DADH reduces cell surface expression of MHC molecules on DCs, we quantified these molecules on the surface of DCs by flow cytometric analysis. As shown in , the expression levels of MHC class I and class II molecules on immature or stimulated DCs were not affected by DADH. We also found that DADH did not affect the expression level of co-stimulatory molecule CD86 (), but rather enhanced the transcription of inflammatory cytokines such as IL-6, TNF-α, and IL-12b (supplemental ). DADH may affect a regulatory network that enhancing the production of inflammatory cytokines; however, the lack of the information makes the molecular target of DADH in DCs remain unknown.
Figure 4. Expression levels of MHC and co-stimulatory molecules on DCs.
Cell surface expression levels of MHC class I, MHC class II, and CD86 molecules were determined by flow cytometric analysis. BMDCs were incubated in the presence (DADH) or absence (CTRL) of DADH for 42 h and then treated with (right panel) or without (left panel) LPS for an additional 6 h. stain, stained with specific Abs; nonstain, without Ab-treatment. Typical profiles are shown, and similar results were observed in two independent experiments.
DADH suppressed T cell proliferation induced by non-stimulated (without LPS) BMDCs in , but not in . Although we cannot explain the mechanism by which this discrepancy is caused, further analysis in the experimental condition that the degree of non-stimulated DC-mediated T cell proliferation is higher than that in this study may clarify this issue.
In the current study, we found that DADH suppressed allogeneic CD8+ T cell proliferation by modulating DC function, which was not observed in ISP-1. These results may enable the development of novel immunomodulators that target DCs.
It is well known that FTY720, a chemical derivative of ISP-1, acquires agonistic function for S1P1 after phosphorylation in vivo. Considering the possibility of in vivo phosphorylation of DADH, experiments using in vivo system should be performed to clarify the function of DADH in regulation of immunoresponse.
In a preliminary experiment, we found that 3 i.p. administrations of approximately 10 mg/kg DADH did not show toxicity in mice. However, detailed analyses showing the mechanisms by which DADH modulates the function of DCs are required to determine whether DADH can be used in immunoregulation.
Abbreviations
BM: bone marrow
DADH: (2S,6R)-diamino-(5R,7)-dihydroxy-heptanoic acid
DCs: dendritic cells.
Author Contribution
T. Y., F. S., and T. Sekimoto performed experiments and analyzed data. T. Shirai, F. H., K. M., S. K., and S. S. performed experiments. K. N. and K. K. analyzed data. M. N. designed research. C. N. designed research and wrote the paper.
DADH-Supplemental_Fig-h.docx
Download MS Word (50 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary materials
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Hasebe F, Matsuda K, Shiraishi T, et al. Amino-group carrier-protein-mediated secondary metabolite biosynthesis in Streptomyces. Nat Chem Biol. 2016;12:967–972.
- Horie A, Tomita T, Saiki A, et al. Discovery of proteinaceous N-modification in lysine biosynthesis of Thermus thermophilus. Nat Chem Biol. 2009;5:673–679.
- Ouchi T, Tomita T, Horie A, et al. Lysine and arginine biosyntheses mediated by a common carrier protein in Sulfolobus. Nat Chem Biol. 2013;9:277–283.
- Fujita T, Inoue K, Yamamoto S, et al. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo). 1994;47:208–215.
- Miyake Y, Kozutsumi Y, Nakamura S, et al. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun. 1995;211:396–403.
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150.
- Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897.
- Wei SH, Rosen H, Matheu MP, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235.
- Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553.
- Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360.
- Kluepfel D, Bagli J, Baker H, et al. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J Antibiot (Tokyo). 1972;25:109–115.
- Aragozzini F, Craveri R, Scolastico C, et al. Isolation and structure determination of a new antifungal alpha-hydroxymhthyl-alpha-amino acid. Tetrahedron. 1972;28:5493.
- Kitamura N, Yokoyama H, Yashiro T, et al. Role of PU.1 in MHC class II expression through transcriptional regulation of class II transactivator pI in dendritic cells. J Allergy Clin Immunol. 2012;129(814–824):e816.
- Nagaoka M, Yashiro T, Uchida Y, et al. The orphan nuclear receptor NR4A3 is involved in the function of dendritic cells. J Immunol. 2017;199:2958–2967.
- Yashiro T, Hara M, Ogawa H, et al. Critical role of transcription factor PU.1 in the function of the OX40L/TNFSF4 promoter in dendritic cells. Sci Rep. 2016;6:34825.
- Nakamura S, Kozutsumi Y, Sun Y, et al. Dual roles of sphingolipids in signaling of the escape from and onset of apoptosis in a mouse cytotoxic T-cell line, CTLL-2. J Biol Chem. 1996;271:1255–1257.
