Abstract
Objective
Published evidence indicated that the leptin receptor (LEPR) gene polymorphisms are associated with polycystic ovary syndrome (PCOS) risk. However, studies on the association between the polymorphisms of LEPR gene are inconsistent or even controversial.
Material and Methods
We conducted this meta-analysis to explore the more precise relationship between LEPR polymorphisms and PCOS risk. Relevant articles were searched with five online databases up to March 1 2023. Odds ratios (OR) with 95% confidence intervals (CI) were selected to examine the statistical strength of each genetic model. Moreover, RNA secondary structure and variant effects of these loci were examined with in silico analysis.
Results
Overall, 11 publications were analyzed, and the pooled results did not present any significant association between rs1137101 A/G polymorphism and PCOS risk in general population and some subgroup analysis. But the significant association were observed in Asian population (AG vs. AA: OR = 0.51, 95%CI = 0.32–0.81, p = .01, I2=0%; AG + GG vs. AA: OR = 0.41, 95%CI = 0.26–0.65, p < .01, I2=25.9%). Moreover, similar positive associations were also observed in rs1805096 polymorphism with PCOS risk.
Conclusion
In summary, our meta-analysis suggested that the LEPR gene polymorphisms might be associated with PCOS susceptibility. Owing to the limited studies and small sample size in our meta-analysis, more well-designed studies from different races were needed to be conducted to verify the current results.
Introduction
Polycystic ovary syndrome (PCOS) is a hormonal disorder common in the reproductive women. It is characterized by several symptoms, such as infertility, gestational diabetes, obesity, hirsutism, and menstrual disorders, impacting the quality of life of patients worldwide. Furthermore, the prevalence of PCOS among women of reproductive age ranges from 6% to 10% [Citation1,Citation2]. Various risk factors contribute to its development, including hypertension, low-grade inflammation, excess androgen levels, high cholesterol level, and unhealthy lifestyle. However, its exact etiology remains unknown, although increasing evidence suggests genetic factors, including mutations and abnormal gene expression, play a role [Citation3].
The leptin receptor (LEPR) is essential for facilitating leptin’s function by receiving and transmitting signals, acting as a bridge between the peptide and its downstream targets [Citation4]. LEPR is located on chromosome 1p31 and comprises 18 exons within the cytokine receptor protein superfamily. It is predominantly located in brain neurons, including the hippocampus, piriform cortex, and thalamic nuclei, regulating hunger, body temperature, sleep, and other functions [Citation5].
Single-nucleotide polymorphisms (SNPs) are the most prevalent type of genetic variation, causing that contribute to single base pair mutations in DNA sequences and leading to individual diversity and disease susceptibility. Several SNPs have been identified in the LEPR gene, and the rs1137100 A/G, rs1137101 A/G, and rs1805096 C/T. These SNPs are associated with metabolic syndrome, obesity, cancer, and other diseases. In 2000, Oksanen et al. conducted the first case-control study examining the association between LEPR gene polymorphisms and the susceptibility to PCOS in the Finnish population but found no significant results. Subsequent studies on this association yielded inconsistent and contradictory results. Therefore, we conducted a meta-analysis by pooling data to precisely assess the relationship between these three LEPR gene polymorphisms and the susceptibility to PCOS.
Materials and methods
This meta-analysis adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). All included data were collected from previously published studies, and no ethical issues existed.
Search strategy
We conducted a comprehensive search for all relevant studies examining the association between LEPR polymorphisms and PCOS risk. This search encompassed five online databases (PubMed, Embase, Web of Science, CNKI, and Wanfang) up to March 1, 2023, without language restrictions. The following search terms were used: ‘leptin receptor’, ‘LEPR’, ‘rs1137101’, ‘rs1137100’, ‘rs1805096’, ‘polymorphism’, ‘variant’, ‘genotyping’, ‘mutation’, ‘polycystic ovary syndrome’, ‘PCOS’. Other relevant studies were manually retrieved from the included references and reviewed.
Eligibility criteria
Included studies adhered to the following criteria: (1) case–control studies focused on the association between LEPR polymorphisms and PCOS risk; (2) studies with sufficient genome information of three genotypes (including homozygous wild-type, heterozygous mutant, and homozygous mutant) in both in the case and control groups and (3) for duplicate data from the same authors, studies with the most recent or largest sample sizes were selected. The following were excluded: (1) reviews, letters, case series, and other unrelated reports; (2) studies lacking sufficient data; (3) fundamental molecular or biology research; (4) studies involving animal models or cell lines.
Quality assessment
A quality assessment on each included study was conducted by two authors with the modified Newcastle–Ottawa Quality Assessment Scale (NOS) (Supplementary Table 1). Six departments, including representativeness of cases, source of controls, Hardy–Weinberg equilibrium (HWE) status in controls, genotyping methods, sample size, and association assessment were evaluated and scored from 0 to 11, and studies with a score of 8 or higher were considered of high quality ().
Table 1. Characteristics of case–control studies on LEPR polymorphisms and PCOS risk in the meta-analysis.
Data extraction
Data extracted from the included studies: surname of the first author, publication year, study location, ethnicity, control design (HB: healthy controls; PB: patients with other diseases as controls), genotyping method, sample sizes, genotype distribution in both case and control groups, subjects count on circulating levels, mean and standard deviation (SD) of circulating leptin, insulin and leptin receptor levels in PCOS groups.
Statistical analysis
Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for dichotomous data on the relationship between LEPR polymorphisms and PCOS risk in the general population. Moreover, Subgroup analyses were conducted based on the HWE status, ethnic diversity, language use, control design, and genotyping methods, when more than two studies on the same theme were available. Allele contrast (M vs. W) and co-dominant (WM vs. WW and MM vs. WW), dominant (WM + MM vs. WW), and recessive (MM vs. WW + WM) models were used to examine the strength of the associations. Heterogeneity among the included studies was examined using the Cochran’s Q test and I2 statistical method. A fixed-effect model was used when the I2 value was less than 40%; otherwise, a random-effect model was used. A meta-regression analysis was conducted to identify the factors contributing to heterogeneity. Cumulative meta-analyses were conducted to evaluate result trends. Moreover, sensitivity analyses were conducted to assess changes resulting from the exclusion of individual studies. Publication bias was examined with Egger’s linear regression and Begg’s funnel plots. For continuous data, the results were calculated as standardized mean differences (SMDs) with 95% CIs. Statistical analyses were performed using STATA version 14.0 (Stata Corporation, College Station, TX, USA), with two-sided P values < 0.05 indicating statistical significance.
Bioinformatic analysis
The secondary structure of RNA and the subsequent impact of the three LEPR polymorphic loci were examined using the RNAsnp software. (http://rth.dk/resources/rnasnp/, accessed May 1, 2023).
Results
Study characteristics
Initially, 122 publications were identified using the search strategy outlined above. After title screening to eliminate duplicates, 69 publications remained; subsequently, 31 studies were excluded during the abstract review phase as they focused on fundamental biology research. Additionally, 11 studies were excluded due to unrelated polymorphisms, insufficient genotyping data, or being review articles (.). Ultimately, we included 11 articles (comprising 18 independent case-control studies) that investigated the associations between the three LEPR polymorphisms and PCOS susceptibility. These articles involved a total of 1,852 patients with PCOS and 1,693 controls [Citation4,Citation6–15].
Of the included studies, six were from the Middle East (ME), four from East Asia (EA), and one from Northern Europe (NE). The power of HWE test in the control group was less than 0.05 in three studies on the rs1137101 A/G polymorphisms and two studies on the rs1137100 A/G polymorphisms. Moreover, one publication provided only allele data, resulting in missing values for HWE assessment.
Specifically, we identified 12 case–control studies focused on the rs1137101 A/G locus, 4 case-control studies on the rs1137100 A/G locus, and two case–control studies on the rs1805096 C/T locus. Among them, 12 were high-quality based on NOS score. Further details on the included studies are presented in . Moreover, due to the limited number of candidate SNPs association studies, only four, three, and two studies examined the circulating leptin, insulin, and leptin receptor levels, in relation to with genotype diversity among the case groups at the rs1137101 A/G locus (Supplementary Tables 2–4).
Quantitative analysis
Rs1137101 a/G polymorphism and PCOS risk
Twelve case–control studies, comprising 1,890 PCOS patients (average age: 29.62 ± 4.51 years) and 1,815 healthy controls (average age: 32.20 ± 4.69 years), investigated the association between the rs1137101 A/G polymorphism and PCOS risk. Overall, no significant association was observed in any of the five genetic models among the general population (). Heterogeneity was detected in these genetic models, but regression analyses did not identify any significant contributing factors. Significant associations were observed in subgroup analyses among Asian individuals for both heterozygote and dominant models (AG vs. AA: OR = 0.51, 95%CI = 0.32–0.81, p = .01, I2=0%; AG + GG vs. AA: OR = 0.41, 95%CI = 0.26–0.65, p < .01, I2=0%) (). No such associations were found among Caucasian descendants. In addition, no significant associations were found in other subgroup analyses based on the HWE status, ethnic differences, or control design ().
Figure 2. OR and 95% CIs of the associations between LEPR rs1137101 a/G polymorphism and PCOS susceptibility in G versus A model.
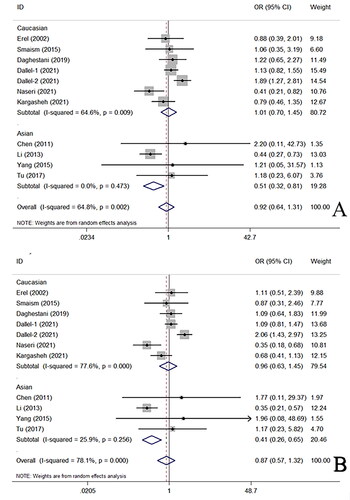
Table 2. Summary ORs and 95% CI of LEPR polymorphisms and PCOS risk.
Cumulative analyses consistently yielded negative results as more studies were added in all genetic models (Supplementary Figure 1 for heterozygote and dominant models). No significant changes were observed with sensitivity analyses in the results (Supplementary Figure 2 for heterozygote and dominant models). In addition, no significant publication bias was observed in the funnel plot examination among the included studies (Supplementary Figure 3 for heterozygote and dominant models), which was further confirmed using the Egger’s test (G vs. A: p = .93; AG vs. AA: p = .67; GG vs. AA: p = .66; AG + GG vs. AA: p = .64; and GG vs. AA + AG: p = .50). Moreover, no significant association was found between the circulating levels of determined parameters and genotypes at the rs1137101 A/G locus (Supplementary Tables 5–7).
Rs1137100 a/G, rs1805096 C/T polymorphisms and PCOS risk
Four case–control studies involving 926 patients with PCOS (average age: 28.89 ± 4.30 years) and 1,089 controls (average age: 33.30 ± 4.61 years) examined the association between the 1137100 A/G polymorphism and PCOS risk and the pooled results showed no association between them ().
Two case–control studies involving 329 patients with PCOS (average age: 31.68 ± 3.80 years) and 250 controls (average age: 31.06 ± 3.68 years) examined the association between the rs1805096 C/T polymorphism and PCOS risk. These studies revealed a higher risk associated with the presence of the T mutation ().
Cumulative analyses, sensitivity analyses, and tests for publication bias were not conducted due to the small sample size for the either rs1137100 A/G and rs1805096 C/T polymorphisms.
Bioinformatics Analysis
Bioinformatics analysis indicated no significant changes in minimum free energy (MFE) changes or thermodynamic alterations in the RNA secondary structure ensemble for rs1137101 A/G (A to G substitution, MFE change from −86.60 to −85.90 kcal/mol, p = .85) (Supplementary Figure 4); and the same results were also observed in 1137100 A/G (A to G substitution, MFE change −72.90 to −74.70 kcal/mol, p = .59) and rs1805096 C/T (C to T substitution, MFE change −92.90 to −90.90 kcal/mol, p = .93) polymorphisms (Supplementary Figure 4).
Discussion
PCOS is a common and complex disease characterized by reproductive endocrine and menstrual cycle disorders and one of the leading causes of fertility issues in women of reproductive age. Most PCOS patients suffer from both reproductive dysfunction and obesity, always accompanied by dysregulate ovarian functions at the leptin and LEPR levels.
Leptin, a 16 kDa peptide hormone secreted by the lipocytes [Citation16], regulates various physiological activities including energy metabolism, immune responses, and cell angiogenesis [Citation17–19]. Elevated leptin levels have been associated with insulin resistance, metabolic disorders, and infertility, which are considered triggers of PCOS. Studies have revealed higher leptin levels in patients with PCOS compared with healthy controls [Citation20]. LEPR is selectively expressed in central and peripheral tissues, binding to leptin and subsequently mediating its effects.
Furthermore, decreased LEPR levels are associated with PCOS and other metabolic disorders [Citation21]. Additionally, the saturation of hypothalamic LEPR and a reduction in the number of available LEPR exacerbate central leptin resistance and accelerate obesity [Citation22]. Moreover, the abnormal expression or mutation of the LEPR gene can disrupt the bidirectional regulatory effects of insulin and leptin, which may lead to leptin resistance, obesity, and sexual hormone metabolism disorders [Citation23,Citation24].
Rs1137101 A/G is the most common variant in the LEPR gene. It is located on chromosome 1p31 and involves a substitution of the 223rd amino acid from Arg to Gln. Moreover, rs1137100 A/G and rs1805096 C/T result in amino acid changes from Lys to Arg and Pro to Pro in the LEPR gene, respectively. These polymorphisms have gained attention for their potential association with PCOS. In 2000, Oksanen et al. conducted the first case–control study on LEPR rs1137100 A/G and rs1137101 A/G polymorphisms in relation to PCOS susceptibility in the Finnish population but found no significant relationships [Citation6]. Two years later, Erel et al. conducted a similar study and no significant association was found between rs1137101 A/G polymorphism and PCOS susceptibility [Citation7]. Moreover, subsequent studies conducted by Chen et al. and Yang et al. demonstrated no significant association between the LEPR rs1137101 A/G polymorphism and PCOS susceptibility in the Chinese population [Citation8,Citation9]. Conversely, Li et al. found that the G mutation at the rs1137101 locus protects against the development of PCOS in Koreans (OR = 0.446, 95% CI = 0.331-0.602, p < .0001) [Citation4]. In 2021, Naseri et al. observed that participants with AG and GG genotypes had a lower risk of PCOS compared with those with an AA genotype (OR = 0.41, 95%CI = 0.21–0.81, and OR = 0.19, 95%CI = 0.075–0.48) [Citation14]. Furthermore, Dallel et al. [Citation13] and Kargasheh et al. [Citation15] reported a positive association between rs1137101 A/G polymorphism and susceptibility to PCOS. To address the existing confusion and inconsistencies, larger sample sizes and more studies were synthesized to yield more precise results regarding LEPR gene polymorphisms and PCOS susceptibility. In 2019, Liang et al. conducted a meta-analysis and concluded that the LEPR rs1137101 A > G polymorphism is significantly associated with the susceptibility to PCOS in Asian populations in the recessive genetic model only [Citation25].
Herein, we conducted an updated meta-analysis, including four newly published studies, to explore the influence of LEPR polymorphisms on the susceptibility to PCOS. The pooled results indicate significant susceptibility in the Asian population and other subgroups with rs1137101 A/G polymorphism, but not with rs1137100 A/G polymorphism. This suggests that rs1137101 A/G polymorphism may be an important, albeit not an independent, factor contributing to PCOS. Its effects are likely influenced by multiple factors, including the spatial structure of RNA and proteins, cytokine signaling networks, lifestyle choices, and racial diversity, which collectively influence disease occurrence. Moreover, a significant association was found observed between the LEPR rs1805096 C/T polymorphism and PCOS susceptibility; however, caution is warranted when interpreting these positive results, as only two studies provided supporting evidence.
Inevitably, some limitations should be addressed in our meta-analysis. First, we examined the current three polymorphic loci separately, without exploring their interactions with each other or with other genetic polymorphisms due to limited sample size and data availability. Second, we observed general heterogeneity in all five genetic models, which we mitigated through subgroup analysis, indicating some inconsistency in the included data. Third, language and ethnic biases were present, as all selected studies included Caucasian or Asian populations, and only English or Chinese publications were included. Therefore, caution is needed when applying our results to other racial groups. Nevertheless, our study has several advantages: (1) a rigorous scientific retrieval strategy was to identify and reviewed relevant literatures; (2) robust statistical methodologies, including cumulative and sensitivity analyses, were used to ensure the validity and reliability of our results; (3) comprehensively stratified analyses, including HWE status, ethnic diversity, language diversity, control design, and genotyping methods, with a substantial sample size were conducted to examine the potential relationships between LEPR polymorphisms and PCOS susceptibility; (4) a silico analysis was performed to validate the current results by examining MFE changes and thermodynamic alterations in the RNA secondary structure ensemble.
Conclusion
In summary, our updated meta-analysis suggests that LEPR polymorphisms could be a potential risk factor for PCOS, providing theoretical evidence for their association with susceptibility to this disease. However, owing to several limitations, additional high-quality clinical studies with larger sample size are needed to confirm these findings.
Supplemental Material
Download Zip (664.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used or analyzed in this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the amsterdam ESHRE/ASRM-Sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97(1):1–7. doi: 10.1016/j.fertnstert.2011.09.024.
- Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril. 2018;110(5):794–809. doi: 10.1016/j.fertnstert.2018.08.021.
- Zheng SH, Du DF, Li XL. Leptin levels in women with polycystic ovary syndrome: a systematic review and a Meta-Analysis. Reprod Sci. 2017;24(5):656–670. doi: 10.1177/1933719116670265.
- Li L, Lee KJ, Choi BC, et al. Relationship between leptin receptor and polycystic ovary syndrome. Gene. 2013;527(1):71–74. doi: 10.1016/j.gene.2013.05.074.
- Wada N, Hirako S, Takenoya F, et al. Leptin and its receptors. J Chem Neuroanat. 2014;61-62:191–199. doi: 10.1016/j.jchemneu.2014.09.002.
- Oksanen L, Tiitinen A, Kaprio J, et al. No evidence for mutations of the leptin or leptin receptor genes in women with polycystic ovary syndrome. Mol Hum Reprod. 2000;6(10):873–876. doi: 10.1093/molehr/6.10.873.
- Erel CT, Cine N, Elter K, et al. Leptin receptor variant in women with polycystic ovary syndrome. Fertil Steril. 2002;78(6):1334–1335. doi: 10.1016/s0015-0282(02)04352-2.
- Chen Y. The relationship between polymorphism of the Gln223Arg gene of leptin receptor and insulin resistance and lipid metabolism in polycystic ovary syndrome. Proc Clin Med. 2011;20:912–914.
- Yang MY, Song QQ, Zhao XK, et al. Association between polymorphism of leptin receptor gene Gln223Arg and polycystic ovary syndrome. Clin J Med Offic. 2015;43:855–858.
- Smaism MF. Assessment of leptin levels in the different genotypes and leptin receptor genes in the women with polycystic ovary syndrome and diabetes mellitus type 2 in Iraq population. Int J PharmTech Res. 2016;9:269–276.
- Tu X, Yu C, Gao M, et al. LEPR gene polymorphism and plasma soluble leptin receptor levels are associated with polycystic ovary syndrome in Han Chinese women. Per Med. 2017;14(4):299–307. doi: 10.2217/pme-2017-0016.
- Daghestani MH, Daghestani MH, Daghistani MH, et al. The influence of the rs1137101 genotypes of leptin receptor gene on the demographic and metabolic profile of normal Saudi females and those suffering from polycystic ovarian syndrome. BMC Women’s Health. 2019;19:10.
- Dallel M, Douma Z, Finan RR, et al. Contrasting association of leptin receptor polymorphisms and haplotypes with polycystic ovary syndrome in Bahraini and Tunisian women: a case-control study. Biosci Rep. 2021;41(1):41.
- Naseri R, Barzingarosi E, Sohrabi M, et al. The effect of leptin receptor gene polymorphisms (R223Q and P1019P) in susceptibility to polycystic ovarian syndrome in kurdish women. Int J Fertil Steril. 2021;15:123–127.
- Kargasheh FB, Ansaripour S, Borumandnia N, et al. Association of leptin G2548A and leptin receptor Q223R polymorphisms and their serum levels with infertility and recurrent pregnancy loss in iranian women with polycystic ovary syndrome. PLoS One. 2021;16(8):e0255920. doi: 10.1371/journal.pone.0255920.
- Villa J, Pratley RE. Adipose tissue dysfunction in polycystic ovary syndrome. Curr Diab Rep. 2011;11(3):179–184. doi: 10.1007/s11892-011-0189-8.
- Atoum MF, Alzoughool F, Al-Hourani H. Linkage between obesity leptin and breast cancer. Breast Cancer (Auckl). 2020;14:1178223419898458. doi: 10.1177/1178223419898458.
- Saxton RA, Caveney NA, Moya-Garzon MD, et al. Structural insights into the mechanism of leptin receptor activation. Nat Commun. 2023;14:1797.
- Cui Q, Zhang Y, Tian N, et al. Leptin promotes angiogenesis via pericyte STAT3 pathway upon intracerebral hemorrhage. Cells. 2022;11(17):11. doi: 10.3390/cells11172755.
- Peng Y, Yang H, Song J, et al. Elevated serum leptin levels as a predictive marker for polycystic ovary syndrome. Front Endocrinol (Lausanne). 2022;13:845165. doi: 10.3389/fendo.2022.845165.
- Rizk NM, Sharif E. Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol. 2015;2015:927805–927810. doi: 10.1155/2015/927805.
- Sandhofer A, Laimer M, Ebenbichler CF, et al. Soluble leptin receptor and soluble receptor-bound fraction of leptin in the metabolic syndrome. Obes Res. 2003;11(6):760–768. doi: 10.1038/oby.2003.106.
- Adiga U, Banawalikar N, Mayur S, et al. Association of insulin resistance and leptin receptor gene polymorphism in type 2 diabetes mellitus. J Chin Med Assoc. 2021;84(4):383–388. doi: 10.1097/JCMA.0000000000000507.
- Aller R, Primo Martín D, Izaola O, et al. Association of the leptin receptor rs 1805134 polymorphism with obesity parameters, dietary intakes, and metabolic syndrome in Caucasian obese subjects. Nutr Hosp. 2023;40:35–40.
- Liang J, Lan J, Li M, et al. Associations of leptin receptor and peroxisome Proliferator-activated receptor gamma polymorphisms with polycystic ovary syndrome: a meta-analysis. Ann Nutr Metab. 2019;75(1):1–8. doi: 10.1159/000500996.