 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Executive function deficits are often reported as a specific weakness in preterm children. Yet, executive function development is still not fully understood. In a prospective longitudinal study, 115 preterm born children, ≤31 weeks of gestation, were recruited at birth and subject to neuropsychological assessments at ages 5.5 and 18 years. By applying Miyake and colleagues’ integrative framework of executive function to our data, two core components of executive function, working memory and cognitive flexibility, were identified through confirmatory factor analysis. Developmental stability was investigated in a serial multiple mediator structural equation model. Biological, medical, and social factors as well as mental development at 10 months were entered as predictors. Both components of executive function were highly stable from 5.5 to 18 years. Gestational age, intrauterine growth, lack of perinatal medical complications, and female sex were positively related to mental development at 10 months, which together with parental education influenced both core executive functions at 5.5 years. Working memory at 5.5 years mediated outcome in working memory at 18 years. In addition to the mediation of cognitive flexibility at 5.5 years, perinatal medical complications and restricted intrauterine growth had a continued direct negative impact on cognitive flexibility at 18 years. The application of a theoretical framework added to our understanding of executive function development in preterm born children. The study supports early identification of executive deficits among children born preterm, as deficits are unlikely to diminish with maturation.
It is well established that preterm birth is associated with negative neurodevelopmental consequences, and that children born very (i.e., ≤31 weeks) and, in particular, extremely (≤27 weeks) preterm are most at risk (Pascal et al., Citation2018; Serenius et al., Citation2016). In the last four decades, the overall survival rates among children born very or extremely preterm have improved greatly as a result of enhancements in neonatal medicine and the centralization of care to specialized neonatal units (Wyatt, Citation2010). Improved survival has been most dramatic among extremely preterm infants (Fellman et al., Citation2009). Advances in neonatal care have also changed the spectrum and rates of morbidity among preterm children (Wyatt, Citation2010). Major disabilities, when present, are usually identified early in development and include moderate or severe intellectual disability, cerebral palsy, visual or auditory impairments, and epilepsy. Milder neurocognitive deficits, which are much more common, will typically become obvious with age as the child falls short of expected development and is challenged by increasing demands. These more subtle dysfunctions are found in 50–70% of children born very preterm (Aylward, Citation2010), and may affect long-term behavioral, educational, and social outcome (Vohr, Citation2010).
Cognitive deficits are related to lower gestational age at birth (Bhutta, Cleves, Casey, Cradock, & Anand, Citation2002), restricted intrauterine growth (Lundequist, Böhm, Forssberg, Lagercrantz, & Smedler, Citation2015; Raz, Debastos, Newman, & Batton, Citation2012), serious perinatal medical complications (Roze et al., Citation2009; Vohr et al., Citation2000), and male sex (Ingemarsson, Citation2003). By contrast, environmental factors such as higher socioeconomic conditions and parental education are related to better outcome (Manley et al., Citation2015; Taylor, Clayton, & Rowley, Citation2004). It is also striking that, in spite of these well-established group differences, the variability is quite large, and individual children of similar background and medical history may exhibit very different outcomes (Anderson, Howard, & Doyle, Citation2010; Lundequist, Böhm, & Smedler, Citation2013; Stålnacke, Lundequist, Böhm, Forssberg, & Smedler, Citation2015).
As a group, children born very or extremely preterm have lower cognitive abilities than their peers born at term (Anderson, Citation2014; Johnson, Citation2007; Taylor, Burant, Holding, Klein, & Hack, Citation2002; Vohr et al., Citation2000). Meta-analyses of general cognitive outcome, typically reported as IQ, have found mean score differences of 0.7–0.8 standard deviations (SDs) between preterm and term born children (Bhutta et al., Citation2002; Kerr-Wilson, Mackay, Smith, & Pell, Citation2011). The difference seems to persist into adolescence and adulthood (Allin et al., Citation2008; Hack, Citation2009; Hack et al., Citation2002; Kormos, Wilkinson, Davey, & Cunningham, Citation2014; Lundequist et al., Citation2015). IQ provides a relevant measure of the overall cognitive level. However, in order to better understand outcome and take individual variability into account, more specific cognitive measures are required. Aside from below average IQ, cognitive deficits reported in children born preterm include visual-motor problems, memory deficits, delayed language skills, and, not least, executive dysfunctions (Anderson et al., Citation2010; Taylor & Clark, Citation2016).
Executive function is an umbrella term for a set of cognitive processes that are important for active and purposeful regulation of thought, emotion, and behavior (Anderson, Citation2008; Diamond, Citation2013). These processes are especially important in novel situations or when automatic responses are maladaptive (Anderson, Anderson, Jacobs, & Spencer-Smith, Citation2008; Diamond, Citation2013), and even a subtle depreciation in executive function may influence cognitive, social, and academic performance (Moffitt et al., Citation2011). A universally accepted theoretical model of executive function is still lacking, but inhibition, working memory, and cognitive flexibility are generally regarded as core components of executive function (e.g., Diamond, Citation2013; Miyake et al., Citation2000). These core functions are not independent of one another. Existing theoretical models of executive function have typically been formulated based on empirical data from adults. It is only in the past two to three decades that typical development of executive function as well as the effects of early brain insult on these functions have been systematically researched, (Anderson, Northam, Hendy, & Wrennall, Citation2001; Garon, Bryson, & Smith, Citation2008). It is still not fully understood to what extent models of executive functions based on adult data are applicable to children, who are in a continuous and dynamic process of cerebral maturation and functional development (Anderson, Citation2002; Best & Miller, Citation2010).
Miyake and collaborators (Citation2000) have proposed an integrative framework of executive function, which has been proved useful also in exploring typical executive function in children (Best & Miller, Citation2010; Garon et al., Citation2008). This framework posits three clearly distinguishable executive functions: inhibition, shifting (cognitive flexibility), and updating (working memory), which share an underlying common factor. In a more recent empirical test of the model, Miyake and Friedman found that inhibition did not explain variance over and above the common factor, leading them to revise the model accordingly (Miyake & Friedman, Citation2012). The revised model consists of a common executive function factor, which includes inhibition and executive attention, as well as a shifting specific and an updating specific factor. Moreover, in a previous investigation of executive function development in children, Miyake’s integrative framework was cross-sectionally tested on four age groups of children (Huizinga, Dolan, & van der Molen, Citation2006). Support was found for the latent factors of working memory and set-shifting, but not for inhibition – which is in line with Miyake and Friedman’s revised model.
There is a growing consensus that children born very or extremely preterm are at risk of executive deficits, over and beyond the risk for lower general cognitive ability. However, studies of executive function in children born preterm vary in sample selection and chosen outcome measures, and many questions regarding the specifics of these deficits warrant further study (Anderson, Citation2014). Two meta-analyses have investigated slightly differing aspects of executive function in children born preterm (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, Citation2009; Mulder, Pitchford, Hagger, & Marlow, Citation2009). Overall, the meta-analyses give at hand that executive functions were significantly weaker in the preterm group compared to controls, with effect sizes varying from small to large. Whenever reported in the primary studies, executive function was positively related to gestational age; in other words, the children born most preterm were most likely to display clinically significant executive deficits.
These deficits may remain in adolescence and young adulthood, according to the relatively few long-term follow-up studies presented to date. It has been suggested that executive dysfunction may mediate the relationship between preterm birth and below average academic achievement and social competence in young adulthood reported in the literature (Anderson et al., Citation2010; Burnett, Scratch, & Anderson, Citation2013; Kroll et al., Citation2017; Luu, Ment, Allan, Schneider, & Vohr, Citation2011). However, studies presented so far are inconclusive (Taylor & Clark, Citation2016). Nosarti and colleagues reported that among children born very preterm in the late 1970s and early 1980s, executive function deficits, identified at school age, persisted into young adulthood. The deficits were unrelated to perinatal medical complications (Nosarti et al., Citation2007). In contrast, Saavalainen and colleagues (Citation2007) found that in adolescents born preterm, working memory was at the same level as peers born at term. These results were contradicted by a report from Wilson-Ching and collaborators (Citation2013), who found that attention remained a weakness at age 17 for extremely preterm adolescents, and that the association with perinatal medical complications was limited. Likewise, in our cohort, executive function remained a specific weakness at age 18 in the extremely preterm group, and this was exacerbated by, but not dependent on, perinatal medical complications (Lundequist et al., Citation2015). Given these limited and slightly diverging findings, there is a need to study executive function development in children born very or extremely preterm in a longitudinal design.
Across studies, there is a great variation in operationalization and choice of tasks in measuring executive function. The results reported thus represent different aspects of executive and often also nonexecutive functions (Howard, Anderson, & Taylor, Citation2008). Most executive function tasks also tax visual-motor skills, as well as information processing efficiency and speed (Anderson, Citation2008; Cepeda, Blackwell, & Munakata, Citation2013), and more complex executive function tasks may be almost indistinguishable from tasks intended to assess fluid intelligence (Blair, Citation2006; Diamond, Citation2013). In sum, measurements of executive functions overlap significantly with measurements of intelligence, and are also influenced by speed and attention proficiency. Therefore, leading researchers in the field have argued that it is not meaningful to control for intelligence when assessing executive function, especially not for developing children and in clinical samples. In effect, controlling for intelligence will also by and large wash out actual differences in executive function (Dekker & Karmiloff-Smith, Citation2011; Dennis et al., Citation2009).
Within the Stockholm Neonatal Project (SNP), a cohort of preterm children has been followed prospectively from birth to 18 years of age. Comprehensive neuropsychological assessments were conducted at age 5.5 and 18 years. From this cohort, we have previously reported that executive function was a specific weakness at 5.5 years and remained so still at 18 years (Böhm, Smedler, & Forssberg, Citation2004; Lundequist et al., Citation2015). We now seek to further investigate executive function development in this cohort, leaning on Miyake and Friedman’s theoretical model (Citation2012) and the empirical support lent to it by Huizinga and colleagues (Citation2006). Specifically, using a latent variable approach, we are investigating two core aspects of executive function.
The aim of this study was to investigate executive function development, specifically
The stability and differentiation in working memory and cognitive flexibility, from preschool age to late adolescence, in children born very or extremely preterm.
To what extent gestational age at birth, intrauterine growth, sex, perinatal medical complications, level of parental education, and mental development at 10 months influence executive function at 5.5 and 18 years.
Method
Participants
The data emanate from the SNP, which is a longitudinal prospective population-based study of children born preterm (delivery before 36 completed weeks of gestation) and with a birth weight less than 1500 g during the years 1988–1993 in Stockholm. Neuropsychological assessment data at ages 5.5 and 18 years, as well as demographic and medical data from the perinatal period and an assessment of mental development at age 10 months (corrected age) were included in the present analyses. The original cohort consisted of 291 preterm born infants recruited at birth, of which 236 survived their first year of life. The assessment at age 5.5 years included 182 participants of which 134 (75%) also completed the assessment at 18 years. In addition, at age 5.5 years, a matched control group was recruited and assessed. The controls were children born ≥37 weeks of gestation, with a birth weight ≥2500 g, classified as healthy babies at birth. Ninety-four term-born controls participated and completed the assessments at both age 5.5 and 18 years.
In this study, 115 participants born very or extremely preterm, that is, at a gestational age ≤31 weeks, were included, and compared to term-born peers (n = 94). Nineteen participants born moderately preterm, after 32 to <37 weeks of gestation, were not included in these analyses. As a result of the very low birth weight inclusion criterion for the SNP cohort (<1500 g), all of the moderately preterm children had suffered intrauterine growth restriction, presumably in late gestation, and demonstrated cognitive deficits of a different pattern and background than the cohort at large (Lundequist et al., Citation2015). Participant characteristics are displayed in . The cohort, attrition analyses, and assessments have been presented in greater depth in previous articles (Böhm, Katz-Salamon, Smedler, Lagercrantz, & Forssberg, Citation2002; Lundequist et al., Citation2015).
Table 1. Participant characteristics.
The SNP was originally approved by the Ethics Committee of Karolinska Hospital (Böhm et al., Citation2002). Throughout, participation has been based on informed consent, obtained for each data collection. The collection of follow-up data at age 18, as well as continued use of the SNP database, was approved by the Regional Ethics Board in Stockholm (2007/46-31/3).
Measurements and latent constructs
In line with Miyake and Friedman’s (Citation2012) framework, executive function measurements at ages 5.5 and 18 years, respectively, were separated into working memory and cognitive flexibility. Four latent variables (working memory and cognitive flexibility at 5.5 years and working memory and cognitive flexibility at 18 years) were formed, each based on three test variables. As an example, the nonobservable latent variable working memory at 5.5 years, was captured using measurements from three observable test scores: Arithmetic, Digit Span, and Knox Cubes (see ). The latent variables thus reflect the shared process tapped by the test variables.
Table 2. Latent variables and the test variables including source and test description.
The tests included were chosen with the aim to reflect the same abilities at the two age points, and are described in . The means, SDs, and range of the included test variables are presented in .
Table 3. Test variable descriptive statistics and group comparison.
Sex, parental education, gestational age at birth, intrauterine growth, and perinatal medical complications were included to test to what extent these variables could predict executive outcome in terms of working memory and cognitive flexibility, respectively. In addition, a measure of mental development at 10 months was added as a predictor. Parental education attainment was classified according to levels defined by Statistics Sweden (2000) with eight levels ranging from no formal education to a doctoral degree. Intrauterine growth was defined as Birth Weight Standard Deviation Score (Niklasson & Albertsson-Wikland, Citation2008). The perinatal medical complication score was based on the incidence of severe levels of intraventricular hemorrhage grade III-IV; periventricular leukomalacia grade III-IV; chronic lung disease; retinopathy of prematurity grade 3+ . At age 10 months (age corrected for gestational age at birth), the majority of the infants in the preterm group (n = 99) were assessed with Griffiths’ Mental Development Scales (Lindstam, Citation1968). The sum of the A to E scales’ stanine scores was used as a measure of early mental development. The descriptives of these variables are found in .
Statistical analysis
The analysis consisted of three steps. First, the test scores of the included variables were compared between the preterm and term groups. Second, the model for executive function was specified and subjected to a confirmatory factor analysis (CFA) to ensure that the test variables provided successful operationalization of the latent constructs, working memory and cognitive flexibility. This CFA was performed at 5.5 years and 18 years, respectively, for the preterm and term group combined (n = 209). In the third step, for the preterm group, the prediction variables and the associations with the latent executive function variables were added and the full serial multiple mediator model was subjected to structural equation modeling (SEM) analysis. The SEM allows for simultaneous estimation of unobserved, latent variables, and investigating relationships between these and predictor variables in a prespecified model. The predictor variables possibly influence outcome at 18 years directly as well as through three indirect pathways: One indirect pathway running through mental development at 10 months; one indirect pathway running through Working Memory 5.5 and Cognitive Flexibility 5.5, respectively; one serial indirect pathway running through the mediators at 10 months and at 5.5 years sequentially. The sample covariance matrix and mean vector of the model are available on request.
As the sample size was smaller than ideal, had some missing values, and not all variables filled the criteria for normal distribution, the estimation was based on full information maximum likelihood with robust standard errors and Yuan–Bentler scaling (MLR estimator) (Yuan & Bentler, Citation2008). The mean vector as well as the covariance matrix were thus used in the model fit estimation. presents the goodness-of-fit measures used.
Table 4. Goodness-of-fit measures.
Analyses of variable and participant characteristics were performed in SPSS 20 and 22 for PC (IBM Corp., Armonk, NY, USA). The CFA and SEM analysis were performed in the Lavaan package in R (R Core Team, Citation2012; Rosseel, Citation2012).
Results
Step 1: The preterm group performed lower on the included test variables as compared to the control group. The group differences were statistically significant for almost all included variables and are reported in .
Step 2: CFAs with two latent variables (working memory and cognitive flexibility), each measured with three test variables as specified in and , were performed on the combined preterm and control group (n = 209) for ages 5.5 and 18 years, respectively, to test the measurement models’ fit to theory. At age 5.5 years, the model fit was good ( = 14.47, p = .07; comparative fit Index (CFI) = .98; root-mean-square error of approximation (RMSEA) = .06 [90% confidence interval (CI) .00–.09, p-value RMSEA ≤ .5 = .62], standardized root mean-square residual (SRMR) = .02). All factor loadings were significant, ranging from .53 to .81. The covariance between the latent variables was .90. The model had a better fit than a competing model where all six test variables loaded on a single executive function latent variable. The difference between the models was borderline significant (pdiff = .05). At age 18 years, the model fit was also good (
= 9.66, p = .29; CFI = .995; RMSEA = .03 [90% CI .00–.12, p-value RMSEA ≤ .5 = .30], SRMR = .03). All factor loadings were significant, ranging from .56 to .79. The covariance between the latent variables was .82. This model had a significantly better fit than a competing model where all six test variables loaded on a single executive function latent variable (pdiff < .0001). We thus concluded that our operationalization of executive function was valid.
Step 3: For the preterm group (n = 115), the full serial multiple moderator model was subjected to a SEM analysis. The model had a good fit. ( = 121.1, p = .06; CFI = .96; RMSEA = .05 [90% CI .00–.07, p-value RMSEA ≤ .5 = .57], SRMR = .05). The factor loadings, regression coefficients, and covariances are presented in and the model with significant associations is depicted in .
Table 5. Factor loadings, regressions coefficients, and covariances among test, predictor, and latent variables for the extremely or very preterm born. N = 115.
Figure 1. Serial multiple mediator model of executive function development with significant associations. Dashed lines represent significant indirect effects. For simplicity, test variables and their factor loadings, and error terms are not included in the figure. N = 115.
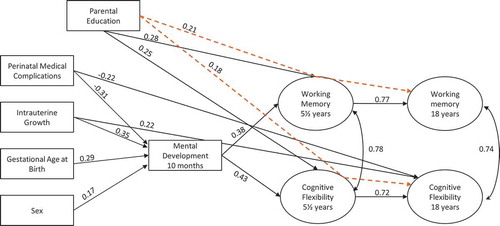
Perinatal factors, sex, and parental education had primarily indirect effects on long-term outcome, mediated by mental development at 10 months and/or core executive function measures at age 5.5. The effects on Working Memory 18 were largely mediated by Working Memory 5.5. There was also a significant indirect effect of parental education mediated by Working Memory 5.5. Seventy-four percent of the variance in Working Memory 18 was explained by the model. For Cognitive Flexibility 18, 72% of the variance was explained by the model. The outcome was largely mediated by Cognitive Flexibility 5.5. In addition, outcome was also directly and negatively influenced by intrauterine growth restriction and perinatal medical complications, as well as indirectly and positively by parental education, mediated by Cognitive Flexibility 5.5.
Working Memory 5.5 and Cognitive Flexibility 5.5 were both directly influenced by level of parental education as well as mental development at 10 months. Gestational age and sex did not reach significance as a direct association. Mental development at 10 months, in turn, was influenced by gestational age at birth, intrauterine growth, and perinatal medical complications. The influence of sex on mental development at 10 months was borderline significant (p = .05). The effect of the perinatal factors thus influenced outcome at age 5.5 largely through their effect on mental development at 10 months.
The indirect and direct mediation effects are found in .
Table 6. Indirect and direct path effects on outcome in executive function at age 18 years.
Discussion
In this report, we have investigated the development and stability of executive function in a cohort born very or extremely preterm, using an empirically tested theoretical framework of executive function as a point of departure. The approach allows us to discuss the development of two core components of executive function, how they differ, and to what extent perinatal factors exert an influence during development.
First, our results showed that executive function could be meaningfully differentiated into two major components, which proved to be highly stable from preschool age to late adolescence. Working memory and cognitive flexibility were differentiated from each other at the ages of both 5.5 and 18 years. They also followed slightly different developmental pathways over time, further indicating that they are indeed separable components of executive function. Working memory performance at 5.5 years essentially predicted working memory performance at 18 years. The prediction of cognitive flexibility performance at age 18 years was more complex. The level of performance was largely determined by cognitive flexibility at age 5.5 years, but in addition, intrauterine growth restriction and perinatal medical complications had a negative direct impact on outcome.
Second, our results also show that male sex, lower gestational age at birth, intrauterine growth restriction, and perinatal medical complications adversely affect outcome. The effects of these known risk factors (Bhutta et al., Citation2002) were apparent already in infancy, as reflected by an assessment of global mental development at 10 months, corrected age. The detrimental effects of the perinatal risk factors on executive function outcome at age 5.5 years were largely mediated through mental development at 10 months. As expected based on previous research, parental education had a positive impact on executive function development at age 5.5 years.
Notably, in our study group of very and extremely preterm born children, the early developmental assessment at 10 months was not influenced by parental level of education. We interpret this to mean that, in this group of medically at-risk infants, medical and biological risk factors overshadowed environmental factors as determinants of development in the first year of life. Environmental factors, such as that of parental education, exert their influence throughout childhood. In our study, parental education contributed significantly and directly to outcome at age 5.5 years, and also had an indirect effect on outcome in late adolescence.
Parental education had a positive influence on both working memory and cognitive flexibility, having exerted its differentiating effect by the end of the preschool years. Along with other studies, this result shows that favorable socioeconomic conditions, notably parental education, are associated with better developmental outcome (Ford et al., Citation2011; Luu et al., Citation2011; Manley et al., Citation2015). In Sweden, where maternal and child medical care is publicly funded and universally provided, differences in outcome can hardly be explained by differences in quality of health care related to family income. Rather, the implication may be that well-educated parents are more likely to ensure and provide favorable learning environments for their children (Davis-Kean, Citation2005). This has implications for interventions. Health professionals working with preterm children of less educated parents are advised to be aware of a possible need for increased support, already during the preschool years, to facilitate an optimal learning environment.
According to our data, male sex was associated with worse long-term outcome. However, in the model, there was merely one borderline statistically significant direct sex effect, namely on mental development at 10 months. The overall significant sex difference in outcome in late adolescence could be attributed to the summed direct and indirect sex effects. The risks associated with male sex have been investigated by others, although the specific mechanisms are yet to be fully explored (DiPietro & Voegtline, Citation2017; Smith, Alexander, Rosenkrantz, Sadek, & Fitch, Citation2014). Previous studies suggest that female brains have a greater potential for reorganization of function, which might contribute to a better recovery from the impact of early disturbances in females as compared to males (Anderson, Anderson, Northam, Jacobs, & Catroppa, Citation2001).
In our study, restricted intrauterine growth, as well as perinatal medical complications, had a direct negative impact on cognitive flexibility task performance in late adolescence, in addition to its impact on earlier developmental assessments. This might reflect a protracted maturation in cognitive flexibility, as compared to working memory.
Executive functions in children develop gradually; more basic and simple processes are available to the child at an earlier age than are more complex functions (Anderson, Citation2002). Consequently, children of preschool age are typically not yet mature enough to solve tasks requiring more advanced cognitive flexibility. In fact, tasks designed to assess cognitive flexibility in preschoolers might largely reflect basic components such as response inhibition, sustained attention, and working memory capacity (Diamond, Citation2013). Neurodevelopmentally, this can be understood to reflect that early disturbances in brain integrity, on which more advanced executive functions rely, become apparent first at the age when these functions typically develop, and increasing demands are put on the individual. Seemingly, the effects become apparent with age, when an expected development does not occur; in effect, the children grow into their problems. Again, this protracted effect may reflect the more complex nature of cognitive flexibility, which presumably depends on neural substrates that develop during adolescence.
Our study suggests that assessment of mental development already at 10 months will give a fair prediction of executive skills in the late preschool years. Furthermore, the stability in executive function performance implies that assessment at late preschool age will give a good indication of the child’s continued executive function performance. The notion of a catch-up effect after late preschool age (e.g., Saavalainen et al., Citation2007; H. G. Taylor et al., Citation2004) is not supported by our findings. Rather, given the stability in function between ages 5.5 and 18 years they imply that any catch-up would occur before the age of 5. This is also in line with findings regarding typical executive function development during the first 5 years of life, in normative groups of children (Diamond, Citation2013; Garon et al., Citation2008; Hendry, Jones, & Charman, Citation2016). An implication of our results is that a wait-and-see approach will more likely lead to secondary effects of a deficit rather than a catch-up over time. Support and interventions to limit the effects of executive function deficits on learning and social interaction should be initiated early and may need to be continued throughout childhood. Moreover, early interventions aimed at strengthening the development of executive function and self-regulation skills are increasingly being implemented and gaining scientific support (Blair & Raver, Citation2015; Center on the Developing Child at Harvard University, Citation2011, Citation2015; Diamond & Lee, Citation2011).
Research on cognitive development poses psychometric challenges. Human functioning is complex, and assessment of a specific function means narrowing the observation down to as pure a measurement as possible. Even at that, all tasks designed to measure executive function will to some extent also tap nonexecutive processes. The problem of test impurity, and indeed of validity, is further accentuated when assessing children (Anderson et al., Citation2001; Best & Miller, Citation2010; Garon et al., Citation2008). Children may fail the more complex cognitive flexibility tasks, because they have difficulties with inhibition or working memory. Also, deficits in various primary functions, such as processing speed, fine motor skills, or visual perception, may negatively affect test performance. Furthermore, the same task might tap different abilities at different ages. By using a latent variable approach, some fundamental measurement problems can be circumvented. Latent constructs extract what is common in the test variables, which reduces the problem of impurity in the specific test. Thereby the latent approach alleviates the construct validity problem of executive function measurements (Miyake et al., Citation2000).
The strengths of this study include its prospective, longitudinal design, with a low drop-out rate, and comprehensive developmental assessments at three points in time. Admittedly, additional assessments, during the toddler and preadolescent years, would have provided a stronger design for investigating developmental pathways and stability. Also, we have not analyzed whether the extremely and very preterm born children typically differ with regard to structure and developmental trajectories of executive function. Still, for our preterm study group and data, the fit to the model was good. The tests used in our study have strong theoretical merits and were chosen to reflect the latent constructs of the model. To our knowledge, we are the first to fit longitudinal data of developing children to the model of executive function proposed by Miyake and colleagues. Given the powerful statistical methods used, the rather limited size of the cohort is a weakness. Even at that, the model had a good fit, indicating a high explanatory value.
In conclusion, applying Miyake et al.’s (Citation2000, Miyake and Friedman, Citation2012) integrative framework of executive function added to our understanding of executive function development in children born very or extremely preterm. Higher parental education, higher gestational age at birth, female sex, and normative development at 10 months were related to better overall executive functioning at late preschool age. The consequences of perinatal medical complications and a restricted intrauterine growth had a continued detrimental effect specifically on cognitive flexibility in late adolescence. On the other hand, executive function performance from preschool to late adolescence was stable enough to imply that significant catch-up effects in preterm children should not be expected beyond the preschool years. From a practical-clinical view, the study poses an argument for identification of executive deficits before school entry among children born preterm and points to the importance of providing extra support to families with low educational attainment.
Acknowledgments
The authors wish to acknowledge the contribution of Hugo Lagercrantz to the Stockholm Neonatal Project, which is a prospective longitudinal cohort study from which results are continually published.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aarnoudse-Moens, C. S. H., Weisglas-Kuperus, N., van Goudoever, J. B., & Oosterlaan, J. (2009). Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics, 124(2), 717–728.
- Allin, M., Walshe, M., Fern, A., Nosarti, C., Cuddy, M., Rifkin, L., … Wyatt, J. (2008). Cognitive maturation in preterm and term born adolescents. Journal of Neurology, Neurosurgery, and Psychiatry, 79(4), 381–386.
- Anderson, P. J. (2002). Assessment and development of executive function (EF) during childhood. Child Neuropsychology, 8(2), 71–82.
- Anderson, P. J. (2014). Neuropsychological outcomes of children born very preterm. Seminars in Fetal and Neonatal Medicine, 19(2), 90–96.
- Anderson, P. J., Howard, K., & Doyle, L. W. (2010). Executive function development in preterm children. In C. Nosarti, R. M. Murray, & M. Hack (Eds.), Neurodevelopmental outcomes of preterm birth. From childhood to adult life (pp. 195–208). Cambridge, UK: Cambridge University Press.
- Anderson, P. J. (2008). Towards a developmental model of executive function. In V. A. Anderson, R. Jacobs, & P. J. Anderson (Eds.), Executive functions and the frontal lobes. A lifespan perspective (pp. 3–21). New York: Psychology Press.
- Anderson, V. A., Anderson, P. J., Jacobs, R., & Spencer-Smith, M. M. (2008). Development and assessment of executive function: From preschool to adolescence. In V. A. Anderson, R. Jacobs, & P. J. Anderson (Eds.), Executive functions and the frontal lobes. A lifespan perspective (pp. 123–154). New York: Psychology Press.
- Anderson, V. A., Anderson, P. J., Northam, E., Jacobs, R., & Catroppa, C. (2001). Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology, 20(1), 385–406.
- Anderson, V. A., Northam, E., Hendy, J., & Wrennall, J. (2001). Developmental neuropsychology. A clinical approach. Hove, UK: Psychology Press.
- Arthur, G. A. (1947). Point scale of performance tests. Revised form II. New York: The Psychological Corporation.
- Aylward, G. P. (2010). Commentary: Environmental influences: Issues of timing and type. Journal of Pediatric Psychology, 35(3), 284–285.
- Best, J., & Miller, P. (2010). A developmental perspective on executive function. Child Development, 81(6), 1641–1660.
- Bhutta, A. T., Cleves, M. A., Casey, P. H., Cradock, M. M., & Anand, K. J. S. (2002). Cognitive and behavioral outcomes of school-aged children who were born preterm. Journal of the American Medical Association, 288(6), 728–737.
- Blair, C. (2006). How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. The Behavioral and Brain Sciences, 29(2), 109–125.
- Blair, C., & Raver, C. C. (2015). School readiness and self-regulation: A developmental psychobiological approach. Annual Review of Psychology, 66, 711–731.
- Böhm, B., Katz-Salamon, M., Smedler, A.-C., Lagercrantz, H., & Forssberg, H. (2002). Developmental risks and protective factors for influencing cognitive outcome at 5½ years of age in very-low-birthweight children. Developmental Medicine & Child Neurology, 44(8), 508–516.
- Böhm, B., Smedler, A.-C., & Forssberg, H. (2004). Impulse control, working memory and other executive functions in preterm children when starting school. Acta Paediatrica, (93), 1363–1371. doi:10.1080/08035250410021379
- Burnett, A. C., Scratch, S. E., & Anderson, P. J. (2013). Executive function outcome in preterm adolescents. Early Human Development, 89(4), 215–220.
- Center on the Developing Child at Harvard University. (2011). Building the brain’s “air traffic control” system: How early experiences shape the development of executive function (Working Paper No. 11). doi:10.1111/cdep.12095
- Center on the Developing Child at Harvard University. (2015). Enhancing and practicing executive function skills with children from infancy to adolescence. Retrieved from http://developingchild.harvard.edu/resources/tools_and_guides/enhancing_and_practicing_executive_function_skills_with_children/
- Cepeda, N. J., Blackwell, K. A., & Munakata, Y. (2013). Speed isn’t everything: Complex processing speed measures mask individual differences and developmental changes in executive control. Developmental Science, 16(2), 269–286.
- Davis-Kean, P. E. (2005). The influence of parent education and family income on child achievement: The indirect role of parental expectations and the home environment. Journal of Family Psychology, 19(2), 294–304.
- Dekker, T. M., & Karmiloff-Smith, A. (2011). The dynamics of ontogeny: A neuroconstructivist perspective on genes, brains, cognition and behavior. Progress in Brain Research, 189, 23–33.
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001). Delis-Kaplan Executive Function System (D-KEFS). Examiner’s manual. San Antonio, TX: The Psychological Corporation.
- Dennis, M., Francis, D. J., Cirino, P. T., Schachar, R., Barnes, M. A., & Fletcher, J. M. (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15(3), 331–343.
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168.
- Diamond, A., & Lee, K. (2011). Interventions shown to aid executive function development in children 4 to 12 years old. Science (New York, N.Y.), 333(6045), 959–964.
- DiPietro, J. A., & Voegtline, K. M. (2017). The gestational foundation of sex differences in development and vulnerability. Neuroscience, 342, 4–20.
- Fellman, V., Hellström-Westas, L., Norman, M., Westgren, M., Källén, K., Lagercrantz, H., … Wennergren, M. (2009). One-year survival of extremely preterm infants after active perinatal care in Sweden. Journal of the American Medical Association, 301(21), 2225–2233.
- Ford, R. M., Neulinger, K., O’Callaghan, M., Mohay, H., Gray, P., & Shum, D. (2011). Executive function in 7–9-year-old children born extremely preterm or with extremely low birth weight: Effects of biomedical history, age at assessment, and socioeconomic status. Archives of Clinical Neuropsychology, 26(7), 632–644.
- Garon, N., Bryson, S. E., & Smith, I. M. (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134(1), 31–60.
- Hack, M. (2009). Adult outcomes of preterm children. Journal of Developmental and Behavioral Pediatrics, 30(5), 460–470.
- Hack, M., Flannery, D. J., Schluchter, M., Cartar, L., Borawski, E., & Klein, N. (2002). Outcomes in young adulthood for very-low-birth-weight infants. The New England Journal of Medicine, 346(3), 149–157.
- Hendry, A., Jones, E. J. H., & Charman, T. (2016). Executive function in the first three years of life: Precursors, predictors and patterns. Developmental Review, 42, 1–33.
- Howard, K., Anderson, P. J., & Taylor, H. G. (2008). Executive functioning and attention in children born preterm. In V. A. Anderson, R. Jacobs, & P. J. Anderson (Eds.), Executive functions and the frontal lobes. A lifespan perspective (pp. 219–241). New York: Psychology Press.
- Hu, L., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. doi:10.1080/10705519909540118
- Huizinga, M., Dolan, C. V., & van der Molen, M. W. (2006). Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia, 44(11), 2017–2036.
- Ingemarsson, I. (2003). Gender aspects of preterm birth. BJOG: An International Journal of Obstetrics and Gynaecology, 110(s20), 34–38.
- Johnson, S. (2007). Cognitive and behavioural outcomes following very preterm birth. Seminars in Fetal & Neonatal Medicine, 12(5), 363–373.
- Kerr-Wilson, C. O., Mackay, D. F., Smith, G. C. S., & Pell, J. P. (2011). Meta-analysis of the association between preterm delivery and intelligence. Journal of Public Health, 34(2), 209–216.
- Korkman, M. (1990). Nepsy, Neuropsychological Assessment 4–7 years. Swedish version. Stockholm: Psykologiförlaget AB.
- Kormos, C. E., Wilkinson, A. J., Davey, C. J., & Cunningham, A. J. (2014). Low birth weight and intelligence in adolescence and early adulthood: A meta-analysis. Journal of Public Health (Oxford, England), 36(2), 213–224.
- Kroll, J., Karolis, V., Brittain, P. J., Tseng, C. J., Froudist-Walsh, S., Murray, R. M., & Nosarti, C. (2017). Real-life impact of executive function impairments in adults who were born very preterm. Journal of the International Neuropsychological Society, 23(5), 381–389.
- Lindstam, R. (1968). Griffiths Mental Development Scales. Swedish version. Stockholm: Skandinaviska testförlaget.
- Lundequist, A., Böhm, B., Forssberg, H., Lagercrantz, H., & Smedler, A.-C. (2015). Cognitive outcome varies in adolescents born preterm depending on gestational age, intrauterine growth and neonatal complications. Acta Paediatrica, 104(3), 292–299.
- Lundequist, A., Böhm, B., & Smedler, A.-C. (2013). Individual neuropsychological profiles at age 5½ years in children born preterm in relation to medical risk factors. Child Neuropsychology, 19(3), 313–331.
- Luu, T. M., Ment, L. R., Allan, W., Schneider, K. C., & Vohr, B. R. (2011). Executive and memory function in adolescents born very preterm. Pediatrics, 127(3), e639–46.
- Manley, B. J., Roberts, R. S., Doyle, L. W., Schmidt, B., Anderson, P. J., Barrington, K. J., … Scapinello, L. (2015). Social variables predict gains in cognitive scores across the preschool years in children with birth weights 500 to 1250 grams. The Journal of Pediatrics, 166(4), 870–876.e2.
- Miyake, A., & Friedman, N. P. (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. doi:10.1177/0963721411429458
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100.
- Moffitt, T. E., Arseneault, L., Belsky, D., Dickson, N., Hancox, R. J., Harrington, H., … Caspi, A. (2011). A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 2693–2698.
- Mulder, H., Pitchford, N. J., Hagger, M. S., & Marlow, N. (2009). Development of executive function and attention in preterm children: A systematic review. Developmental Neuropsychology, 34(4), 393–421.
- Niklasson, A., & Albertsson-Wikland, K. (2008). Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatrics, 8, 8.
- Nosarti, C., Giouroukou, E., Micali, N., Rifkin, L., Morris, R. G., & Murray, R. M. (2007). Impaired executive functioning in young adults born very preterm. Journal of the International Neuropsychological Society, 13(4), 571–581.
- Pascal, A., Govaert, P., Oostra, A., Naulaers, G., Ortibus, E., & Van den Broeck, C. (2018). Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: A meta-analytic review. Developmental Medicine & Child Neurology, 1–14. doi:10.1111/dmcn.13675
- R Core Team. (2012). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Raz, S., Debastos, A. K., Newman, J. B., & Batton, D. (2012). Intrauterine growth and neuropsychological performance in very low birth weight preschoolers. Journal of the International Neuropsychological Society, 18(2), 200–211.
- Rosseel, Y. (2012). Lavaan : An R package for structural equation modeling. Journal of Statistical Software, 48(2), 1–36.
- Roze, E., Van Braeckel, K. N. J. A., van der Veere, C. N., Maathuis, C. G. B., Martijn, A., & Bos, A. F. (2009). Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatrics, 123(6), 1493–1500.
- Saavalainen, P., Luoma, L., Bowler, D., Määttä, S., Kiviniemi, V., Laukkanen, E., & Herrgård, E. (2007). Spatial span in very prematurely born adolescents. Developmental Neuropsychology, 32, 769–785.
- Serenius, F., Ewald, U., Farooqi, A., Fellman, V., Hafström, M., Hellgren, K., … Källén, K. (2016). Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatrics, 170(10), 954.
- Smith, A. L., Alexander, M., Rosenkrantz, T. S., Sadek, M. L., & Fitch, R. H. (2014). Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Experimental Neurology, 254, 54–67.
- Stålnacke, J., Lundequist, A., Böhm, B., Forssberg, H., & Smedler, A.-C. (2015). Individual cognitive patterns and developmental trajectories after preterm birth. Child Neuropsychology, 21(5), 648–667.
- Taylor, H. G., Burant, C. J., Holding, P. A., Klein, N., & Hack, M. (2002). Sources of variability in sequelae of very low birth weight. Child Neuropsychology, 8(3), 163–178.
- Taylor, H. G., & Clark, C. A. C. (2016). Executive function in children born preterm: Risk factors and implications for outcome. Seminars in Perinatology, 40(8), 520–529.
- Taylor, H. G., Minich, N., Bangert, B., Filipek, P. A., & Hack, M. (2004). Long-term neuropsychological outcomes of very low birth weight: Associations with early risks for periventricular brain insults. Journal of the International Neuropsychological Society, 10(7), 987–1004.
- Taylor, L. C., Clayton, J. D., & Rowley, S. J. (2004). Academic socialization: Understanding parental influences on children’s school-related development in the early years. Review of General Psychology, 8(3), 163–178.
- Vohr, B. R. (2010). Cognitive and functional outcomes of children born preterm. In C. Nosarti, R. M. Murray, & M. Hack (Eds.), Neurodevelopmental outcomes of preterm birth: From childhood to adult life (pp. 141–163). Cambridge, UK: Cambridge University Press.
- Vohr, B. R., Wright, L. L., Dusick, A. M., Mele, L., Verter, J., Steichen, J. J., … Kaplan, M. D. (2000). Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics, 105(6), 1216–1226.
- Wechsler, D. (1991). Wechsler Intelligence Scale for Children: WISC- III. Swedish version. Stockholm: Psykologiförlaget AB.
- Wechsler, D. (1999). Wechsler Preschool and Primary Scale of Intelligence - Revised. Swedish version. Stockholm: Psykologiförlaget AB.
- Wechsler, D., Nyman, H., Johansson, C., Bragesjö, M., Bothén, P., Granath, K., & Waaler, E. (2004). Wechsler Adult Intelligence Scales - III NI. Swedish version for neuropsychological assessment. Stockholm: Pearson Education Ltd.
- Wilson-Ching, M., Molloy, C. S., Anderson, V. A., Burnett, A. C., Roberts, G., Cheong, J. L. Y., & Anderson, P. J. (2013). Attention difficulties in a contemporary geographic cohort of adolescents born extremely preterm/extremely low birth weight. Journal of the International Neuropsychological Society, 19(10), 1097–1108.
- Wyatt, J. (2010). The changing face of intensive care for preterm newborns. In C. Nosarti, R. M. Murray, & M. Hack (Eds.), Neurodevelopmental outcomes of preterm birth. From childhood to adult life (pp. 17–29). Cambridge, UK: Cambridge University Press.
- Yuan, K.-H., & Bentler, P. M. (2008). Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociological Methodology, 30(1), 165–200.
