Abstract
Purpose: Despite the availability of several markers for the evaluation of ovarian reserve, the correlation between the markers has not been reported clearly in the existing studies. Therefore, this study investigated the level of subfertility by comparing the physical parameters such as age and BMI in the subfertile women. In addition, the study compared the ultrasound and hormonal parameters with the physical parameters in subfertile women.
Methods: A total of 200 subfertile patients presented to outpatient department were considered in this study. The selected candidate was 29 to 39 years old and was investigated after two years of unprotected sexual intercourse. A consecutive enumerative sampling method has been employed for data collection. The collected data are processed to determine correlation and regression coefficients using Statistical Package for Social Sciences (SPSS) version 20.0.
Results: The results revealed that follicle stimulating hormone (FSH) level and anti-Mullerian hormone (AMH) values varied among the above 30 age group of respondents with BMI values 25–30. There is no relationship between the respondents’ right and left ovarian volume by comparing age within BMI.
Conclusion: In conclusion, there is a strong relationship between the FSH level, AMH and physical parameters age and BMI. The reproductive age of women will be older than or younger than the actual age of the women. The reproductive age will be calculated with the ovarian volume, ovarian reserve, ovary size and time to menopause.
Introduction
Delayed childbearing, involuntary or voluntary, is a generalized feature in couples who visit fertility clinics. Alternatively, (ovarian reserve) has a significant role in achieving pregnancy by following any treatment in subfertile women. Alternatively, estimation has been performed routinely through several ovarian reserve tests (ORTs) that predict the response and outcome of the couples choosing in vitro fertilization [Citation1]. Frequently used tests are basal follicle stimulating hormone (FSH), anti-Mullerian hormone (AMH) [Citation2] and antral follicle count (AFC).
ORT provides an approximate estimation of the ovarian follicular pool. Ideal ORT must be easier to reproduce and perform, and the corresponding decision based on their results must help to differentiate between women with poor and normal ovarian responses [Citation3]. Thus, this helps identify and counsel the couples with a minimum chance for conception against repeated and expensive treatment. Nevertheless, the availability of various reserve markers suggested that nothing seemed ideal [Citation4]. These tests were utilized in the subfertile women former to the first IVF attempt to predict poor ovarian response. In recent years, several studies have investigated their values in predicting hyper-response and utilizing a safer stimulation step for preventing ovarian hyper-stimulation syndrome (OHSS). Reducing the ovarian reserve is noted in the female during the mid to late 30s, reflecting the deteriorating oocyte quality and follicular pool [Citation5]. Assuming fixed time variations between the reproductive milestones, the fertility will not get lost completely, on average, for four years [Citation6]. The primary evidence proposed that several ORTs possess better predictive values for pregnancy [Citation7].
Nevertheless, recently, it was understood that these tests were more efficient in predicting ovarian response only for stimulation purposes but not predicting pregnancy. Furthermore, the interpretation of results was complicated by inadequate uniform threshold values and uniform definitions for hyper or poor responders for identifying the abnormal results. In addition, the study utilized histological, biophysical, biochemical and biological tests to find the ovarian reserve.
The ovarian reserve tests possess a moderate ability to predict hyper and poor responses. It is essential for providing balance in the prediction of exogenous gonadotrophins. Thus, it prevents hypo-response, leading to cycle missing and low pregnancy rates. Furthermore, the pretreatment workup that measures the right combination of the ovarian reserve parameter was tried in literature several times. However, the group of ovarian markers correctly predicting ovarian function is still under debate. To overcome these existing confusions, a comprehensive analysis of the ovarian reserve parameters should be implemented.
Premature ovarian failure impacts about 10% of the overall general population and is a principal reason for infertility. Furthermore, body mass index (BMI) is a major component of subfertility [Citation8]. The most frequently utilized techniques incorporate valuation of biochemical markers like AFC, anti-mullerian, inhibin, estradiol and FSH per ovary. Furthermore, the antral follicles are 2–8 m in diameter, which can be counted, seen and measured by transvaginal ultrasound (TVU).
Research gap
The following are research gaps in the prevailing articles associated with the present study. Nevertheless, the prevailing evidence depicts that AMH and AFC are useful ovarian reserve markers. In addition, AMH can be applied to the general population to find the weakening ovarian reserve before reaching its critical level, which cannot be effectively treated. It may help women who wish to delay pregnancy to make an informed decision [Citation9]. The study’s main contribution is to evaluate the association of dependent variables, the ovarian reserve hormonal and ultrasound parameters with independent variables age, BMI and menstrual cycle length in subfertile women.
Need for the study
Generally, the ovarian function of a female depends on not only age but also the strength of the ovary. Hence childbearing complexities and decisions about the rectification through assisted reproductive technology (ART) should not be made only with physical parameters like age. Awareness must be created in young women with diminished ovarian reserve (OR) that despite their age, they are in the urge to uptake ART. On the other hand, women with a good ovarian reserve and belonging the age >30 have to be motivated by the fact that they can conceive without the aid of ART. Hence, the proper assessment of physical, hormonal and biological parameters enables the precise utilization of ART among females. Hence, the present study focused on establishing the correlation between physical parameters such as BMI, menstrual cycle length and age with ultrasound and hormonal parameters.
Objectives
To assess the level of subfertility based on physical parameters comparison.
To compare the ovarian reserve hormonal and ultrasound parameters with physical parameters like age and BMI in subfertile women.
Materials and methodology
Subfertility diagnosis has been performed following the hormonal, ultrasound and physical parameters, followed by comparing these parameters.
Study design
Cross-sectional prospective single-centered observational study
Sample size
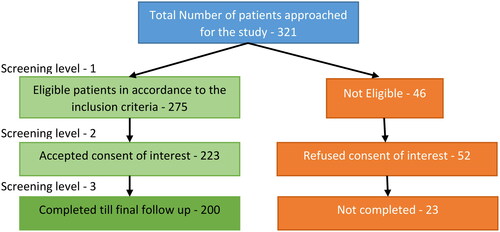
The total sample size was 200 infertile patients who presented to OPD. This sample size is finalized by the patient inflow diagram shown in . Initially, 321 patients were approached in this study, and 46 patients were excluded following the eligible criteria. Meanwhile, 275 patients met the eligibility criteria, but 52 refused the consent of interest. Furthermore, another 23 patients did not follow up on the clinical investigation until the end. Hence finally, 200 patients were included as a sample size.
Study area
Department of OBG, Saveetha Medical College, Chennai
Study duration
February 2019 to January 2021.
Inclusion criteria
The study participants are 29 to 39 years old with infertility complaints after two years of unprotected sexual intercourse. The patients possessing detailed complaints of infertility, menstrual and past medical and obstetric history were considered.
Exclusion criteria
The study excluded patients with fertility issues like Polycystic ovary syndrome (PCOS), tubal blockage, male factor infertility, endometriosis and current breastfeeding, also injectable hormonal contraception in the previous year. The study utilized a consecutive enumerative sampling method for data collection.
Methods
The research noted patients’ age, BMI and requested the patients to be present for the investigation on the second or third day of the menstrual cycle. The patients were followed up during their next cycle on the second and third day, and consequently, their blood samples were collected for serum extraction to determine FSH by fluoroimmunoassay. Furthermore, the study employed enzyme-linked florescent assay-phage and immuno concentration technology to estimate AMH. Furthermore, a transvaginal probe performed an ultrasound examination to determine AFC. These predicted markers are employed for assessing the patient’s ovarian reserve, followed by a comprehensive comparison.
Statistical analysis
The descriptive, inferential statistics have been utilized to determine the correlation coefficient, and consequently, the detection of association with the marker’s correlation coefficient was also performed. Various ultrasound and hormonal parameters were noted in the excel sheets, and a comparative analysis was performed with Statistical analysis. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 20.0. Mean standard deviation and range of values were estimated, and the correlation coefficients were determined with every set of values and Microsoft Excel 2010 was used for all statistical analyses. The frequency analysis presented the demographic, physical parameters, age and BMI as the count and percentage. ANOVA (analysis of variance) is a test used to test the significant difference among the groups. If the p value is smaller than .05, then it is shown that respondents differ significantly in this study. The two-way ANOVA test was used to assess the differences between the mean values of ultrasound and hormonal parameters in the different physical parameter groups. Bonferroni’s method revealed the significant difference between the different types of treatment. In this study, Bonferroni t test, multiple comparisons test was used to compare the physical with hormonal and ultrasound parameters and the relationship between these parameters is assessed. The study represents a multivariate analysis and is presented in a table to depict the interrelation of all the parameters to determine their corresponding significant value.
Compliance with ethical standards and consent: Informed consent was obtained from all individual participants included in the study after explaining the study’s nature. The study was approved by the Institutional Human Ethics committee, Saveetha Institute of Medical and Technical Sciences (007/01/2020/IEC/SMCH) on Jan 28, 2020. Permissions were obtained from the hospital authority Saveetha Medical College and Hospital for the recruitment of subfertile women seeking help through assisted reproductive techniques from (Jan 2020 to Jan 21). Confidentiality was maintained throughout the study. Wherever necessary, the relevant information was given to the treating doctor. Details about the ultrasound scan were kept in a password-protected computer, accessed only by the principal investigator. The currently enforced PCPNDT act did not do the gender determination of the fetus.
Results
This section provides the results obtained by the research in detail. Furthermore, this section initially discusses the parameter involved in this research ().
Table 1. Description of ovarian reserve parameters evaluated in the analysis.
Description of ovarian reserve parameters included in this study
Demographic analysis
From , it is inferred that most of the respondents are above the 30 age group, and most of the respondents’ BMI level is in the range of 25–30. Taylor [Citation10] found a strong relationship with the physical factors of age, BMI and subfertility. The chances of conceiving spontaneously in women in their late 30s and early 40s is about half that of women aged 19–26 years, and also women with BMI greater than 30 and less than 20 are less likely to get a chance to conceive.
Table 2. Physical parameter analysis.
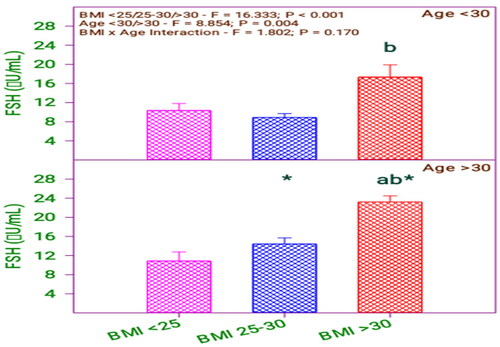
From and , it is found that there is a significant difference in FSH between the BMI group (0.001) and the age group (0.004). The letters in the graphs indicates the scale level of the measure, b indicates the higher level, ab indicates the level between a and b, and (*) asterisk indicates the effect and interaction between the variable. This applies to all the comparison graphs used in the study. Moreover, there is no significant interaction between BMI category and age category (0.170).
Table 3. Follicular stimulating hormone (FSH) levels of subfertile women in comparison with body mass index (BMI) and age (years).
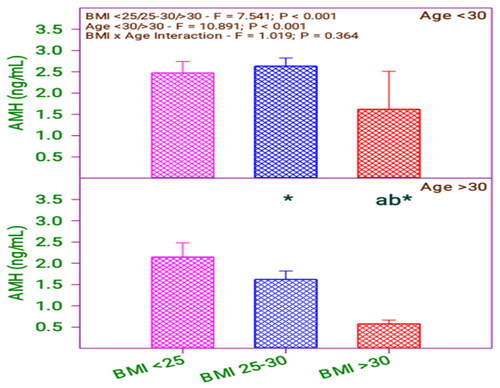
and show that AMH level significantly differs in the BMI group (0.001) and age group (0.001). Moreover, there is no significant interaction between BMI and age category (0.364).
Table 4. AMH levels of subfertile women in comparison with BMI and age (years).
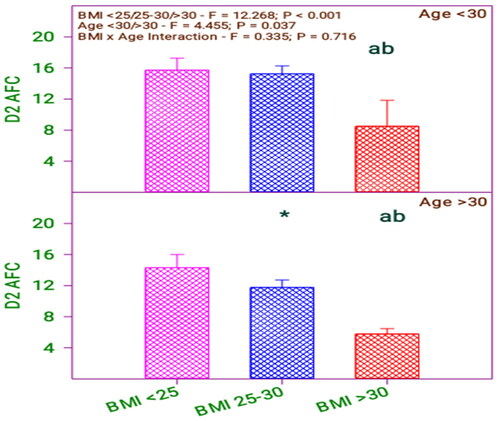
From and , it is inferred that there is a significant difference in Day 2-AFC concerning the BMI group (0.001) and age group (0.037). Moreover, there is no significant interaction between BMI and age category (0.716).
Table 5. Day 2-AFC of subfertile women in comparison with BMI and age (years).
and show that serum E2 is significantly different in the BMI group (0.001) and no significant difference with the age group (0.668). Moreover, there is no significant interaction between BMI category and age category (0.131).
Figure 5. Serum E2 levels of subfertile women in comparison with body mass index (BMI) and age (years).
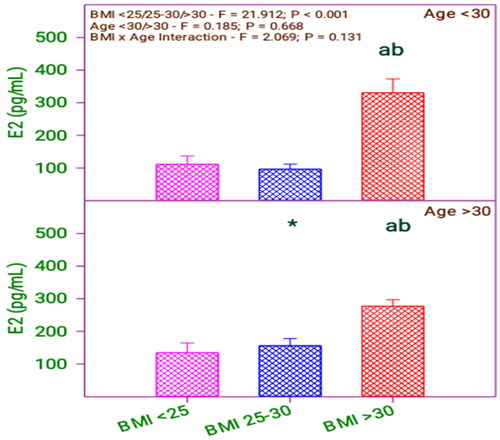
Table 6. Serum E2 levels of subfertile women in comparison with BMI and age (years).
From and , it is found that there is a significant difference in right ovarian volume concerning the BMI group (0.001) and no significant difference in the age group (0.888). Moreover, there is no significant interaction between BMI category and age category (0.142).
Figure 6. Right ovarian volume of subfertile women in comparison with body mass index (BMI) and age (years).
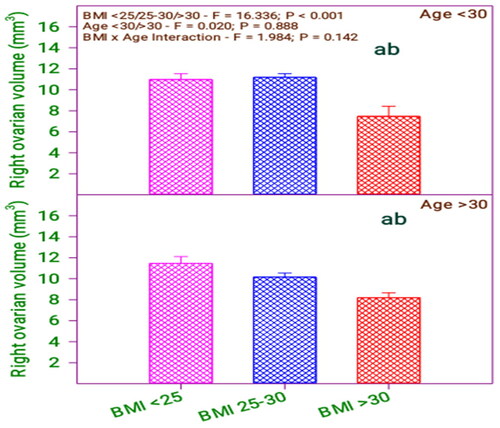
Table 7. Right ovarian volume of subfertile women in comparison with BMI and age (years).
From and , it is found that there is a significant difference in left ovarian volume in the BMI group (0.001) and no significant difference in the age group (0.965). Furthermore, there is no significant interaction between BMI category and age category (0.089).
Figure 7. Left ovarian volume of subfertile women in comparison with body mass index (BMI) and age (years).
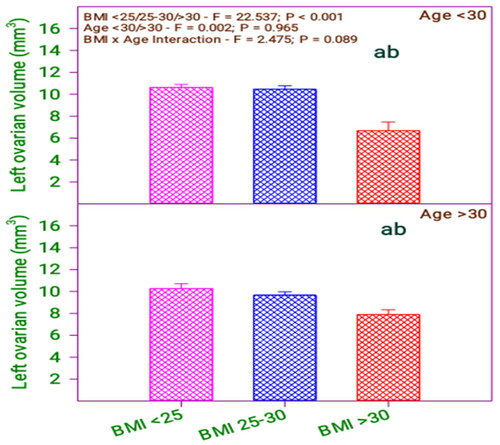
Table 8. Left ovarian volume of subfertile women in comparison with BMI and age (years).
Comparisons of physical and hormonal and ultrasound parameters
From , it is found that there is a difference in FSH among the respondents having BMI > 30 concerning BMI < 25 and BMI 25–30. Moreover, there exists a difference between the respondents in the age group above 30 and below 30 years. Analyzing the age and BMI with FSH level revealed a difference between respondent age and BMI 25–30 and above 30. Likewise, comparing the age with BMI, the FSH level of below 30 age group respondents differed with their BMI levels > 30 and <25. Furthermore, the FSH level of the above 30 age group respondents does not differ when their BMI level is <25 or 25–30.
Table 9. Bonferroni t test comparisons of FSH and BMI and age.
A result of the ‘Do not test’ occurs for a comparison when no significant difference is found between two means that enclose that comparison. shows that AMH significantly differs among the respondents with BMI <25, 25–30 with respect to BMI > 30. The respondents’ AMH values have differed concerning the age of the respondents. By analyzing the age and BMI with AMH value, it is revealed that there is a difference between respondent age and BMI of 25–30 and above 30. Likewise, comparing the age with BMI, the AMH level of the below 30 age group respondents does not differ with their BMI levels > 30 and 25–30. Furthermore, the AMH level of the above 30 age group respondents does not differ when their BMI level is <25 or 25–30 ().
Table 10. Bonferroni t test comparisons of AMH and BMI and age.
shows that Day-2 AFC is significantly different among the respondents with BMI <25, 25–30 concerning BMI >30. The respondents’ Day-2 AFC values differ concerning the respondents’ age. By analyzing the age and BMI with Day-2 AFC value, there is a difference between respondent’s age and BMI of 25–30 only. Likewise, comparing the age with BMI, the Day-2 AFC of below 30 age group respondents has not differed with their BMI levels <25 and 25–30. Moreover, the Day-2 AFC of above 30 age group respondents did not differ when their BMI level was <25 or 25–30 but differed in BMI <25, 25–30 to BMI > 30.
Table 11. Bonferroni t test comparisons of Day-2 AFC and BMI and age.
shows that serum E2 level significantly differs among the respondents with BMI <25, 25–30 with respect to BMI > 30.
Table 12. Bonferroni t test comparisons of serum E2 level and BMI and age.
The respondents’ serum E2 levels do not differ from the age respondents. However, by analyzing the age and BMI with serum E2 level, it is revealed that there is a difference between respondent’s age and BMI of 25–30 only. Likewise, comparing the age with BMI, the serum E2 level of the below 30 age group and above 30 respondents does not differ with their BMI levels <25 and 25–30 but differed in BMI <25, 25–30 concerning BMI > 30.
shows that right ovarian volume significantly differs among the respondents with BMI < 25, 25–30 with respect to BMI > 30. However, the respondents’ right ovarian volume does not differ in age. Therefore, by analyzing the age and BMI with right ovarian volume, it is revealed that there is no difference between respondents’ age and BMI. Likewise, comparing the age with BMI, the right ovarian volume of the below 30 age group and above 30 respondents does not differ with their BMI levels <25 and 25–30 but differed in BMI < 25, 25–30 concerning BMI > 30.
Table 13. Bonferroni t test comparisons of right ovarian volume and BMI and age.
shows that left ovarian volume significantly differs among the respondents with BMI < 25, 25–30 with respect to BMI > 30. However, the respondents’ left ovarian volume does not differ in age. Therefore, by analyzing the age and BMI with left ovarian volume, it is revealed that there is no difference between respondents’ age and BMI. Likewise, comparing the age with BMI, the left ovarian volume of the below 30 age group and above 30 respondents does not differ with their BMI levels <25 and 25–30 but differed in BMI < 25, 25–30 concerning BMI > 30.
Table 14. Bonferroni t test comparisons of left ovarian volume and BMI and age.
Correlation of hormonal and ultrasound parameters with physical parameters
FSH level and AMH – age and BMI
FSH level and AMH values are varied among the above 30 age group of respondents with BMI values of 25–30.
FSH level and AMH values of the below 30 age group are not similar when their BMI values are less than 25 and greater than 30.
There is a strong relationship between the FSH level, AMH and physical parameters, age and BMI.
Day 2 AFC and serum E2 level – age and BMI
For the respondents with BMI 25–30, their Day 2 AFC and serum E2 levels varied regarding age. Furthermore, it is revealed that the Day2 AFC varies among the respondents, those with BMI less than 25 and BMI 25–30. So there is a strong relationship between the Day 2 AFC, serum E2 level and physical parameter age and BMI.
Ovarian volume – age and BMI
There is a variation in the left and right ovarian volume between the respondents with BMI <25 and 25–30 with BMI > 30. So there is a relationship between ovarian volume and BMI value.
There is no relationship between the respondents’ right and left ovarian volume by comparing age within BMI. Therefore, the reproductive age will be used for fertility assessment. The reproductive age of women will be older than or younger than the actual age of the women. The reproductive age will be calculated with the ovarian volume, ovarian reserve, ovary size and time to menopause.
Discussion
Recently, women’s career advancement has become more important. Since the age of women when they get married is generally higher these days, there is an increased number of pregnancies at an advanced maternal age. Despite their advanced Age, some women possess comparatively good ovarian functions. On the other hand, despite their youth, others have weakened ovarian reserve. Moreover, the ability to reproduce for women with diminished ovarian function leads to a rise in the rate of ART. Hence, it is essential to remind women with decreased ovarian age that, even though they get pregnant in earlier thirty’s, they could predict a prognosis similar to advance age pregnancy.
Additionally, ORT is a term that clearly explains the ovary’s functional potential, composes the ovarian follicle’s size and reflects the quality and number of oocytes. The assessment of ovarian reserve assists in reflecting women’s reproductive potential. Several markers are available that can access the parameters of ovarian reserve. Our study established the correlation between physical parameters such as BMI, day and age with ultrasound and hormonal parameters. Moreover, age is considered a more predominant predictor of miscarriage and complications in pregnancy. Our study stated that age remains a major factor impacting reproduction. Besides, women with a BMI value of more than thirty and less than twenty have a chance to conceive.
Similar to our study, recent studies [Citation11] have shown that biological age is more important in predicting pregnancy complications. The study [Citation11] determined the correlation between the OR biomarkers and the reproductive potential among late reproductive-aged women. This study observed that the diminished OR estimated by low AMH was associated with women’s infertility during late reproductive age. It has been established that OR reduces with increasing age, leading to infertility. Therefore, a precise estimation of OR and the corresponding ovarian response to the stimulation must complement the age of women in achieving live birth and pregnancy [Citation12].
In the present study, there is significant variation in the FSH following the BMI group and no considerable interaction between age and BMI category. In addition, the study was observed to be significant in AMH, FSH, AFC, and Day 2 according to age and BMI. Study by Revelli et al. [Citation13] found a positive correlation between physical parameter age, BMI and AMH. Study by Jehan and Syed [Citation14] also strengthens our study results that there is an association between ages and BMI. In contrast to the present study, Adak et al. [Citation15] reported no relationship between AMH and BMI and a significant weak to moderate negative linear correlation with age. However, Adak et al. [Citation15] concluded that AMH appears to be the most useful biochemical marker of ovarian reserve in addition to chronological age.
Furthermore, AMH has a high diagnostic value and could be utilized for women to decide to delay pregnancy as informed. FSH levels in the subfertile women were investigated, and it concluded that the respondents aged 35 and above 33 possess decreased fertility compared to young women. In addition, Okunola et al. [Citation16] stated that or, as estimated by basal serum FSH and random serum FSH, considerably decreased in the subfertile women with regular cycles. In support of the present study, this study states that statistically, significant variation prevails in OR of infertile women compared to fertile Nigerian women. Hence OR reduction was associated with subfertility or early ovarian aging. However, the reported study found no significant rate in the E2 level of BMI women. Revelli et al. [Citation13] found a positive correlation between physical parameter age, BMI and AFC. Study by Rehman et al. [Citation17] concludes that BMI negatively correlates with serum E2 levels.
In addition, Albu and Albu [Citation18] analyzed and supported our study in the case of serum E2 levels. This study paper obtained a considerable difference between BMI and right ovarian volume. However, no relationship with age was observed. Similarly, a correlation existed between left ovarian volume and BMI and not with age. Analyzing the age and BMI with FSH level revealed a difference between respondents’ age and BMI of 25–30 and above 30. Likewise, comparing the age with BMI, the FSH level of below 30 age group respondents differed with their BMI levels of more than 30 and less than 25. It was supported by the existing studies [Citation19,Citation20]. The following observations are made when comparing Day 2 AFC with BMI and age using the Bonferroni t test. It has been revealed that there is a difference between respondents’ age and BMI of 25–30. Likewise, comparing the age with BMI, the Day-2 AFC of below 30 age group respondents does not differ with their BMI levels less than 25 and 25–30. By analyzing the age and BMI with serum E2 level, it is revealed that there is a difference between respondent’s age and BMI of 25–30 only. The respondents’ right ovarian volume does not differ concerning the age of the respondents, and the respondents’ left ovarian volume does not differ concerning the age of respondents. The study [Citation21] concludes that the ovarian volume decreases when the BMI value increases, which leads to a decrease in fertility. The assessment of ovarian volume reported by Sun et al. [Citation22] revealed no relationship between the actual age and the ovarian volume. Similarly, Zafar [Citation23] determined the correlation between BMI and subfertility among Pakistan’s young women. Hence, this study recommended that there exists a requirement of interventions for controlling BMI that increase the level of fertility among women.
The study by Hong et al. [Citation24] assessed AMH for early pregnancy loss. It made a comparative analysis of AMH with age as a major factor in the determination of pregnancy loss in subfertile women. The study stated that AMH could be a marker of decreased reproductive potential because of the association with pregnancy loss. In another retrospective study by Pasternak et al. [Citation25], in which 12,526 cycles were addressed, the infertile women were categorized into two groups of ages above 35 and below 35. A negative correlation has been found between AMH and BMI levels in women greater than 35 age which was not found in women less than 35 years of Age. In subfertile women, BMI is a favorable predictor of inducing ovulation with US monitoring [Citation26,Citation27]. The description of anovulatory cycles, total cycle length, luteal and follicular phase length, days and intensity of non-menstrual and menstrual bleeding in women without known subfertility for more than one year has been investigated [Citation28]. In a recent meta-analysis [Citation29], OR markers such as FSH and AMH were significantly lower in obese and normal in weighted women. Universally, BMI is negatively correlated with AMH. Hence, estimating basal FSH is controversial despite considerable significance in some studies [Citation30,Citation31].
The cumulative pregnancy rate of the subfertile couple after training on fertility awareness has been investigated by Frank-Herrmann et al. [Citation32]. The authors believed that integrating fertility awareness into subfertility care would likely lead to significant cost savings in subfertility management. Apart from that, studies on the acceptability of learning fertility awareness in the condition of subfertility are recommended. The study by Shrestha and Shrestha [Citation33] assessed the knowledge regarding subfertility among reproductive age women. The findings highlighted a lack of knowledge regarding subfertility in women. Hence, knowledge should be provided through mass media and different health campaigns to improve knowledge regarding subfertility among reproductive-age women. These prevailing evidence depicted that AFC and AMH are the most relevant markers of OR in addition to chronological Age. In addition, AMH could apply to the general population to detect diminishing OR before reaching a critical level where no efficient treatment could be offered. It could help women who are interested in delaying pregnancy [Citation9].
Conclusion
Ovarian reserve parameters depict the ovary’s reproductive potential, so insight plays a significant role in conception. To minimize the cost and time duration of the fertility treatment, the study assessed the ovarian reserve markers to find the correlation between them effectively. Various studies focus on the role of ovarian markers in determining the ovarian reserve potential before planning counseling and treatment. This study concludes that there is a strong correlation between the AMH, FSH and physical parameters such as age and BMI. It is also observed that the reproductive age of women is older or younger than the actual Age of the women. Hence progressing age could be utilized as an important marker for declining OR.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ulrich ND, Marsh EE. Ovarian reserve testing: a review of the options, their applications, and their limitations. Clin Obstet Gynecol. 2019;62(2):1–9.
- Xu H, Zeng L, Yang R, et al. Retrospective cohort study: AMH is the best ovarian reserve markers in predicting ovarian response but has unfavorable value in predicting clinical pregnancy in GnRH antagonist protocol. Arch Gynecol Obstet. 2017;295(3):763–770.
- Scheffer JB, Carvalho RFd, Souza Aguiar APd, et al. Which ovarian reserve marker relates to embryo quality on day 3 and blastocyst; age, AFC, AMH? JBRA Assist Reprod. 2021;25(1):109–114.
- Tehraninezhad ES, Mehrabi F, Taati R, et al. Analysis of ovarian reserve markers (AMH, FSH, AFC) in different age strata in IVF/ICSI patients. Int J Reprod Biomed. 2016;14(8):501–506.
- Al Rashid K, Taylor A, Lumsden MA, et al. Association of the functional ovarian reserve with serum metabolomic profiling by nuclear magnetic resonance spectroscopy: a cross-sectional study of∼ 400 women. BMC Med. 2020;18(1):1–14.
- Abrahami N, Izhaki I, Younis JS. Do young women with unexplained infertility show manifestations of decreased ovarian reserve? J Assist Reprod Genet. 2019;36(6):1143–1152.
- Moolhuijsen LM, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361–3373.
- Siristatidis C, Pouliakis A, Sergentanis TN. Special characteristics, reproductive, and clinical profile of women with unexplained infertility versus other causes of infertility: a comparative study. J Assist Reprod Genet. 2020;37(8):1923–1930.
- Liu X, Li T, Wang B, et al. Mild stimulation protocol vs conventional controlled ovarian stimulation protocol in poor ovarian response patients: a prospective randomized controlled trial. Arch Gynecol Obstet. 2020;301(5):1331–1339.
- Taylor A. ABC of subfertility: extent of the problem. BMJ. 2003;327(7412):434–436.
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367–1376.
- Borges E, Jr, Zanetti BF, Setti AS, et al. FSH dose to stimulate different patient’ages: when less is more. JBRA Assist Reprod. 2017;21(4):336–342.
- Revelli A, Gennarelli G, Biasoni V, et al. The ovarian sensitivity index (OSI) significantly correlates with ovarian reserve biomarkers, is more predictive of clinical pregnancy than the total number of oocytes, and is consistent in consecutive IVF cycles. J Clin Med. 2020;9(6):1914.
- Jehan S, Syed S. Association of ovarian reserve with age, BMI and serum FSH level in subfertile women. J Pak Med Assoc. 2016;66:409–413.
- Adak SR, Ghosh C, Pal S, et al. Biochemical parameters serving as prognostic indicators of ovarian reserve in females with unexplained sub-fertility. IOSR J Dent Med Sci. 2017;16(05):61–65.
- Okunola T, Ajenifuja KO, Loto OM, et al. Follicle stimulating hormone and anti-Müllerian hormone among fertile and infertile women in Ile-Ife, Nigeria: is there a difference? Int J Fertil Steril. 2017;11:33.
- Rehman R, Hussain Z, Faraz N. Effect of estradiol levels on pregnancy outcome in obese women. J Ayub Med College Abbottabad. 2012;24:3–5.
- Albu D, Albu A. The relationship between anti-Müllerian hormone serum level and body mass index in a large cohort of infertile patients. Endocrine. 2019;63(1):157–163.
- Siddiqui QUA, Anjum S, Zahra F, et al. Ovarian reserve parameters and response to controlled ovarian stimulation in infertile patients. Pak J Med Sci. 2019;35(4):958–962.
- Raffas HA, Al-Janabi HA, Jawad RG. Thyroid status in iraqi subfertile women: a case control study. Ind J Public Health Res Dev. 2019;10(4):710.
- Zaidi S, Usmani A, Shokh IS, et al. Ovarian reserve and BMI between fertile and subfertile women. J Coll Physicians Surg Pak. 2009;19:21–24.
- Sun X, Luo M, Ma M, et al. Ovarian aging: an ongoing prospective community-based cohort study in Middle-aged chinese women. Climacteric. 2018;21(4):404–410.
- Zafar M. Association of body mass index (BMI) and sub-fertility among young women in karachi, Pakistan. Fertil Sci Res. 2019;6(1):23.
- Hong S, Chang E, Han EJ, et al. The anti-Mullerian hormone as a predictor of early pregnancy loss in subfertile women. Syst Biol Reprod Med. 2020;66(6):370–377.
- Pasternak MC, Christos P, Spandorfer SD. The relationship between body mass index and anti-mullerian hormone levels in reproductive-age women; is there a negative correlation? Fertil Steril. 2018;109(3):e42–e43.
- Ahn SH, Lee I, Cho S, et al. Predictive factors of conception and the cumulative pregnancy rate in subfertile couples undergoing timed intercourse with ultrasound. Front. Endocrinol. 2021;12:363.
- Van Eekelen R, Eijkemans M, Mochtar M, et al. Cost-effectiveness of medically assisted reproduction or expectant management for unexplained subfertility: when to start treatment? Hum Reprod. 2020;35(9):2037–2046.
- Najmabadi S, Schliep KC, Simonsen SE, et al. Menstrual bleeding, cycle length, and follicular and luteal phase lengths in women without known subfertility: a pooled analysis of three cohorts. Paediatr Perinat Epidemiol. 2020;34(3):318–327.
- Moslehi N, Shab BS, Ramezani TF, et al. Systematic review of the association between BMI and antimullerian hormone (AMH) levels in reproductive aged women. Int J Endocrinol Metab. 2018;20(2):89–101.
- Islam Y, Aboulghar MM, AlEbrashy AE-D, et al. The value of different ovarian reserve tests in the prediction of ovarian response in patients with unexplained infertility. Middle East Fertil Soc J. 2016;21(2):69–74.
- Zhang Y, Xu Y, Xue Q, et al. Discordance between antral follicle counts and anti-Müllerian hormone levels in women undergoing in vitro fertilization. Reprod Biol Endocrinol. 2019;17(1):1–6.
- Frank-Herrmann P, Jacobs C, Jenetzky E, et al. Natural conception rates in subfertile couples following fertility awareness training. Arch Gynecol Obstet. 2017;295(4):1015–1024.
- Shrestha R, Shrestha K. Knowledge regarding subfertility among reproductive age women in selected VDC of baglung district. J Chitwan Med Coll. 2018;8(2):49–56.