Abstract
Objective: This study investigates the effects of CangFu Daotan Decoction (CDD) on m6A methylation and Wnt/β-catenin pathway in rats with polycystic ovary syndrome (PCOS).Methods: The PCOS rat model was established by letrozole gavage. The rats were fed high-fat chow, and their body weight and blood glucose were recorded. The expressions of follicle-stimulating hormone(FSH), luteinizing hormone(LH), and testosterone(T) were quantified by ELISA. Chemical components in CDD were analyzed using UPLC-Q/TOF-MS. Based on network pharmacology methods, related targets of CDD on PCOS were screened. An enrichment analysis according to Tokyo Encyclopedia of Genes and Genomes (KEGG) was conducted to predict the potential signaling pathway of CDD in PCOS. The expressions of Wnt-1, β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, Bax, and PCNA were detected by western blotting. The expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 were monitored by RT-PCR. The expressions of Mettl3, Fto, and Ythdf1 were detected by western blotting.Results: Letrozole and a high-fat diet induced ovarian dysfunction in rats, which was attenuated by CDD. CDD decreased blood glucose, LH, and T concentrations and increased FSH expression in PCOS. After removing duplicates, a total of 71 compounds were identified by UHPLC-Q/TOF-MS, among which terpenoids and flavonoids account for the main proportion. The clustering analysis showed that the active site of CDD might be in the Wnt/β-catenin pathway. CDD decreased the expressions of Wnt-1, β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, and Bax and increased PCNA expression in the ovarian tissue of PCOS rats. CDD decreased the m6A gene expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 in peripheral blood and ovarian tissue of PCOS rats. CDD reduced the m6A proteins expressions of Mettl3, Fto, and Ythdf1 in the ovarian tissue of PCOS rats.Conclusion: CDD can regulate m6A modification and inhibit the Wnt/β-catenin signaling pathway in PCOS rats, thereby reducing body weight, lowering blood glucose levels, improving sex hormone disorders, and decreasing autophagy and apoptosis in ovarian tissue to promote the recovery of ovarian morphology.
Introduction
Polycystic ovary syndrome (PCOS) is the most common reproductive endocrine disorder in women of reproductive age, with a global prevalence of 6%–20% [Citation1]. The clinical features are highly heterogeneous and include reproductive (infertility, hyperandrogenemia, and hirsutism), metabolic (insulin resistance, impaired glucose tolerance, type 2 diabetes, and adverse cardiovascular risk), and psychological features (increased anxiety and depression), as well as a deterioration in the quality of life [Citation2]. Polycystic ovarian changes are the main morphologic feature of PCOS, which is defined according to the Rotterdam criteria as the presence of 12 or more follicles of 2–9 mm in diameter in one ovary and/or an increase in ovarian volume (>10 ml) [Citation3].
Methylation is the most common type of RNA modification in eukaryotes. More than 150 types of RNA methylation have been identified, among which N6-methyladenosine (m6A) is the most representative post-transcriptional RNA modification [Citation4]. Specific inactivation of METTL3, a key m6A methyltransferase in oocytes, leads to DNA damage, follicle development defects, and abnormal ovulation [Citation5]. PCOS is often associated with overweight/obesity and insulin resistance, and the obesity-associated gene (FTO), a m6A demethylase, regulates adipogenesis and obesity progression [Citation6–8]. Several meta-analyses have revealed that genetic variants of FTO are associated with the risk of developing PCOS and insulin resistance [Citation9–12].
Wnts are secreted glycoproteins that regulate multiple signaling pathways through β-catenin (CTNNB1)-dependent, non-CTNNB1-dependent, and Wnt/Ca2+-related mechanisms [Citation13]. There is growing evidence that Wnt signaling plays an important role in follicle development as Wnt2 and Wnt4 are expressed in ovarian granulosa cells at all follicular development stages [Citation13]. The classical Wnt signaling pathway, namely Wnt/β-catenin signaling pathway, is involved in cell development and affects cell proliferation, differentiation, and apoptosis [Citation14]. The Wnt/β-catenin signaling pathway is also involved in the apoptosis of PCOS granulosa cells, which affects follicular development and maturation [Citation15]. Gene expression profiling confirmed that Wnt1 and GSK3β, genes of Wnt/β-catenin signaling pathway, are increased in oocytes of PCOS patients [Citation16]. A prospective comparative study found that upregulation of FOXO3 mRNA expression in luteinized granulosa cells of non-obese PCOS patients after controlled superovulation was associated with m6A modification [Citation17]. FOXO is linked to several signaling pathways, including the Wnt signaling pathway [Citation18], and m6A modification can affect the expression of related Wnt/β-catenin signaling pathway protein expression [Citation19].
Traditional Chinese Medicine (TCM) has been widely used as a complementary and alternative therapy for treating chronic diseases. For example, CangFu Daotan Decoction (CDD) can improve endometrial blood flow and insulin resistance (IR), increase endometrial tolerance, and improve the pregnancy rate in PCOS patients [Citation20]. Animal experiments revealed that CDD could improve follicular development and IR, inhibit apoptosis and inflammatory cytokine production, reduce morphological damage to the ovary, as well as regulate lipid metabolism and sex hormone secretion [Citation21,Citation22].
Although CDD is effective for treating PCOS in clinical practice, its mechanism of action has not been fully elucidated. Therefore, this study investigated the mechanism of action of CDD in a PCOS rat model.
Materials and methods
Ethical statement
All experimental operations were approved by the Animal Experimental Research Center of Zhejiang Chinese Medical University (ethics reference number: 20210809-08).
Preparation and identification of CDD
CDD was provided by the pharmacy of Zhejiang Hospital of Integrated Traditional Chinese and Western Medicine consisted of Rhizomam Atractylodes 12 g, Rhizoma Cyperi 12 g, Pericarpium Citri Reticulatae 9 g, Arisaematis Rhizoma 6 g, Fructus Aurantii 12 g, Poria Cocos 9 g, Licorice 6 g, Zingiber officinale Roscoe 6 g, and Massa Medicata Fermentata 6 g. The daily doses for adults and rats are 1.3 g/kg and 1.625 g/kg, respectively. According to the standard method of Chinese Pharmacopeia, all crude drugs were soaked in 12 times the volume of water for 40 min and boiled for 40 min. The extract was then filtered, the filtrate was boiled eight times the volume of water for another 40 min, and the solution was filtered. The two batches of drug filtrate were mixed and concentrated to 1.3 g/mL in preparation for gavage administration. The main chemical components of CDD were characterized by UHPLC-Q/TOF-MS on a Waters ACQUITY I-Class Plus UPLC system (Waters, USA) combined with a SCIEX X-500R Q/TOF-MS (AB SCIEX, USA). The analysis was conducted in positive and negative ionization modes, with accurate relative molecular mass and tandem mass spectrometry information for assisted interpretation through UNIFI software (Waters).
Experimental animals
Fifty 8-week-old female specific-pathogen-free (SPF) SD rats weighing 110 ± 10 g were obtained from the Animal Experiment Center of Zhejiang Chinese Medical University [Animal Certificate of Conformity No. SYXK (Zhe) 2021-0012]. The rats were housed in an SPF environment at a constant temperature (24 °C) and relative humidity (45 ∼ 55%). They had free access to sterile food and water and were exposed to a 12h light/dark cycle.
Animal grouping and PCOS model
The rats were randomly divided into normal and experiment groups(n = 10 in each group). The normal group was fed with normal chow and received 0.5% carboxymethylcellulose(CMC) by gavage, and the experimental rats were fed with high-fat chow and received letrozole 1.0 mg/kg (1 ml/kg/d, dissolved in 0.5% CMC) by gavage for 21 days. The body weight and estrous cycles were recorded, and vaginal smears were performed to assess PCOS establishment. After successful model establishment, the rats were randomly divided into model, CDD, metformin and inhibitor groups. The CDD group was administered 1.625 g/kg/d CDD, the metformin group received 200 mg/kg/d metformin, and the inhibitor group was administered 10 µM/kg/d IWR by gavage for four weeks.
HE staining
After four weeks, the rats were anesthetized with 3% pentobarbital to harvest the ovaries. The ovaries were washed with cold saline, dried on filter paper, and fixed in 10% formalin for 48 h before embedding in paraffin. Then, 5-μm sections were cut and baked for 1 h, dewaxed, and stained with hematoxylin at 37 °C for 5 min before rinsing with tap water for 10 min. The sections were then soaked in ethanol containing 1% hydrochloric acid for 1 ∼ 5 s, rinsed in tap water for 10 min, and distilled water for a final washing 1 ∼ 2 s, then 90% and 95% for 5 min each and 100% alcohol for 10 min. The sections were dewaxed by washing with xylene for 10 min, coated with neutral fragrance oil, and allowed to dry before visualization under a light microscope.
Elisa
The expressions of serum FSH, LH, and T were quantified by ELISA according to the manufacturer’s instructions. The assay was performed in double replicate wells for all samples.
RT-PCR
Total RNA was extracted by Trizol and reverse transcribed using PrimeScript RT kit. The primers for Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, Ythdf2, and GADPH listed in were synthesized by Shanghai Thermo Fisher Biotechnology Co. RT-PCR was performed using SYBR® PreMix Ex TaqTM II kit according to the manufacturer’s protocol. The reaction mix (15 μL) contained 7.5 μL of SYBR®PreMix Ex TaqTM II (2×), 0.5 μL of forward primer, 0.5 μL of reverse primer, 0.05 μL of ROX reference dye (50×), 2 μL of template, and 5.5 μL of double-distilled water (DDH2O). RT-PCR was performed on an ABI Prism® 7000 instrument (Applied Biosystems, California, USA) with the following cycling conditions: pre-denaturation at 95 °C for 30 s, followed by denaturation at 95 °C for 5 s and annealing extension at 60 °C for 30 s for 40 cycles. U6 and GADPH were used as internal standards. The relative expression of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 were calculated by the 2-ΔΔCT method, and all experiments were performed independently three times.
Table 1. Primer sequences for RT-PCR.
Table 2. Compounds in CDD with OB larger than 30% and DL larger than 0.18, which combined with the results of UHPLC-Q/TOF-MS.
Western blotting
The proteins were extracted, quantified by BCA method, and electrophoresed on 10% SDS-PAGE gels for 90 min. The proteins were transferred to PVDF membranes and blocked in 5% skimmed milk for 2 h before incubation overnight (4 °C) with the primary antibodies anti-Wnt-1, β-Catenin, GSK-3β, C-MYC, P62, Beclin1, LC3II, Bax, and PCNA. Then, the membranes were incubated with horseradish peroxidase-labeled secondary antibodies for 2 h at room temperature, and the proteins were detected by enhanced chemiluminescence (ECL).
Active ingredients and target prediction of CDD
The TCMSP database(http://lsp.nwu.edu.cn/tcmsp.php) was searched for all the active ingredients of components of CDD. They were screened according to the criteria of oral bioavailability(OB ≥ 30%) and drug-likeness (DL ≥ 0.18) [Citation23]. Then, each target of the active ingredient was obtained from TCMSP.
Related targets of PCOS
The disease target genes related to PCOS were retrieved from the GeneCards database(https://www.genecards.org/) and OMIM database(https://www.omim.org/) with ‘polycystic ovary syndrome’ as the keyword. In the GeneCards database, targets were screened with a relevance score > 5. The results of the two databases were pooled, and duplicates were removed to obtain the final disease targets for PCOS.
Drug-disease common targets
Drug and disease targets were imported into the jvenn website (http://jvenn.toulouse.inra.fr/) for visual analysis, and their common targets were obtained by establishing a Venn diagram.
Construction of protein-protein interaction (PPI) network
The data of protein-protein interaction (PPI) are from STRING (https://string-db.org/,version 11.5) with the species limited to ‘Homo sapiens’ and the minimum required interaction score > 0.4. Each node represents a protein, and each edge represents an interaction. More edges mean more importance of the node.
Gene ontology (GO) and KEGG pathway enrichment analysis
The GO and KEGG enrichment analysis was performed using the functional annotation tool of DAVID Bioinformatics Resources 6.7 (https://davidd.ncifcrf.gov/). With Benjamini–Hochberg method to control the false discovery rate (FDR) for multiple hypothesis tests, the adjusted p-value < 0. 05 was used as the significance cutoff.
Statistical analysis
All data were analyzed using SPSS 25.0 software (IBM, Armonk, New York, USA) and are expressed as mean ± standard deviation. T-tests were employed to compare two groups, and a one-way analysis of variance (ANOVA) was utilized for comparisons between multiple groups. A p < 0.05 was considered statistically significant.
Results
Changes of estrous cycle in PCOS rat models
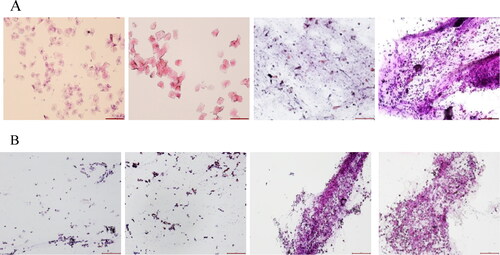
Vaginal smears of rats in normal control and polycystic ovary syndrome groups are displayed in . The estrous cycle of the control rats is generally 4–5 days, including the proestrus, estrus, metestrus, and diestrus stages (). Nucleated epithelial cells were observed in the proestrus, stageirregularly shaped and interconnected keratin-forming cells in the estrus stage, nucleated epithelial cells, irregular epithelial keratin-forming cells, and leukocytes in the metestrus stage and numerous leukocytes in the diestrus stages. However, after letrozole administration, PCOS group rats had an irregular cycle with a prolonged diestrus stages and a large number of leukocytes.
Effect of CDD on ovarian morphology in PCOS rat models
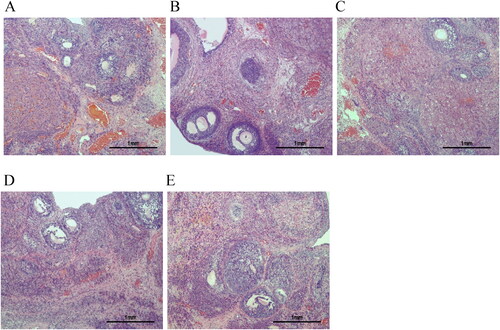
HE staining showed normal ovarian histological morphology in the control group () with multiple corpus luteum and follicles, as well as normal oocytes and multiple layers of granulosa cells in the follicles. Multiple follicles were visible in the PCOS group () with obvious cystic dilatation, a reduced number of granulosa cell layers, and an absence of oocytes in the follicles. After treatment, the morphological changes in the ovaries of PCOS rats were partially restored, with the appearance of oocytes, an increased number of granulosa cell layers, and decreased cystic follicles ().
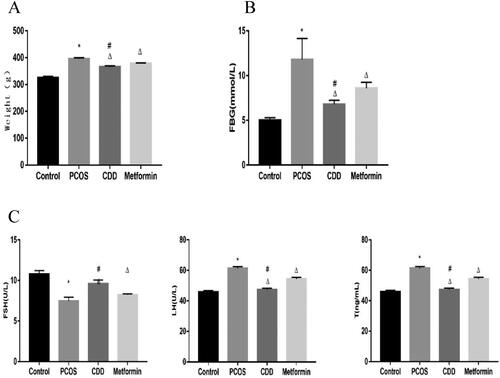
Effect of CDD on body weight, blood glucose, and serum hormones in PCOS rats
As depicted in , the weight and blood glucose significantly increased in PCOS group, while the weight and blood glucose in CDD and metformin groups decreased (p < 0.05). Compared to metformin group, the weight and blood glucose decreased in CDD group (p < 0.05). FSH significantly decreased in PCOS group, while FSH in CDD and metformin groups increased (p < 0.05). Compared to metformin group, FSH increased in CDD group (p < 0.05). LH and T significantly increased in PCOS group, while LH and T in CDD and metformin groups decreased (p < 0.05). Compared to metformin group, LH and T decreased in CDD group (p < 0.05).
Figure 3. Effect of CDD on body weight, blood glucose, and serum hormones in PCOS rats. A: body weight. B: blood glucose. C: serum hormones levels including FSH, LH, and T. Data are shown as means ± SD (n = 10), and data between multiple groups were compared by one-way ANOVA. Compared with the normal group, *p < 0.05; compared with the PCOS group, △p < 0.05; compared with the metformin group, #p < 0.05.
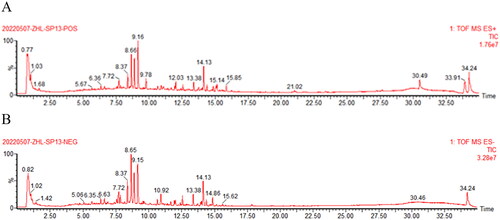
Chemical components in CDD
manifested the total ion chromatogram of CDD in positive ion mode () and negative ion mode (). In positive ion mode,65 kinds of components were identified. In negative ion mode, 28 kinds of components were identified, some of which were the same as those in the positive mode (Supplementary Table S1). A total of 71 compounds were identified after removing duplicates. There were 26 flavonoids (36.62%), 27 terpenoids (38.03%), and 18 others (25.35%), including fatty acids, phenols, coumarins, quinones, etc.
Figure 4. Characteristics of chemical components in CDD by UHPLC-Q/TOF-MS analysis. A: positive ion mode. B: negative ion mode.
According to the criteria of OB ≥ 30% and DL ≥ 0.18 in TCMSP database,155 chemical ingredients were screened out (Supplementary Table S2). After taking out the duplicated parts, combined with the results of UHPLC-Q/TOF-MS assay,141 chemical constituents were accepted. Finally,12 chemical ingredients were screened out for further target prediction analysis .
Identification of CDD-related targets
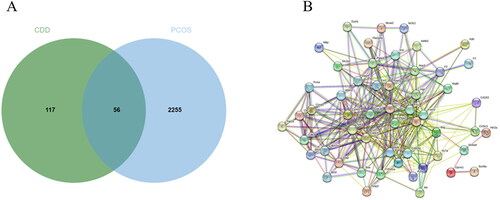
As a result, GenesCard produced 2,311 distinct targets associated with PCOS, including almost all the targets related to PCOS that have already been identified or are currently being investigated. Potential targets of CDD include genes associated with PCOS progression or treatment. 56 of the 2,311 PCOS-related genes were closely associated with CDD. shows the number of overlaps between CDD-related genes and PCOS-related genes. 57 nodes and 381 edges were included in a global view of the PPI network in . These findings suggested that CDD interacted with PCOS in a multi-target, multi-pathway, and overall integrative manner.
Enrichment analysis and construction of the regulatory network
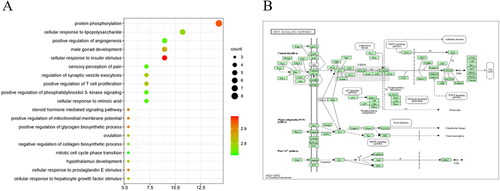
The above possible CDD target genes were introduced into GO functional enrichment analysis, mainly enriched in glucose homeostasis, cellular response to insulin stimulus, protein phosphorylation, positive regulation of mitochondrial membrane potential, etc. In , the results were sorted according to the significance from low to high, and the top 20 of the significance were selected for bubble chart display. The above possible CDD target genes were introduced into KEGG pathway analysis to study the potential signaling pathway of CDD in the treatment of PCOS. Wnt signaling pathway is involved in biological processes such as apoptosis, proliferation or autophagy of PCOS ().
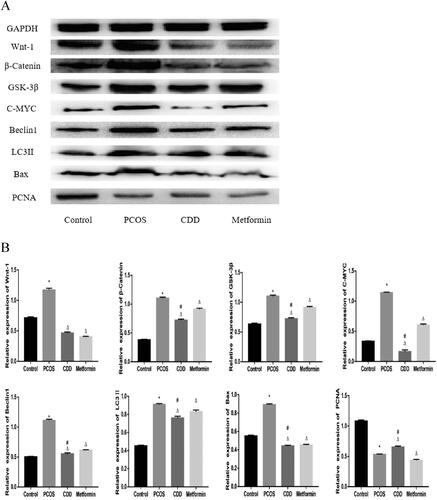
Effect of CDD on the Wnt/β-catenin signaling pathway, autophagy, apoptosis, and proliferation proteins in the ovarian tissue of PCOS rats
As depicted in , compared to the normal group, the expressions of Wnt-1, β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, and Bax were elevated, and PCNA expression decreased in PCOS group (p < 0.05). Compared to PCOS group, CDD and metformin groups had lower expressions of Wnt-1, β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, and Bax, with increased PCNA expression (p < 0.05). Compared to metformin group, the expressions of β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, and Bax expressions were decreased, and PCNA expression increased in CDD group (p < 0.05).
Figure 7. Effect of CDD on the Wnt/β-catenin signaling pathway, autophagy, apoptosis, and proliferation proteins in the ovarian tissue of PCOS rats. A: The image of western blot band. B: The expression of proteins including Wnt-1, β-Catenin GSK-3β, C-MYC, Beclin1, LC3II, Bax, and PCNA. Data are shown as means ± SD (n =3), and data between multiple groups were compared by one-way ANOVA. Compared with the normal group, *p < 0.05; compared with the PCOS group, △p < 0.05; compared with the metformin group, #p < 0.05.
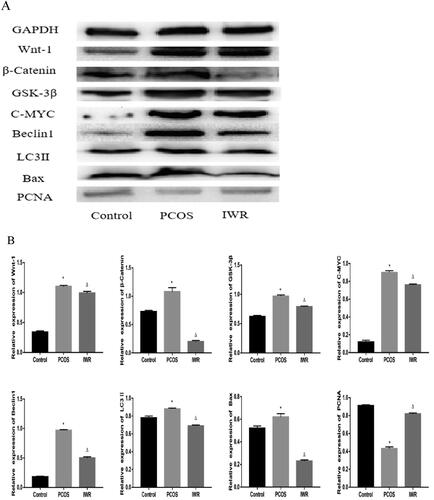
Wnt/β-catenin signaling pathway is involved in the therapeutic effect of CDD on PCOS
To investigate the role of the Wnt/β-Catenin signaling pathway in PCOS treatment by CDD, PCOS rats were gavaged with 10 µM IWR-1 (Wnt pathway inhibitor) for four weeks. As shown in , compared to the normal group, the expressions of Wnt-1, β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, and Bax were elevated, and PCNA expression decreased in PCOS group (p < 0.05). Compared to PCOS group, IWR group exhibited decreased expressions of Wnt-1, β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, and Bax, and increased expression of PCNA (p < 0.05).
Figure 8. Wnt/β-catenin signaling pathway is involved in regulating autophagy, apoptosis and proliferation in ovarian tissues of PCOS rats. A: The image of western blot band. B: The expression of proteins including Wnt-1, β-Catenin, GSK-3β, C-MYC, Beclin1, LC3II, Bax, and PCNA. Data are shown as means ± SD (n =3), and data between multiple groups were compared by one-way ANOVA. Compared with the normal group, *p < 0.05; compared with the PCOS group, △p < 0.05.
Effect of CDD on the m6A gene in the peripheral blood of PCOS rats
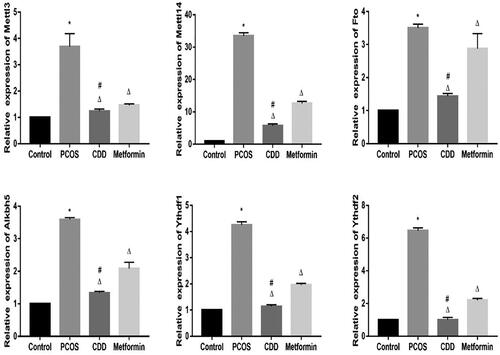
The m6A gene expression in peripheral blood of PCOS rats was detected by RT- PCR (). Compared to the normal group, the expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 were elevated in PCOS group (p < 0.05). Compared to PCOS group, the expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 in CDD and metformin groups were reduced (p < 0.05). Compared to metformin group, the expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 expressions were reduced in CDD group (p < 0.05).
Figure 9. Effect of CDD on the m6A gene in the peripheral blood of PCOS rats. The expression of m6A genes including Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2. Data are shown as means ± SD (n =10), and data between multiple groups were compared by one-way ANOVA. Compared with the normal group, *p < 0.05; compared with the PCOS group, △p < 0.05; compared with the metformin group, #p < 0.05.
Effect of CDD on the m6A gene in the ovarian tissue of PCOS rats
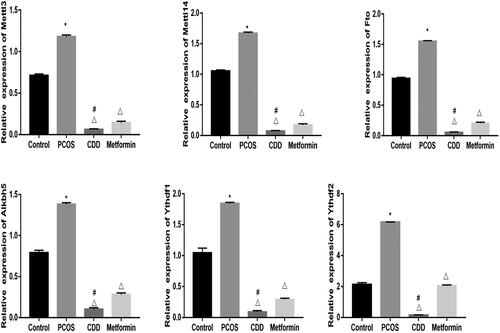
The m6A gene expression in ovarian tissue of PCOS rats was detected by RT- PCR (). Compared to the normal group, the expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 were elevated in PCOS group (p < 0.05). Compared to PCOS group, the expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 in CDD and metformin groups were reduced (p < 0.05). Compared to metformin group the expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 expressions were reduced in CDD group (p < 0.05).
Figure 10. Effect of CDD on the m6A gene in the ovarian tissue of PCOS rats. The expression of m6A genes including Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2. Data are shown as means ± SD (n =10), and data between multiple groups were compared by one-way ANOVA. Compared with the normal group, *p < 0.05; compared with the PCOS group, △p < 0.05; compared with the metformin group, #p < 0.05.
Effect of CDD on the m6A proteins in the ovarian tissue of PCOS rats
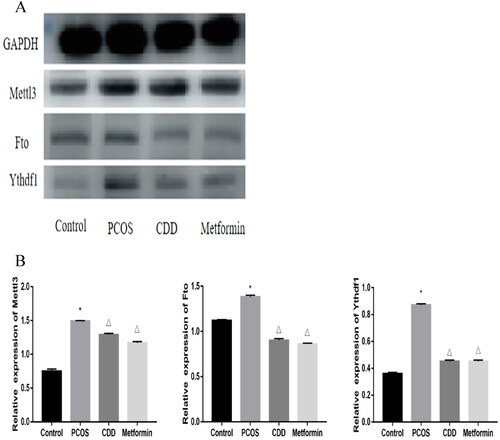
The m6A proteins expression in the ovarian tissue of PCOS rats was detected by Western blot (). Compared to the normal group, the expressions of Mettl3, Fto, and Ythdf1 were elevated in PCOS group (p < 0.05). Compared to PCOS group, the expressions of Mettl3, Fto, and Ythdf1 in CDD and metformin groups were reduced (p < 0.05).
Figure 11. Effect of CDD on the m6A proteins in the ovarian tissue of PCOS rats. A: The image of western blot band. B: The expression of proteins including Mettl3, Fto, and Ythdf1. Data are shown as means ± SD (n =3), and data between multiple groups were compared by one-way ANOVA. Compared with the normal group, *p < 0.05; compared with the PCOS group, △p < 0.05.
Discussion
In TCM, CangFu Daotan Decoction(CDD) is a complementary and alternative drug for PCOS treatment [Citation24]. Based on the characteristic study using UHPLC-Q/TOF-MS analysis, 71 compounds were identified in CDD under positive and negative ion modes, and we found that terpenoids and flavonoids widely existed in CDD. Studies have shown that terpenoids can lose weight and improve metabolic status either by inhibiting lipogenesis or by increasing lipolysis [Citation25]. Also, glycyrrhizic acid, a terpenoid compound, is the principal bioactive constituent in licorice, which reduces blood glucose levels, decreases serum insulin levels, and enhances insulin sensitivity [Citation26]. Many flavonoids have been reported to improve glucose tolerance and insulin sensitivity, modulate lipid metabolism, and suppress inflammation and apoptosis [Citation27]. Current evidence supports the positive properties of flavonoids on ovarian histomorphology and hormonal status in animal models of PCOS [Citation28]. It can be speculated that CDD may improve the metabolism of glucose and lipid, regulate hormone levels, and promote oocyte growth and development in PCOS rats through natural components such as terpenoids and flavonoids.
By using multiple technologies, network pharmacology establishes the multi-level association between ‘drug-compound-target pathway-disease’, which expose the fundamental molecular targets and pharmacodynamics methods of TCM in a systematic manner [Citation29]. In this study, we investigated the potential targets and pathways of CDD in PCOS.
This study investigated the mechanism of action of CDD in a rat model of PCOS, revealing that CDD treatment reduced body weight, improved blood glucose and sex hormone levels, and restored ovarian morphological damage in PCOS rats. Further experiments revealed that CDD could inhibit the Wnt/β-catenin signaling pathway and reduce m6A genes expressions.
Androgen overload can cause follicular dysplasia or atresia, ultimately leading to anovulation or poor ovulation [Citation30]. LH and T levels of PCOS rats treated with CDD decreased compared to the model group, and ovarian sections suggested the appearance of oocytes, increased number of granulosa cell layers, a decrease in cystic follicles, and restoration of ovarian morphology consistent with a previous study [Citation21].
The Wnt/β-catenin signaling pathway is a highly conserved and tightly controlled molecular mechanism that regulates embryonic development, cell proliferation, and differentiation [Citation31] and is triggered by Wnt1, Wnt2, Wnt3, Wnt3a, and Wnt8 [Citation32]. β-catenin is a key protein in Wnt/β-catenin signaling pathway with important roles in embryonic development and stem cell maintenance [Citation33]. GSK-3β is a conserved serine/threonine kinase that regulates key cellular processes, including cell proliferation, DNA repair, and cell cycle progression [Citation34]. C-MYC is a transcription factor to stimulate cell cycle progression and apoptosis [Citation35]. Autophagy is important in inducing apoptosis and maintaining intracellular environment homeostasis and occurs at all follicular development stages [Citation36]. Defective autophagy in follicular cells at different developmental stages occurs in the ovaries of PCOS patients [Citation1], and Wnt/β-catenin pathway can induce autophagy and apoptosis [Citation37]. The present study confirmed that the expressions of Wnt-1, β-catenin, GSK-3β, and C-MYC, the main components of Wnt signaling pathway, decreased in the treated group compared to PCOS group, while the expressions of autophagy proteins Beclin1, LC3II, and pro-apoptotic protein Bax decreased, and the expression of proliferation protein PCNA increased. The Wnt/β-catenin pathway was significantly inactivated in the ovarian tissues of the treated rats, with reduced apoptosis and autophagy and increased proliferation. The trend in the expressions of autophagy, apoptosis, and proliferation proteins in ovarian tissues of PCOS rats after adding Wnt pathway inhibitors was the same as those in the treated group. Therefore, it was hypothesized that CDD can treat PCOS by inhibiting Wnt/β-catenin signaling pathway and increasing ovarian cell proliferation. However, further studies are required to confirm this speculation.
The m6A methylation plays an important role in follicle development, and METTL3 is an important component of the m6A methyltransferase complex. Animal experiments revealed that METTL3 knockdown in mouse germ cells reduced the translation efficiency of mRNA, which severely inhibited oocyte maturation [Citation38]. Enrichment analysis revealed that m6A methylation targets key genes for many critical biological processes associated with transcription, cell proliferation, and cancer-related pathways, such as the Wnt signaling pathway [Citation39]. Multiomics analysis confirmed that the most frequently occurring gene as a potential target interacting with m6A modification in cancer is the protein-coding gene BCL9L, an important member of the Wnt signaling pathway [Citation40].
The m6A methylation is catalyzed by the methyltransferase complex (MTC) composed of Mettl3 and Mettl14 and their cofactors, which exert biological functions by binding to proteins containing YTH structural domain, including Ythdf1-3 [Citation41]. RNA methylation process can be reversed by RNA demethylases, which include Fto and Alkbh5 [Citation42]. In this study, the expressions of Mettl3, Mettl14, Fto, Alkbh5, Ythdf1, and Ythdf2 were significantly elevated in the peripheral blood and ovarian tissue of PCOS rats compared to the normal group, and the expressions of these genes decreased after treatment with CDD or metformin. Also, the m6A proteins expressions of Mettl3, Fto, and Ythdf1 in the ovarian tissue of PCOS rats reduced after treating with CDD. These results confirmed that m6A methylation was altered in PCOS rats and CDD could affect m6A gene expression.
Conclusion
CDD can prevent ovarian apoptosis and autophagy, and promote proliferation by inhibiting Wnt/β-catenin signaling pathway, thus revealing new therapeutic strategies for treating PCOS. This study also provides a new piece of evidence that m6A methylation was altered in ovarian tissue of PCOS rats and CDD can regulate m6A methylation.
Authors’ contributions
ZYW and DCF designed the study. ZYW drafted the manuscript. ZYW and ZHL performed the experiments, and proofread the manuscript. All the authors have read and approved the final manuscript. ZYW and DCF confirm the authenticity of all the raw data.
Ethics approval and consent to participate
All animal experiments are conducted under the approval of the Animal Experimental Research Center of Zhejiang Chinese Medicine University to minimize the suffering of animal subjects. The ethics number is 20210809-08.
Supplemental Material
Download Zip (60.6 KB)Availability of data and materials
The data and materials used to support the findings of this study are available from the corresponding author upon request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kumariya S, Ubba V, Jha RK, et al. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):1–12.
- Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41.
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Sterility. 2003;81(1):19–25.
- Dai X, Ren T, Zhang Y, et al. Methylation multiplicity and its clinical values in cancer. Expert Rev Mol. 2021;23:1–10.
- Mu H, Zhang T, Yang Y, et al. METTL3-mediated mRNA N6-methyladenosine is required for oocyte and follicle development in mice. Cell Death Dis. 2021;12(11):989.
- Chen J, Du B. Novel positioning from obesity to cancer: FTO, an m6A RNA demethylase, regulates tumour progression. J Cancer Res Clin Oncol. 2019;145(1):19–29.
- Deng X, Su R, Stanford S, et al. Critical enzymatic functions of FTO in obesity and cancer. Front Endocrinol (Lausanne). 2018;9:396.
- Lan N, Lu Y, Zhang Y, et al. FTO - a common genetic basis for obesity and cancer. Front Genet. 2020;11:559138.
- Liu Y, Chen Y. Fat mass and obesity associated gene polymorphism and the risk of polycystic ovary syndrome: a meta-analysis. Iran J Public Health. 2017;46(1):4–11.
- Liu AL, Xie HJ, Xie HY, et al. Association between fat mass and obesity associated (FTO) gene rs9939609 a/T polymorphism and polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med Genet. 2017;18(1):89–95.
- Kowalska I, Malecki MT, Straczkowski M, et al. The FTO gene modifies weight, fat mass and insulin sensitivity in women with polycystic ovary syndrome, where its role may be larger than in other phenotypes. Diabetes Metab. 2009;35(4):328–331.
- Tan S, Scherag A, Janssen OE, et al. Large effects on body mass index and insulin resistance of fat mass and obesity associated gene (FTO) variants in patients with polycystic ovary syndrome (PCOS). BMC Med Genet. 2010;11:12–20.
- Li L, Shi X, Shi Y, et al. The signaling pathways involved in ovarian follicle development. Front Physiol. 2021;12:730196.
- Liu X, Chen D, Wu Z, et al. Ghrelin inhibits high glucose-induced 16HBE cells apoptosis by regulating wnt/β-catenin pathway. Biochem Biophys Res Commun. 2016;477(4):902–907.
- Wu XQ, Wang YQ, Xu SM, et al. The WNT/β-catenin signaling pathway may be involved in granulosa cell apoptosis from patients with PCOS in North China. J Gynecol Obstet Hum Reprod. 2017;46(1):93–99.
- Ismail AB, Naji M’S, Nebih İ, et al. The expression profile of WNT/β-catanin signalling genes in human oocytes obtained from polycystic ovarian syndrome (PCOS) patients. Zygote. 2022;30(4):536–542.
- Zhang S, Deng W, Liu Q, et al. Altered m6 a modification is involved in up-regulated expression of FOXO3 in luteinized granulosa cells of non-obese polycystic ovary syndrome patients. J Cell Mol Med. 2020;24(20):11874–11882.
- Maiese K. Forkhead transcription factors: Formulating a FOXO target for cognitive loss. Curr Neurovasc Res. 2017;14(4):415–420.
- Uddin MB, Wang Z, Yang C. The m6A RNA methylation regulates oncogenic signaling pathways driving cell malignant transformation and carcinogenesis. Mol Cancer. 2021;20(1):61.
- Ding CF, Wang CY, Yang X, et al. Effect of modified cangfu daotan decoction in improving endometrial receptivity in infertility patients with polycystic ovarian syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(11):1297–1301.
- Wang C, Ding C, Hua Z, et al. Cangfudaotan decoction alleviates insulin resistance and improves follicular development in rats with polycystic ovary syndrome via IGF-1-PI3K/Akt-Bax/bcl-2 pathway. Mediators Inflamm. 2020;2020:8865647.
- Yi W, Li X, Chen K, et al. Effects of cangfu daotan decoction on obese polycystic ovary syndrome and its mechanism. Steroids. 2021;165:108740.
- Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminf. 2014;6:13.
- Xu W, Tang M, Wang J, et al. Identification of the active constituents and significant pathways of cangfu daotan decoction for the treatment of PCOS based on network pharmacology. Evid Based Complement Altern Med. 2020;2020:4086864.
- Yang XD, Ge XC, Jiang SY, et al. Potential lipolytic regulators derived from natural products as effective approaches to treat obesity. Front Endocrinol (Lausanne. 2022;13:1000739.
- Wang LY, Cheng KC, Li Y, et al. Glycyrrhizic acid increases glucagon like peptide-1 secretion via TGR5 activation in type 1-like diabetic rats. Biomed Pharmacother. 2017;95:599–604.
- Gandhi GR, Vasconcelos ABS, Wu DT, et al. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: a systematic review of in vitro and in vivo studies. Nutrients. 2020;12(10):2907.
- Zhang J, Zhang H, Xin X, et al. Efficacy of flavonoids on animal models of polycystic ovary syndrome: a systematic review and meta-analysis. Nutrients. 2022;14(19):4128.
- Gao Y, Dai H, Zhang N, et al. The ameliorative effect of mahuang fuzi and shenzhuo decoction on membranous nephropathy of rodent model is associated with autophagy and wnt/β-Catenin pathway. Front Pharmacol. 2022;13:820130.
- Song L, Yu J, Zhang D, et al. Androgen excess induced mitochondrial abnormality in ovarian granulosa cells in a rat model of polycystic ovary syndrome. Front Endocrinol (Lausanne. 2022;13:789008.).
- He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851.
- Xu X, Zhang M, Xu F, et al. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19(1):165.
- Yang F, Xiong H, Duan L, et al. MiR-1246 promotes metastasis and invasion of A549 cells by targeting GSK-3β–mediated wnt/β-Catenin pathway. Cancer Res Treat. 2019;51(4):1420–1429.
- Pecoraro C, Faggion B, Balboni B, et al. GSK3β as a novel promising target to overcome chemoresistance in pancreatic cancer. Drug Resist Updates. 2021;58:100779.
- Vermeulen K, Berneman ZN, Van Bockstaele DR. Cell cycle and apoptosis. Cell Prolif. 2003;36(3):165–175.
- Zhou J, Peng X, Mei S. Autophagy in ovarian follicular development and atresia. Int J Biol Sci. 2019;15(4):726–737.
- Chu CW, Ko HJ, Chou CH, et al. Thioridazine enhances P62-Mediated autophagy and apoptosis through wnt/β-Catenin signaling pathway in glioma cells. Int J Mol Sci. 2019;20(3):473.
- Sui X, Hu Y, Ren C, et al. METTL3-mediated m6A is required for murine oocyte maturation and maternal-to-zygotic transition. Cell Cycle. 2020;19(4):391–404.
- Zhang SY, Zhang SW, Fan XN, et al. Global analysis of N6-methyladenosine functions and its disease association using deep learning and network-based methods. PLoS Comput Biol. 2019;15(1):e1006663.
- Shen S, Zhang R, Jiang Y, et al. Comprehensive analyses of m6A regulators and interactive coding and non-coding RNAs across 32 cancer types. Mol Cancer. 2021;20(1):67.
- Lan Q, Liu PY, Haase J, et al. The critical role of RNA mA methylation in cancer. Cancer Res. 2019;79(7):1285–1292.
- Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29(13):1343–1355.