Abstract
Objective: To investigate unintended pregnancy and changes in mood, acne, and weight in NOMAC-E2 vs levonorgestrel-containing COC (COCLNG) users under 25 years.
Methods: In this large, observational study, new users (first-ever users of an eligible COC or restarting with the same or a new eligible COC after a break of at least 2 months) of NOMAC-E2 and COCLNG were recruited in 12 countries in Europe, Australia, and Latin America and followed up via questionnaires for up to 2 years. Unintended pregnancy was expressed by the Pearl Index (PI; contraceptive failures/100 women-years). Crude (HRcrude) and adjusted hazard ratios (HRadj) were calculated. Mood and acne changes were defined as change of score from baseline. Weight change was defined as percent change of body weight.
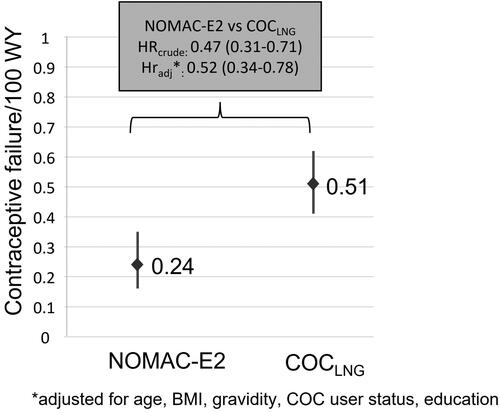
Results: Overall, 12,829 NOMAC-E2 users and 17,095 COCLNG users under 25 were followed-up. The risk of unintended pregnancy was statistically significantly lower in the NOMAC-E2 cohort; confirmed events: 30 NOMAC-E2 (PI 0.24; 95% CI, 0.16–0.35) vs 94 COCLNG (PI 0.51; 95% CI, 0.41–0.62). The HRcrude for unintended pregnancy comparing NOMAC-E2 to COCLNG was 0.47 (95% CI, 0.31–0.71) and the HRadj was 0.52 (95% CI, 0.34–0.78). No differential effect on acne, mood, and weight was observed between cohorts.
Conclusions: NOMAC-E2 shows a significantly better contraceptive effectiveness in young women and has no differential effect on acne, mood, and weight compared to COCLNG.
Introduction
Combined oral contraceptives (COCs) are among the most widely used and acceptable methods of birth control [Citation1]. Besides the primary contraceptive action through inhibition of ovulation, COCs were shown to offer a variety of non-contraceptive benefits, including positive effects on menstrual pain, heavy menstrual bleeding and acne [Citation2]. COCs are associated with a ‘perfect-use’ (COC used consistently and exactly as instructed) contraceptive failure rate of around 0.3% during the first year of use [Citation1, Citation2], while ‘real-use’ failure rates can largely differ and show marked geographical variation [Citation3–5]. The higher failure rates observed in ‘real-life’ users are mostly attributed to non-compliant use (deviating from instructions on the package inserts, e.g. missed pills [Citation6, Citation7]). Overall, the contraceptive effectiveness of a COC is determined by a combination of its efficacy and other factors, such as the individual fecundability or medication adherence and persistence of the user.
Contraception is especially important for young women, as unintended pregnancies are particularly high in this age group [Citation8]. This may be associated with their tendency for incorrect or inconsistent contraceptive use [Citation9] in combination with their higher fertility [Citation10]. A recent study that investigated the unmet need in contraceptive counseling and choice showed that women aged <30 years were more likely to forget to take their pill than women aged >30 years (54% vs. 47%, p < .05) [Citation11]. Another study has shown that young women (aged 18–24) have the highest rate of general practitioner consultations for contraception and unplanned pregnancies [Citation12]. Factors negatively impacting COC adherence include difficulties in establishing a correct pill-taking routine, user errors associated with the hormone-free interval, failure to read and understand the instructions of use, and the occurrence or even the fear of side effects [Citation13, Citation14], such as weight or mood changes.
Since the introduction of COCs, mood-related side effects have been one of the major reasons for discontinuation. Approximately 4–10% of COC users complain of depressed mood, irritability or increased anxiety, but drug-related causality has been difficult to prove [Citation15]. Two studies (a double-blind, randomized, and placebo-controlled trial [Citation16] and a Danish cohort study [Citation17]) have shown an association of COC use with antidepressant use, first diagnosis of depression or general well-being. Interestingly, a double-blind placebo-controlled randomized trial has shown both positive and negative mood effects of COCs depending on the menstrual cycle phase [Citation18]. Overall, it seems difficult to make strong conclusions about which COC users are at risk for adverse mood effects [Citation19].
Also, the influence of COC use on body weight effects is the subject of debate [Citation20]. Clinical studies investigating this association show contradictory results, and a Cochrane systematic database review concluded that the evidence was not strong enough to exclude an effect of hormonal contraceptives on weight, but a major effect was unlikely [Citation21].
To improve adherence to COC use, younger women could benefit from a regimen with a shortened, and non-pill-free hormone-free interval as regimens in which placebo pills are taken during the hormone-free interval have been associated with fewer missed pills [Citation22]. In addition, several studies investigating COCs with a 24/4 day regimen (24 days active treatment and 4-day hormone-free interval) showed reduced ovarian activity and improved efficacy compared to the traditional 21/7 regimen [Citation5, Citation23].
NOMAC-E2 is a monophasic COC containing a fixed dose of the progestin nomegestrol acetate (NOMAC, 2.5 mg) and the synthetic analogue of the endogenous estrogen 17β-estradiol (E2, 1.5 mg), which is taken in a 24/4 day regimen with 4 days of placebo. At dosages of 1.5 mg/day or more, NOMAC effectively suppresses gonadotropic activity and ovulation in women of reproductive age [Citation24]. Results from the PRO-E2 study, a large, prospective, observational study designed to compare the risks of NOMAC-E2 versus levonorgestrel-containing COCs (COCLNG) in a real-world setting, have shown that NOMAC-E2 use was not associated with a higher risk of thromboembolic events (primary outcome of the study) [Citation25] and showed a statistically significant better effectiveness [Citation26] compared with COCLNG. We performed a sub-population analysis limited to participants under 25 years to specifically investigate contraceptive effectiveness and effects on acne, weight, and mood in young users. This age threshold was based on the World Health Organization (WHO) definition for ‘young people’ [Citation18].
Materials and methods
PRO-E2 was a large, multinational, controlled, prospective, active surveillance study that followed new users (starters and restarters) of NOMAC-E2 and COCLNG. Starters were defined as first-ever users of any COC, and restarters were defined as those restarting COC use after a minimum break of 2 months. Ethical approval was obtained as required by local law and an independent Safety Monitoring and Advisory Council (SMAC) monitored the study.
Study population
Women participating in the PRO-E2 study were recruited between August 2014 and September 2019 by health care professionals (HCPs) in Australia, Austria, Colombia, France, Germany, Hungary, Italy, Mexico,Footnote1 Poland, Russia, Spain, and Sweden during routine clinical practice. All women newly prescribed an eligible COCFootnote2 could participate if they had not used a COC in the past 2 months, signed an informed consent form and completed a baseline questionnaire in the local language. In line with the non-interventional design of PRO-E2, the study did not interfere with the usual prescribing behavior of physicians and the physician provided the woman with information about the study only after a prescription had been decided upon.
Baseline survey and follow-up
Participants completed a baseline questionnaire to capture demographic data, reason(s) for their COC prescription, personal and family medical history, concomitant medication, exposure to hormonal contraceptives and lifestyle factors. Subsequently, women were contacted at 6, 12 and 24 months via mail or e-mail and were asked follow-up questions about contraceptive use, pregnancy, and the occurrence of other outcomes of interest.
Self-reported outcomes of interest were reviewed by medical advisers. If necessary, study participants and/or the treating physicians were contacted for further information.
Evaluation
The here presented data are based on a sub-population analysis limited to participants under 25 years. Unintended pregnancy, a secondary outcome of PRO-E2, was expressed by the Pearl Index (PI), defined as the number of unintended pregnancies per 100 women-years (WY) of exposure. Pregnancy events were assigned to the exposure at the time of conception (which may have differed from the COC prescribed at study entry). Only first confirmedFootnote3 pregnancies during study participation were included in this analysis. Confirmed pregnancies were included regardless of the reasons for contraceptive use (e.g. contraceptive or non-contraceptive reasons) reported by study participants at baseline.Footnote4
Inferential statistics were based on Cox regression analysis and crude hazard ratios (HRcrude) and adjusted hazard ratios (HRadj) were calculated. An a priori expert model defined in collaboration with the SMAC included the following pre-defined prognostic factors: age, body mass index (BMI = kg/m2), gravidity, COC user status (starter, restarter), and education level at study entry. All statistical analyses were conducted using SAS 9.4 [Citation27].
Further secondary outcomes of PRO-E2 were changes in body weight, acne, and mood. Study participants were asked to record their weight at baseline and at each follow-up time point (i.e. 6 months, 12 months, and 24 months after study entry). The percent change of body weight was then calculated by comparing the body weight at each follow-up time point to the body weight at baseline.
Acne-related questions were asked at baseline and follow-up to assess patient-perceived acne conditions and their impact on self-esteem or social interactions. The response was scored and summarized into a single acne score scaled from 0 to 100 (with a higher acne score indicating a better skin condition). The scores at each follow-up time point were then compared with the score at baseline to determine the change in acne over time.
Baseline and follow-up questionnaires also contained mood-related questions. The responses to these questions were transformed into a mood score scaled from 0 to 100 (with a higher score indicating a better mood) and score changes from baseline to each follow-up time point were compared.
Changes in body weight, mood and acne scores were calculated for constant users (i.e. women who continued using their baseline prescription through follow-up).
Results
Out of 91,313 women who began using an eligible COC at baseline, 44,559 women were initially prescribed with NOMAC-E2 and 46,754 with COCLNG. Of these, 12,829 NOMAC-E2 users and 17,095 COCLNG users were under 25 years old.
Loss to follow-up and dropout
Of all participants under 25 years old, 3,591 (12%) were lost to follow-up: 1,509 NOMAC-E2 users (11.7%) and 2,084 COCLNG users (12.2%). The dropout rate of women under 25 who indicated they no longer wished to participate at any point in time during the study, did also not differ substantially between user cohorts: 2,755 NOMAC-E2 users (21.5%) and 3,834 COCLNG users (22.4%).
Baseline characteristics
Exposure times (in WY) and selected baseline characteristics of women under 25 are described by user cohort in . There were no substantial differences between the cohorts with respect to most characteristics.
Table 1. Selected baseline characteristics of women under 25 years by user cohort.
Mean weight (NOMAC-E2: 59.8 kg; COCLNG: 59.7 kg) and mean BMI (NOMAC-E2: 22.0; COCLNG: 22.1) were very similar in both cohorts. A slightly lower proportion of NOMAC-E2 users reported ever having been pregnant (NOMAC-E2: 17.9%; COCLNG: 19.2%) and out of them slightly less NOMAC-E2 users reported having had at least one live birth at study entry (NOMAC-E2: 58.2%; COCLNG: 65.4%).
At study entry, 51.1% of NOMAC-E2 users and 53.3% of COCLNG users reported taking the prescribed COC for contraceptive reasons only. Contraceptive and/or non-contraceptive reasons were reported by 47.3% and 45.6% of NOMAC-E2 and COCLNG users, respectively. Of these, 16.6% NOMAC-E2 users and 12.6% COCLNG users reported acne as reason for COC use. The most frequent other non-contraceptive reasons were cycle regulation (NOMAC-E2 44.6% vs COCLNG 49.1%) and painful menstrual bleeding (NOMAC-E2 48.8% vs COCLNG 50.9%).
NOMAC-E2 users reported having a slightly higher level of education, with 22.5% having had more than a university entrance level education compared to 20.9% of COCLNG users.
Only a minor proportion of all COC users reported suffering from depression requiring treatment (1.7% of NOMAC-E2 users and 2.2% of COCLNG users).
Unintended pregnancies
In the PRO-E2 sub-population under 25 years, there were 30 confirmed unintended pregnancy events in NOMAC-E2 users (PI 0.24; 95% CI, 0.16–0.35) and 94 confirmed unintended pregnancy events in COCLNG users (PI 0.51; 95% CI, 0.41–0.62), . The risk of unintended pregnancy was statistically significantly lower in the NOMAC-E2 cohort compared to the COCLNG cohort (p<.0001). The HRcrude for unintended pregnancy comparing NOMAC-E2 to COCLNG was 0.47 (95% CI, 0.31–0.71) and the HRadj was 0.52 (95% CI, 0.34–0.78).
Effects on weight, mood, and acne
Body weight changes from baseline to 12-months follow-up did not differ substantially between cohorts. displays the proportions of users who had an increase or decrease in body weight in predefined percentage ranges in both cohorts. The percentages are rounded to the nearest tens for display. Both cohorts had a similar distribution profile of weight change, with more than half of constant NOMAC-E2 and COCLNG users having a minor change in body weight (increase or decrease) between 0 and <5%. Body weight changes from baseline to 6- and 24-months follow-up are shown in , respectively.
Figure 2. Changes from baseline to 12-months follow-up in weight (a), mood score (b), and acne score (c) by user cohort; (–) indicates weight loss and mood/acne score worsening, respectively; (+) indicates weight gain and mood/acne score improvement, respectively.
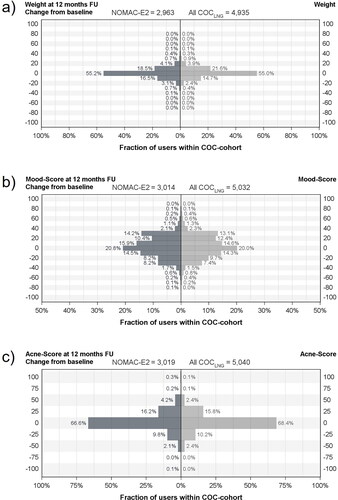
The distribution of mood score changes (improvement or worsening) are displayed in and did not differ substantially between both cohorts. The majority of constant users in both cohorts had a change in mood score from baseline to 12-month follow-up between 0 and <25%. The percentages are rounded to the nearest tens for display. Mood score changes from baseline to 6- and 24-months follow-up are shown in , respectively.
Similar results were observed for changes in acne score (), which also showed a comparable distribution profile of improvement or worsening between cohorts. The majority of constant NOMAC-E2 and COCLNG users had a change in acne score from baseline to 12-month follow-up between 0 and ≤ 25%. Exact acne score-values are multiples of 25 ranging from −100 to 100. Changes in acne score from baseline to 6- and 24-months follow-up are shown in , respectively. In addition, Supplementary Figure S3 displays acne score changes from baseline to 6- 12- and 24-months follow-up in users who reported acne as reason for COC use at baseline.
Discussion
Previous studies have shown that young women often struggle to use contraceptives correctly and consistently [Citation8, Citation14], which contributes to a higher risk of unintended pregnancy in this age group.
This subpopulation analysis of the PRO-E2 study showed that the risk of unintended pregnancy in women under 25 years was significantly lower in NOMAC-E2 users than in COCLNG users. Baseline characteristics did not differ substantially between cohorts, apart from minor differences in relation to parity and educational level. Around half of the women in both cohorts reported to use the prescribed COC due to contraceptive and/or non-contraceptive reasons.
Potential explanations for the reduced incidence of unintended pregnancies in young NOMAC-E2 users compared to young users of the COCLNG include: (i) NOMAC has a longer half-life (approximately 46 h) [Citation27] in comparison to levonorgestrel (LNG) (about 22 h [Citation28]); (ii) An intake regimen with a shorter hormone-free interval (4 days compared to 7 days for most LNG COCs) has shown to make NOMAC-E2 more ‘forgiving’ (contraceptive effectiveness is maintained even with missed pills) [Citation22]. The differences in effectiveness challenge the position of COCLNG as the first-line recommendation in several guidelines. A previously conducted sensitivity analysis of the overall cohort [Citation26] showed that the lower rate of unintended pregnancy among NOMAC-E2 users was not associated with their higher educational level (and presumably more compliant use).
Concerns about weight gain or negative effects on acne and mood can influence contraceptive adherence and persistence in young users [Citation13, Citation14]. Besides unintended pregnancy, this can have the consequence that women discontinue COC use and choose a less effective birth control method, leading to a higher rate of abortion [Citation28], maternal depressive symptoms [Citation29], or difficulties in family planning [Citation30]. This sub-population analysis showed no differential effect on body weight, mood, and acne over time between young users of NOMAC-E2 and young users of COCLNG, based on self-reported outcomes at baseline and follow-up.
Besides the usual limitations of observational research [Citation31], such as the lack of randomization and blinding to exposure, the PRO-E2 study had a higher loss to follow-up (LTFU) rate than in previous similar studies. This results in part from implementing the General Data Protection Regulation (GDPR) in May 2018 and emergence of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic toward the end of the study, adversely impacting the ability of the investigators to communicate with the study participants. Importantly, however, LTFU rates were non-differential between the NOMAC-E2 and COCLNG cohorts. Another limitation was that a direct comparison with a COCLNG, which has the same 24/4 day regimen as NOMAC-E2, was not possible, as such a preparation is not currently on the market [Citation32]. Mood and acne outcomes were derived solely from self-reported information given by participants in response to specific questions as part of the baseline and follow-up questionnaires. These outcomes were not further validated by clinical records or contact with treating physicians.
The study benefited from several strengths. Only new users (first-ever users of an eligible COC or restarting use after a break of at least 2 months) participated; selection bias introduced by the inclusion of prevalent users was eliminated. Relying upon patient reports ensured that almost all outcomes of interest were captured, and pregnancy cases were subsequently confirmedFootnote5 via the women or their physicians. The study design also enabled the capture of important potential confounders such as age, BMI, gravidity, and education level. Furthermore, it captured precise information on COC use (specific COCs, stopping and switching patterns) and women contributed WY to several (sub-)cohorts depending upon their real-life use. The PRO-E2 study was designed to capture real-world COC use. Study participants were recruited by a broad range of HCPs (e.g. gynecologists, general practitioners, midwives) and all eligible new users could participate (e.g. there were no medical inclusion or exclusion criteria). Therefore, the generalizability of the results to the general population is high.
In conclusion, this sub-population analysis from the PRO-E2 study found a significantly better contraceptive effectiveness of NOMAC-E2 vs COCLNG in young women. It showed no differential impact on mood, acne, and weight between cohorts. These results contribute to a better understanding of COC effectiveness in young women in routine clinical practice and provide valuable information to users that may improve contraceptive compliance by reducing the fear of side effects.
Supplemental Material
Download MS Word (873.3 KB)Acknowledgements
The authors would like to acknowledge the valuable scientific contribution of the SMAC. Their critical review of the data and challenging discussions substantially enhanced the overall quality of the study and the interpretation of the results. SMAC members included David Grimes, Michael Lewis, Robert Reid, Samuel Shapiro,Footnote6 Carolyn Westhoff, Ulrich Winkler and Stephanie Teal. Advisors to the SMAC included Jochen Albrecht and Fritz von Weizsäcker.Footnote7 The authors would also like to thank the colleagues in the local field organisations who conducted the day-to-day field work. Their remarkable dedication and flexibility despite the challenging circumstances caused by the pandemic ensured the success of the study. The authors would also like to thank the ZEG Berlin staff members (statisticians, data managers, event validation staff, medical advisers) whose contribution to the study was invaluable.
Disclosure statement
SVS, KB, AB, CF, and KH are full-time employees at ZEG Berlin. FF has received honoraria from Bayer, Gedeon Richter, EffiK, MSD, Pfizer and Theramex for educational or advisory activities. JC has received honoraria from Bayer, Gedeon Richter, Lilly, MSD, and Theramex for educational or advisory activities. CK has received honoraria for educational or advisory activities from Theramex, Theva, Exeltis, Besins, Merck, Organon, Henry Schein, Gedeon Richter, Jenapharm, Bayer, Serono.
Additional information
Funding
Notes
1 Due to non-compliance with study procedures, data contributed by 625 study participants in Mexico were excluded from these analyses as advised by the SMAC. The local field organization deviated from the required storage requirements and destroyed 545 of the informed consent forms. After recontacting the women, proof of consent was available for 235 women.
2 (1) NOMAC-E2, (2) COCLNG monophasic preparation containing 20–30 mcg of ethinylestradiol, (3) COCLNG multiphasic preparation containing up to 40 mcg of ethinylestradiol.
3 If there was ambiguity concerning the date of conception or COC use, the woman and/or her HCP were contacted for clarification. The consent form included permission to contact any treating physician to follow up on specific outcomes.
4 Study participants reported their reason(s) for COC use only at study entry; their reason(s) for use may have changed during follow-up (e.g. a woman who began using a COC for contraceptive only reasons may have experienced a relationship change and continued using the COC for non-contraceptive reasons).
5 If there was ambiguity concerning the date of conception or COC use, the woman and/or her HCP were contacted for clarification.
6 Died 19 April 2016.
7 Died 19 November 2019.
References
- Festin MPR. Overview of modern contraception. Best Pract Res Clin Obstet Gynaecol. 2020;66:1–6.
- Regidor P-A, Schindler AE. Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone. Oncotarget. 2017;8(47):83334–83342.
- Trussell J, Portman D. The creeping pearl: why has the rate of contraceptive failure increased in clinical trials of combined hormonal contraceptive pills? Contraception. 2013;88(5):604–610.
- Dinger JC, Cronin M, Möhner S, et al. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. Am J Obstet Gynecol. 2009;201(3):263.e1-9–263.e9.
- Dinger J, Minh TD, Buttmann N, et al. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol. 2011;117(1):33–40.
- QuickStats: percentage of women who missed taking oral contraceptive pills * – among women aged 15-44 years who used oral contraceptive pills and had sexual intercourse, overall and by age and number of pills missed - National Survey Of Family Growth, United States, 2013-2015. MMWR Morb Mortal Wkly Rep. 2017;66:965.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011;(5):CD004861.
- Caetano C, Peers T, Papadopoulos L, et al. Millennials and contraception: why do they forget? An international survey exploring the impact of lifestyles and stress levels on adherence to a daily contraceptive regimen. Eur J Contracept Reprod Health Care. 2019;24(1):30–38.
- Hooper DJ. Attitudes, awareness, compliance and preferences among hormonal contraception users: a global, cross-sectional, self-administered, online survey. Clin Drug Investig. 2010;30(11):749–763.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline Committee opinion no. 589. Fertility and Sterility. 2014;101:633–634.
- Merki-Feld GS, Caetano C, Porz TC, et al. Are there unmet needs in contraceptive counselling and choice? Findings of the european TANCO study. Eur J Contracept Reprod Health Care. 2018;23(3):183–193.
- Mazza D, Harrison C, Taft A, et al. Current contraceptive management in Australian general practice: an analysis of BEACH data. Med J Aust. 2012;197(2):110–114.
- Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995;51(5):283–288.
- Fumero A, Marrero RJ, Peñate W, et al. Adherence to oral contraception in young women: beliefs, locus of control, and psychological reactance. IJERPH. 2021;18(21):11308.
- Poromaa IS, Segebladh B. Adverse mood symptoms with oral contraceptives. Acta Obstet Gynecol Scand. 2012;91(4):420–427.
- Zethraeus N, Dreber A, Ranehill E, et al. A first-choice combined oral contraceptive influences general well-being in healthy women: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2017;107(5):1238–1245.
- Skovlund CW, Mørch LS, Kessing LV, et al. Association of hormonal contraception With depression. JAMA Psychiatry. 2016;73(11):1154–1162.
- Lundin C, Danielsson KG, Bixo M, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle-A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology. 2017;76:135–143.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21(5):347–355.
- Contraception: do hormonal contraceptives cause weight gain? In: informedHealth.org [internet] : Institute for quality and efficiency in health care (IQWiG); 2017.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2006;(1):CD003987.
- MacGregor EA, Guillebaud J. The 7-day contraceptive hormone-free interval should be consigned to history. BMJ Sex Reprod Health. 2018;44(3):214–220.
- Klipping C, Duijkers I, Trummer D, et al. Suppression of ovarian activity with a drospirenone-containing oral contraceptive in a 24/4 regimen. Contraception. 2008;78(1):16–25.
- Ruan X, Seeger H, Mueck AO. The pharmacology of nomegestrol acetate. Maturitas. 2012;71(4):345–353.
- Reed S, Koro C, DiBello J, et al. Prospective controlled cohort study on the safety of a monophasic oral contraceptive containing nomegestrol acetate (2.5mg) and 17β-oestradiol (1.5mg) (PRO-E2 study): risk of venous and arterial thromboembolism. Eur J Contracept Reprod Health Care. 2021;26(6):439–446.
- Reed S, Koro C, DiBello J, et al. Unintended pregnancy in users of nomegestrol acetate and 17β-oestradiol (NOMAC-E2) compared with levonorgestrel-containing combined oral contraceptives: final results from the PRO-E2 study. Eur J Contracept Reprod Health Care. 2021;26(6):447–453.
- SAS Institute Inc., The SAS system for Windows. Cary, NC: SAS; 2013.
- Kantorová V. Unintended pregnancy and abortion: what does it tell us about reproductive health and autonomy? Lancet Glob Health. 2020;8(9):e1106–e1107.
- Surkan PJ, Strobino DM, Mehra S, et al. Unintended pregnancy is a risk factor for depressive symptoms among socio-economically disadvantaged women in rural Bangladesh. BMC Pregn Childbirth. 2018;18(1):490.
- Bearak J, Popinchalk A, Alkema L, et al. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: estimates from a bayesian hierarchical model. Lancet Glob Health. 2018;6(4):e380–e389.
- Susser M. What is a cause and how do we know one? A grammar for pragmatic epidemiology. Am J Epidemiol. 1991;133(7):635–648.
- Read CM. New regimens with combined oral contraceptive pills–moving away from traditional 21/7 cycles. Eur J Contracept Reprod Health Care. 2010;15 Suppl 2(Suppl 2):S32–S41.