Abstract
In order to replace the antibiotic treatment for control of Vibrio harveyi, a causal agent of luminous disease in Black tiger shrimp, anti-V. harveyi IgY was produced and showed its potential in our preliminary study. However, for further use as feed additive, the IgY stability should be evaluated. The titre of specific IgY was enhanced with an immunostimulant, C-phosphate guanosine oligodeoxynucleotide (CpG-ODN). IgY was stable at natural pH while the activity was decreased below pH 4 or above pH 10. The supplementation of 30% sorbitol significantly enhanced the IgY stability at tested temperatures in excess of 70°C. The activity of IgY remained at 9 and 94% after 4 h incubation in simulated gastric and intestinal fluids, respectively. A storage temperature at −20°C was found to be the best condition. IgY at the concentrations of 1, 5 and 10 mg/ml efficiently inhibited V. harveyi in vitro within 24 h after exposure.
Introduction
Vibrio harveyi is a gram-negative, short rod-shaped luminous bacterium, which is widely dispersed in the marine environment (Ramesh & Venugopalan, Citation1989). The organism has recently emerged as a serious pathogen of marine animals. It is a primary pathogen of cultured penaeid shrimp, especially in South America and Asia. In Thailand, luminous disease in black tiger shrimp (Penaeus monodon) caused by V. harveyi results in massive mortality in both post-larvae in nurseries and juvenile shrimps in grow-out ponds (Jiravanichpaisal, Miyazaki, & Limsuwan, Citation1994). The disease usually occurs during the first month of culture and can cause mortality in excess of 50% (Chen, Citation1992). In order to control this bacterium, chemicals and antibiotics have been widely applied for many years. However, some of their residues cause serious impacts on both the environment and the consumers’ health. In addition, antibiotics resistant strains have also been reported in many countries (Karunasagar, Pai, Malathi, & Karunasagar, Citation1994) and mass mortality caused by this pathogen is still occurring up to the present time.
Passive immunisation with pathogen-specific antibodies raised in chickens has been reported to be a promising approach to control infections, since it can be readily obtained in large quantities from egg yolk, and presents a more cost–effective, convenient and hygienic alternative to mammalian antibodies (Carlander, Kollberg, Wejaker, & Larsson, Citation2000). This method has been shown to be effective against a number of enteric pathogens such as Streptococcus mutans (Hamada et al., Citation1991), Staphylococcos sp. (LeClaire, Hunt, & Bavari, Citation2002), Escherichia coli, Salmonella sp., rotavirus (Mine & Kovacs-Nolan, Citation2002) and Helicobacter pylori (Nomura et al., Citation2005). In aquatic animals, specific egg yolk immunoglobulin (IgY) protection against Edwardsiella tarda in Japanese eel (Gutierrez, Miyazaki, Hatta, & Kim, Citation1993), and Yersinia ruckeri in rainbow trout (Lee, Mine, & Stevenson, Citation2000) by oral passive immunisation has been demonstrated. Kim et al. (Citation2004) produced the anti-white spot syndrome virus (WSSV) IgY by immunising truncated fusion protein of WSSV which was found to provide a good protection from viral infection in Penaeus chinensis by passive immunisation.
In our preliminary research (Srisapoome, Punyokun, Hongprayoon, & Areechon, Citation2006), the chicken antibodies were raised against V. harveyi and used as feed additive in order to evaluate the neutralisation of the bacterial infection. The results proved that it renders a good potential to effectively control the luminous disease in black tiger shrimp. However, for further use as feed additive in control measure, the optimal condition to maintain the IgY stability needs to be determined.
Materials and methods
Preparation of antigen
Vibrio harveyi strain AQVH001 was grown in tryptic soy broth (TSB) containing 1.5% NaCl and cultured at 37°C for 18 h, then the cells were harvested and treated with 2% glutaraldehyde for 3 h. The pellets were dialysed three times in phosphate buffered saline (PBS, pH 7.4; Allen & Kelman, Citation1987) and then resuspended in PBS using a Vortex mixer with a concentration adjustment to 1×109 cells/ml. The cells were kept at 4°C with 0.02% NaN3 until used.
Immunisation of laying hens
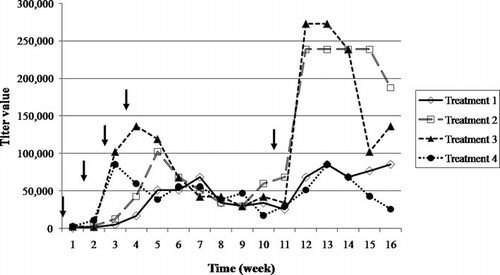
Twelve Roman-Brown laying hens, aged 32 weeks, were used for immunisation and egg production. V. harveyi (2×108 cells/ml) was emulsified with an equal volume of Freund's adjuvant to prepare the antigen. An immunostimulant, C-phosphate guanosine oligodeoxynucleotide (CpG-ODN), was supplemented to enhance the chicken immune response in accordance with Rankin et al. (Citation2001). A specific sequence number 2135 of CpG-ODN (5′-TCGTCGTTTGTCGTTTTGTCGTT-3′) was ordered from Fermentas Inc., Canada. The immunisation program was performed as shown in . One millilitre of the antigen was subcutaneously injected into three hens (each treatment). The immunisation treatment was repeated at weeks 2, 3, 4 and 10. The eggs were collected daily, beginning at 7 days subsequent to the first injection, and were stored at 4°C until use.
Table 1. Immunisation program including four treatments (three hens each).
Partial purification of IgY
IgY was prepared from egg yolk with the water dilution method developed by Akita and Nakai (Citation1992). The egg yolk was separated from the egg white and rolled on paper. The yolk was punctured and mixed gently with six volumes of cold distilled water pH 3 with the mixture adjusted to pH 5.0 and incubated at 4°C for 18 h. The aggregated lipoproteins were then removed by centrifugation (10,000×g, 1 h, 4°C). The WSF was divided into two parts. To one half, 390 g/l of solid ammonium sulphate was added and the mixture was stirred for 20 min at 4°C. The precipitate was collected by centrifugation and resuspended in 0.5×PBS containing 0.02% NaN3, then stored at −20°C for the determination of antibody titre by ELISA. The other half was neutralised with 0.1 N NaOH and lyophilised with the freeze dryer model LyoAlfla 6 (Telstar, USA) to obtain the IgY powder for the stability tests.
Characterisation of IgY powder
The protein concentration of the IgY solution was determined by Coomassie Plus (Bradford) Assay Reagent (Thermo Scientific, USA). Purified chicken IgG (Promega, USA) was used as the IgG standard (62.5–1000 µg/ml).
The total IgY determination was performed by ELISA. According to Barua, Furusaw, and Yoshimura (Citation2000) as described below, except that the plate was coated with 50 µl of rabbit anti-chicken IgG (5 µg/ml). Specific or non-specific IgY powder was diluted 1:10,000 with PBS. Two-fold serial dilutions of purified chicken IgG (1 mg/ml) in PBS (250–15.625 ng/ml) were used to prepare a standard curve. The standard curve (data not shown) was used to provide a relative measurement of total IgY concentration. Purity (%) of IgY was calculated as (concentration of total IgY÷concentration of total protein)×100.
The specific IgY concentration in IgY powder was determined by immunoprecipitation (Japanese Biochemical Society, Citation1986). A volume of 0.5 ml IgY solution (1 mg/ml IgY powder in PBS) and 1 ml of 0.01% antigen solution in PBS were added to the identical tube and incubated overnight at 37°C. The supernatant was separated by centrifugation (3000×g, 30 min), and the absorbance was measured at 280 nm. The specific IgY concentration was calculated as follows:
ELISA
A polystyrene plate was coated with V. harveyi 108 cells/ml in 50 µl/well and incubated at 37°C overnight. The plate was washed three times with PBS containing 0.05% Tween 20 (PBST), 200 µl/well. A blocking buffer (5% Skim milk in PBS) was added at 100 µl/well and incubated at 37°C for 1 h. The plate was then washed three times with PBST. Crude IgY was diluted two-fold serial (1:200–1:409,600) then 50 µl was added to each well and incubated at 37°C for 1 h. The plate was washed again as above and 50 µl of alkaline-phosphatase-conjugated rabbit anti-chicken IgG (Sigma Chemical Co., USA) diluted 5000-fold with PBS was added to each well. After incubation at 37°C for 1 h, the plate was washed and followed by the addition of 100 µl of p-nitrophenyl phosphate in diethanolamine buffer (pH 8.9; Sigma Chemical Co., USA). After incubation at 37°C for 30 min, 50 µl of 3 N NaOH was applied to arrest the reaction. The colour developed was read at 405 nm with an ELISA reader (Labsystem, USA). Crude IgY from nonimmunised hens was used as a negative control.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed (Laemmli, Citation1970) using 6 µl% stacking gel and 12% separating gel. The samples were dissolved in 2× loading buffer (1% SDS, 2% 2-mercapto- ethanol, 0.625 mol/l Tris, pH 6.8) and boiled for 5 min. Electrophoresis was carried out at 40 V for 30 min then followed with 100 V for 100 min. The gels were stained with 2% Coomassie blue R-250 and destained with destaining solution (25% methanol, 7% acetic acid).
Storage stability of IgY
Approximately 10 mg of IgY powder was stored in 1.5 ml paraffin-sealed microcentrifuge tubes at −20, 4 and 18°C and room temperature (RT) (annual average 30.6±2.5°C), respectively. The residual binding activity of IgY to V. harveyi antigen was investigated by ELISA every month for the duration of 1 year. Each measured temperature was tested in triplicate. The activity (%) was calculated as (absorbance at 405 nm of treated IgY÷absorbance at 405 nm of untreated IgY)×100.
Thermal stability of IgY
Approximately 10 mg/ml of IgY solution in 1.5 ml paraffin-sealed microcentrifuge tubes were thermally treated at 60, 70 (120 min) and 80°C (10 min) in a temperature-controlled water bath for various periods of time. To evaluate the effect of thermal protectants, 30% glucose, 30% sucrose or 30% sorbitol were added to the IgY solution. The samples were incubated at 60 and 70°C and sampled for evaluation at 0, 10, 30, 60 and 120 min, respectively. A further set of samples was treated at 80°C and the samples were taken at 0, 1, 2, 5 and 10 min. Evaluation of the IgY activity was determined by ELISA technique. Each treatment was tested in triplicate.
pH stability of IgY
IgY powder was dissolved in 1× PBS. The pH was adjusted to 2, 4, 6, 8, 10 and 12 with 0.1 N HCl or 0.1 N NaOH, after that the samples were kept at 37°C for 0, 0.5, 1, 2 and 4 h. Sampling was conducted at the desired period of time, and the pH was immediately adjusted to 7 with 2 M Tris buffer (pH 8.0). The IgY activity was determined by ELISA technique. Each pH treatment was tested in triplicate.
In vitro stability of IgY to simulated gastric conditions
The stability of the IgY to the gastric conditions was evaluated with simulated gastric fluid (SGF). The SGF consisted of 3.2 mg/ml of pepsin (Sigma Chemical Co., USA) in 0.03 M NaCl, at pH 1.2 (United States Pharmacopeial Convention Council of Experts, Citation2004a). The SGF was added to the IgY to give an enzyme to substrate ratio of 1:20 (Shimizu, Fitzsimmons, & Nakai, Citation1988) and was incubated at 37°C with agitation at intervals of 0, 0.5, 1, 2, 3 and 4 h. The samples were neutralised with 2 M Tris–HCl (pH 8.0). The IgY activity was assessed by ELISA technique. The treatment was tested in triplicate.
In vitro stability of IgY to simulated intestinal conditions
Simulated intestinal fluid (SIF) was prepared as described in the United States Pharmacopoeia Convention Council of Experts (Citation2004b). The SIF consisted of 10 mg/ml Pancreatin (Sigma Chemical Co., USA) in 0.05 M KH2PO4, at pH 6.8. The SIF was added to IgY to give an enzyme to substrate ratio of 1:50 (Mouécoucou, Villaume, Sanchez, & Méjean, Citation2004) and was incubated at 37°C with agitation at intervals of 0, 0.5, 1, 2, 3 and 4 h. The samples were removed and placed on ice to halt the reaction. The IgY activity was subsequently assessed by ELISA. The treatment was tested in triplicate.
Bacterial growth inhibition assay
Even though, the specific binding activity of IgY from various stability tests could be demonstrated by ELISA, the effect on growth inhibition was even more important in terms of the bacterial control measure. To evaluate the remaining effectiveness on bacterial growth inhibition, the samples collected both at the end of the storage stability experiment of 1 year and the end of the incubation time from each stability test involving temperature, pH and effects of proteolytic enzymes on IgY were tested using a bacterial growth inhibition assay. A volume of 0.5 ml×107 CFU/ml live V. harveyi was mixed with an equal volume of 10 mg/ml IgY from each treatment and incubated for 2 h. One hundred microlitres of the sample was taken to perform the plate count as described below.
In vitro neutralisation antibody test
The IgY powder was dissolved in 1.5% NaCl solution to attain the concentrations of 2, 10 and 20 mg/ml compared to a negative control in which the IgY powder was absent, and then mixed at a ratio of 1:1 in 1.5 ml microcentrifuge tubes with a bacterial suspension of 107 CFU/ml of live V. harveyi contents. The final concentrations of IgY and bacteria were 0, 1, 5 and 10 mg/ml and 5×106 CFU/ml, respectively. The triplicates of each concentration were conducted in 1.5 ml microcentrifuge tubes. After incubation at 37°C for 1, 3, 6 and 24 h, 100 µl of each tube was diluted by serially decreased 10-fold dilutions with 1.5% NaCl. One hundred microlitres was taken from each dilution and spread onto thiosulphate citrate bile-salt sucrose (TCBS) agar. The cultured plates were incubated at 37°C for 24 h. The numbers of living cells were counted and calculated as colony forming unit per millilitre (CFU/ml).
Statistical analysis
The results were constructed to determine one-way analysis of variance (ANOVA) which followed a completely randomised design (CRD), and Duncan's new multiple range test (DMRT) was used to compare the divergences of the observed average values among the groups at the level of significance of α=0.01 or 0.05.
Results and discussion
Immunisation of laying hens
The antibody titres in crude IgY from the salting out method were monitored by means of ELISA for 16 weeks (). An increase in the antibody titres was detected at the second week subsequent to the first immunisation, and of the groups supplemented with CpG-ODN (treatment 1–3), both 10 and 20 µg were found to show both a faster and higher immune response. Furthermore, when boosting was conducted at the 10th week, the antibody titre recovered quickly. When observed at the 12th week, the antibody titre of treatments 2 and 3 reached a maximum of 350 and 400% as compared to the non-supplemented with CpG-ODN (treatment 1). Lévesque, Martinez, and Fairbrother (Citation2007) also observed the increase in specific IgY against fimbrial adhesion F4 of enterotoxigenic E. coli in hens subsequent to the use of Incomplete Freund's adjuvant (IFA) supplemented with CpG-ODN as an adjuvant. The response was established to increase in excess of 500% and peaked at 942% in comparison to that with the use of only IFA. Krieg et al. (Citation1995) found the CpG motifs in bacterial DNA and synthetic oligodeoxynucleotides to induce the production of B cells and induce immunoglobulin secretion both in vitro and in vivo test.
Partial purification of IgY
After immunisation, the IgY obtained from egg yolk by means of the water dilution method with subsequent freeze drying was characterised. The average (±standard deviation) protein concentration and total IgY obtained were recorded as 29.0±2.8 and 9.8±0.4 mg/ml of egg yolk, respectively. The yield of the total IgY from egg yolk can vary depending on the type of antigen (Sunwoo, Nakano, Dixon, & Sim, Citation1996), strain of chicken and age (Li et al., Citation1998) and adjuvant (Erhard et al., Citation1997). The purity and specificity of IgY are an important consideration in the wide utilisation and the large-scale production with high yield recovery and high purity. The water dilution method produced 34.2±3.4% of purity and 12.2±4.2% of specific IgY. Moreover, we found that CpG-ODN can enhance the yields of specific IgY in egg yolk.
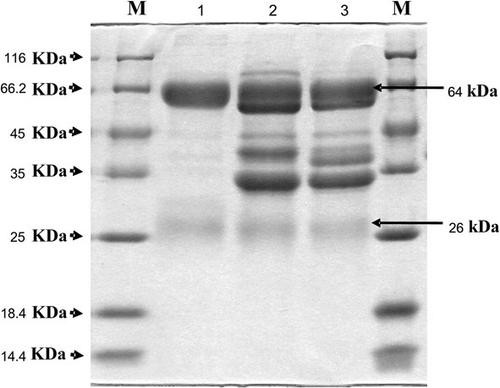
The protein patterns from the two different methods were characterised by the application of SDS-PAGE (), which revealed two bands of 64 and 26 kDa which corresponded to both the heavy chain and light chain of IgY, respectively. Freeze-dried IgY was prepared and used for the stability determination.
Storage stability of IgY
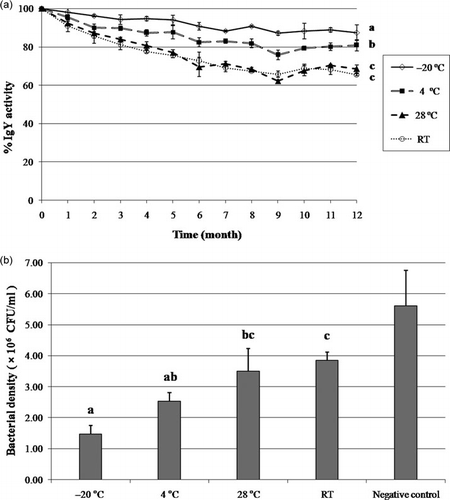
The effect of storage temperatures on the IgY over 12 months was investigated by means of comparison of its activity in ELISA (a). When IgY was stored at 28°C and RT, the colour was observed to change to be brownish, accompanied by an odour which decreased in the solubility beginning from the fourth month, but the IgY stored at –20 and 4°C remained unaffected throughout the total identical period. The result of the samples taken from −20, 4, 28, and RT revealed the remnant activity at 86, 80, 67 and 64%, respectively subsequent to 12 months of storage (P < 0.01). Furthermore, the activity of IgY stored at −20°C was found to be significantly different from those stored at RT or 28°C. There was no significant difference on the inhibitory effect of IgY stored at 4°C compared with the storage at −20°C, but the IgY stored at −20°C was revealed to have the highest inhibitory effect on bacterial growth (b).
Thermal stability of IgY
The IgY solution was thermally treated at various temperatures such as 60, 70 and 80°C for various time periods (a). The IgY activity was found not too significantly (P < 0.01) decrease until the temperature reached at 60 and 70°C a duration of 10 min. The results are similar to those reported by Shimizu et al. (Citation1988), who found the activity of IgY to be decreased by heating at 70°C or above for a duration of 15 min, with a serious denaturation when thermally treated at temperatures in excess of 75°C (Chang, Ou-Yang, Chen, & Chen, Citation1999). Since sugar was reported to be an effective preventer of the denaturation and the destruction of proteins, in our research monosaccharides (glucose), polysaccharides (sucrose) and sugar alcohol (sorbitol) were investigated for potential influences on the heat denaturation of IgY. The result showed that the addition of 30% glucose, 30% sucrose and 30% sorbitol to the IgY solution effectively prevented thermal denaturation of proteins at conditions in excess of 70°C; 30% sorbitol, in particular, proved to maintain superior activity prevention at both 70 and 80°C. The protective effect of sugars on thermal denaturation of proteins was previously described to be due to the enhancement of hydrophobic interactions within the protein molecule (Shimizu, Nagashima, Hashimoto, & Suzuki, Citation1994) and the changes in preferential solvation of protein molecules, which facilitates the stabilisation of proteins during the thermal treatment (Timasheff, Citation1993).
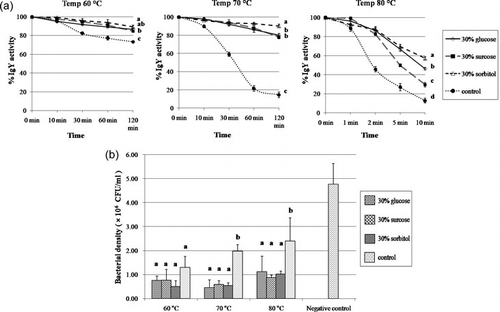
The results on growth inhibition assay from the temperature test correlate with those obtained by ELISA. However, there no significant difference was observed (P < 0.05) among the various kinds of sugars and the control group at 60°C (b). In contrast, the inhibitory effect of IgY in the presence of sugar, at both 70 and 80°C, was found to be significantly different (P < 0.05) from the control groups, but no significant difference (P<0.05) between each sugar group was observed.
pH stability of IgY
The stability of IgY at pH 2–12 was determined by ELISA as shown in a. No significant change in the IgY activity at pH levels 6 and 8 throughout a duration of 4 h was observed. The activity of the IgY was established to gradually decrease at pH 4 and to be almost completely lost at pH 2, with only 27% of the total IgY activity remaining after 4 h. The IgY activity was proven to rapidly decrease at low pH levels due to the conformational change as a result of the variations charge on the protein surface and damages in the Fab portion which include the antigen-binding site (Shimizu et al., Citation1988). In the present study research, IgY was found to be rather stable under basic conditions with a gradual decline from pH 10 to 12. However, the IgY activity was established to remain constant at 63 and 42% after 4 h at pH 11 and 12, respectively.
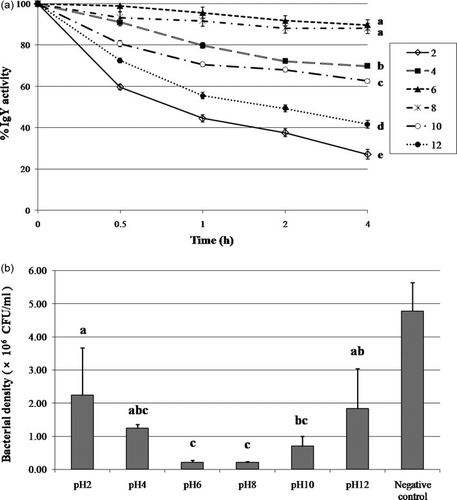
The result from the inhibition assay showed a correlation to the IgY activity by ELISA with the best bacterial growth inhibition observed at pH 6 and 8. Furthermore, we noted that the low binding of IgY at strong acidity or basicity (pH 2, 12) still exhibited good results in bacterial growth inhibition (2.23±1.43×106 CFU/ml at pH2 and 1.83±1.21×106 CFU/ml at pH4) compared to the negative control (4.77±0.86 ×106 CFU/ml) as shown in a,b. However, the result showed a high variation on the inhibition effect which is probably due to the conformational change at the strong condition, resulting in loose non-covalent interactions between the epitope and the variable region (VH/VL) domain, particularly the complementarity-determining regions (CDR) (Kuby, Citation1997). The observation suggests that the IgY structure exhibits some reversible alteration at strong pH condition.
Stability of IgY against proteases
The stability of the IgY under simulated gastric conditions or simulated intestinal conditions was assessed with incubation of the IgY in SGF or SIF for 4 h. At 0.5, 1, 2 and 4 h of incubation, the samples were taken and neutralised for the determination of the IgY activity by ELISA (). The result demonstrated a significant (P < 0.01) loss of the IgY activity in the SGF condition, which 25 and 9% of the binding activity remnant subsequent 30 min and 4 h of exposure, respectively. This result quite confirms those of the SIF conditions in which IgY was observed to maintain an activity in excess of 94% after 4 h of exposure. The results from pepsin treatment confirm those reported by Hatta et al. (Citation1993) who established high susceptibility of the IgY to pepsin at pH 2 but at higher pH levels, for example, pH 4, IgY activity was observed to remain up to 91 and 63% subsequent to 1 and 4 h, respectively. Previous work revealed that the tryptic digestion of IgY to retain its antigen-binding and cell-agglutinating activities in spite of a definite breakdown of the polypeptides. No definite cleavage of the IgY chains was detected in the trypsin digestion; although the IgY activity was observed to remain for the chymotryptic digestion at a high level (Otani, Matsumoto, Saeki, & Hosono, Citation1991; Shimizu et al., Citation1988).
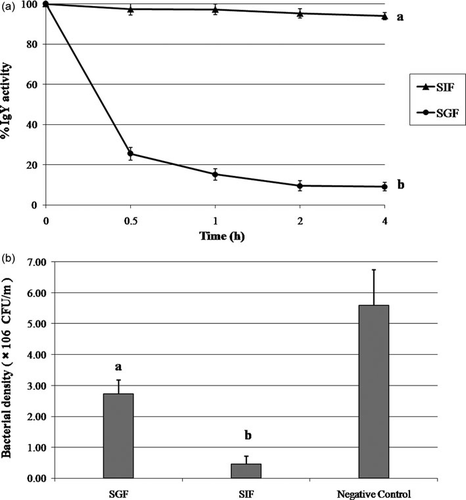
Although ELISA was found to exhibit a great reduction of the IgY activity in SGF conditions, the sample taken after 4 h exposure was still observed to maintain half of the inhibitory effect in the neutralisation assay (a,b). This is likely due to the cleavage of the Fc portion of the IgY by pepsin, which did not demolish the binding activity of the Fab portions which render the inhibition function of the IgY; however, the ELISA was revealed to show low OD readings since the secondary antibody did not bind to the IgY at the Fc.
Neutralisation antibody test in vitro
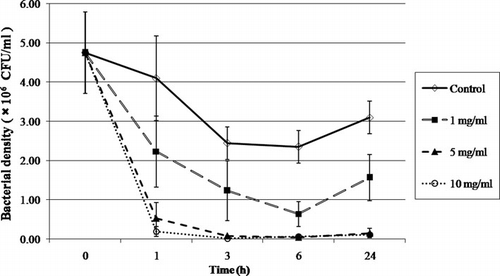
Various concentrations of IgY (0, 1, 5 and 10 mg/ml) were used to neutralise the live V. harveyi 5×106 CFU/ml. The viable cells were counted in a TCBS medium by means of the plate count method. Significant differences of live Vibrio bacteria among the control and treatment groups were observed during the first 24 h post exposure. The bacterial growth was found decrease in the presence of highly concentrated levels of IgY, but no difference in the bacterial growth between the concentrations of 5 and 10 mg/ml of IgY was evident. At 24 h of incubation, the treated groups still showed significantly lower levels (P<0.01) of live bacterial cells in comparison to the control group (). The mechanism of growth inhibition by antibodies is not clearly understood. Agglutination, which is the interaction between antibody and antigen, was found to result in visible clumps, is the intermediary one of growth inhibition (Kubo, Zimmerman, & Grey, Citation1973). Moreover, the agglutination between the antibodies and the bacterial cells may lead to a reduced rate of motility and opportunity to take nutrients than free-motile single cells (Sadziene, Thomopson, & Barbour, Citation1993). Sim, Sunwoo, and Lee (Citation2000) found the essential role in the bacterial growth, which causes specific binding between antibodies and surface components of bacteria, which potentially leads to a reduction of biological functions.
Conclusion
Laying hens were immunised with glutaraldehyde fixed cells of V. harveyi. The immune response of the specific antibody IgY was enhanced with the CpG-ODN sequence in consideration of an increase of 400% in the specific IgY titre. IgY was isolated from egg yolk by means of water dilution method and was concentrated to IgY powder by freeze drying. Large amounts of specific IgY was detected by the application of the ELISA technique. The advantageous utilisation of IgY for food application or feed supplementation, which prevents the contamination by pathogenic bacteria and reduced the pathogen to cause infections in animals, was demonstrated.
Furthermore, the characteristics of the IgY powder demonstrated by the ELISA technique and confirmed by growth inhibitory assay which indicated a IgY stability property in diverse conditions which involve pH levels, temperature, effects of proteolytic enzyme and storage temperature. The results show IgY to be stable at natural pH levels. The thermal destruction of IgY at temperatures in excess of 70°C and the prevention of IgY appeared to be of importance. The addition of 30% sorbitol to the solutions was found to be highly effective to preserve the IgY activity. Simulated gastric and intestinal solutions that imitate the conditions present in the stomach and intestine was used to test the stability of IgY. IgY was found to be relatively resistant to trypsin digestion, but fairly sensitive to pepsin digestion. Moreover, storage of lgY at a temperature of −20°C was determined to be most appropriate.
Acknowledgements
This research is supported by the Center for Agricultural Biotechnology, Postgraduate Education and Research Development Office, Commission on Higher Education, Ministry of Education and Kasetsart University Research and Development Institute (KURDI).
References
- Akita, E.M., & Nakai, S. (1992). Immunoglobulins from egg yolk: Isolation and purification. Journal of Food Science, 57, 629–634.
- Allen, E., & Kelman, A. (1987). Immunofluorescent stain procedures for detection and identification of Erwinia carotovara var. atroseptica. Phytopathology, 6, 1305–1312.
- Barua, A., Furusaw, S., & Yoshimura, Y. (2000). Influence of aging and estrogen treatment on the IgY concentration in the egg yolk of chicken, Gallus domesticus. Journal of Poultry Science, 37, 280–288.
- Carlander, D., Kollberg, H., Wejaker, P.E., & Larsson, A. (2000). Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunologic Research, 21, 1–6.
- Chang, H.M., Ou-Yang, R.F., Chen, Y.T., & Chen, C.C. (1999). Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype c in chicken egg yolk (IgY). Journal of Agricultural and Food Chemistry, 47, 61–66.
- Chen, S. (1992). Coping with diseases in shrimp farming. In H. Saram, & T. de Singh (Eds.), Proceedings of the First Global Congress on the Shrimp Industry (pp. 113–117). Kuala Lumpur: INFOFISH.
- Erhard, M.H., Schmidt, P., Hofmann, A., Bergmann, J., Mittermeier, P., et al. (1997). The lipopeptide, Pam3Cys-Ser-(Lys)4: An alternative adjuvant to Freund's adjuvant for the immunisation of chicken to produce egg yolk antibodies. Alternatives to Laboratory Animals, 25, 173–181.
- Gutierrez, M.A., Miyazaki, T., Hatta, H., & Kim, M. (1993). Protective properties of egg yolk IgY containing anti-Edwardsiella tarda antibody against paracolo disease in the Japanese eel, Anguilla japonica Temminck & Schlegel. Journal of Fish Diseases, 16, 113–122.
- Hamada, S., Horikoshi, T., Minami, T., Kawabata, S., Hiraoka, J., Fujiwara, T., et al. (1991). Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect and Immunity, 59, 4161–4167.
- Hatta, H., Tsuda, K., Akachi, S., Kim, M., Yamamoto, T., & Ebina, T. (1993). Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Bioscience Biotechnology & Biochemistry, 57, 1077–1081.
- Japanese Biochemical Society. (1986). Meneki Seikagaku Kenkyuho. Tokyo: Kagaku Dojin.
- Jiravanichpaisal, P., Miyazaki, T., & Limsuwan, C. (1994). Histopathology, biochemistry, and pathogenicity of Vibrio harveyi infecting black tiger prawn Penaeus monodon. Journal of Aquatic Animal Health, 6, 27–35.
- Karunasagar, I., Pai, R., Malathi, G.R., & Karunasagar, I. (1994). Mass mortality of Penaeus monodon larvae due to antibiotic resistant Vibrio harveyi infection. Aquaculture, 128, 203–209.
- Kim, D.K., Jang, I.K., Seo, H.C., Shin, S.O., Yang, S.Y., & Kim, J.W. (2004). Shrimp protected from WSSV disease by treatment with egg yolk antibodies (IgY) against a truncated fusion protein derived from WSSV. Aquaculture, 237, 21–30.
- Krieg, A.M., Yi, A.K., Matson, S., Waldschmidt, T.J., Bishop, G.A., Teasdale, R., et al. (1995). CpG motifs in bacterial DNA trigger direct B-cell activation. Nature, 374, 546–549.
- Kubo, R.T., Zimmerman, B., & Grey, H.M. (1973). Phylogeny of immunoglobulins. In M. Sela (Ed.), The antigens (pp. 417–477). New York: Academic Press.
- Kuby, J. (1997). Antigen-antibody interactions. In J. Kuby (Ed.), Immunology (3rd ed., pp. 143–164). New York: W.H. Freeman and Company.
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
- LeClaire, R.D., Hunt, R.E., & Bavari, S. (2002). Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect and Immunity, 70, 2278–2278.
- Lee, S.B., Mine, Y., & Stevenson, R.M. (2000). Effects of hen egg yolk immunoglobulin in passive protection of rainbow trout against Yersinia ruckeri. Journal of Agricultural and Food Chemistry, 48, 110–115.
- Lévesque, S., Martinez, G., & Fairbrother, J.M. (2007). Improvement of adjuvant systems to obtain a cost-effective production of high levels of specific IgY. Poultry Science, 86, 630–635.
- Li, X., Nakano, T., Sunwoo, H.H., Paek, B.H., Chae, H.S., & Sim, J.S. (1998). Effects of egg yolk weights on yolk antibody (IgY) production in laying chickens. Poultry Science, 77, 266–270.
- Mine, Y., & Kovacs-Nolan, J. (2002). Chicken egg yolk antibodies as therapeutics in enteric infectious disease: A review. Journal of Medicinal Food, 5, 159–169.
- Mouécoucou, J., Villaume, C., Sanchez, C., & Méjean, L. (2004). Betalactoglobulin/polysaccharide interactions during in vitro gastric and pancreatic hydrolysis assessed in dialysis bags of different molecular weight cut-offs. Biochimica et Biophysica Acta, 1670, 105–112.
- Nomura, S., Suzuki, H., Masaoka, T., Kurabayashi, K., Ishii, H., Kitajima, M., et al. (2005). Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter, 10, 43–52.
- Otani, H., Matsumoto, K., Saeki, A., & Hosono, A. (1991). Comparative studies on properties of hen egg yolk IgY and rabbit serum IgG antibodies. Lebensmittel-Wissenschaft und-Technologie, 24, 152–158.
- Ramesh, A., & Venugopalan, V.K. (1989). Response of enteric luminous bacteria to environmental conditions in the gut of the fish. Journal of Apply Bacteriology, 66, 529–533.
- Rankin, R., Pontarollo, R., Ioannou, X., Krieg, A.M., Hecker, R., Babiuk, L.A., et al. (2001). CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense and Nucleic Acid Drug Development, 11, 333–340.
- Sadziene, A., Thomopson, P.A., & Barbour, A.G. (1993). In vitro inhibition of Borrelia burgdorferi growth by antibodies. Journal of Fish Diseases, 167, 165–172.
- Shimizu, M., Fitzsimmons, R.C., & Nakai, S. (1988). Anti-E. coli immunoglobulin Y isolated from egg yolk of immunized chickens as a potential food ingredient. Journal of Food Science, 53, 1360–1366.
- Shimizu, M., Nagashima, H., Hashimoto, K., & Suzuki, T. (1994). Egg yolk antibody (IgY) stability in aqueous solution with high sugar concentrations. Journal of Food Science, 59, 763–765.
- Sim, J.S., Sunwoo, H.H., & Lee, E.N. (2000). Ovoglobulin IgY. In A.S. Naidu (Ed.), Natural food antimicrobial systems (pp. 227–252). New York: CRC Press.
- Srisapoome, P., Punyokun, K., Hongprayoon, R., & Areechon, N. (2006). Passive Immunization of anti-Vibrio harveyi egg yolk immunoglobulin against luminous disease in black tiger shrimp (Penaeus monodon). Proceedings of the 44th Kasetsart University Annual Conference (pp. 365–374). Bangkok: Kasetsart University.
- Sunwoo, H.H., Nakano, T., Dixon, W.T., & Sim, J.S. (1996). Immune responses in chickens against lipopolysaccharide of Escherichia coli and Salmonella typhimurium. Poultry Science, 75, 342–345.
- Timasheff, S.N. (1993). The control of protein stability and association by weak interactions with water: How do solvents affect these processes? Annual Review of Biophysics and Biomolecular Structure, 22, 67–97.
- United States Pharmacopeial Convention Council of Experts. (2004a). Simulated gastric fluid, TS. In Board of Trustees (Ed.), The United States pharmacopeia 27, the national formulary (Vol. 22, p. 2728). Rockville, MD: Author.
- United States Pharmacopeial Convention Council of Experts. (2004b). Simulated intestinal fluid, TS In Board of Trustees The United States pharmacopeia 27, the national formulary (Vol. 22, p. 2728). Rockville, MD: Author.