?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Debregeasia orientalis, an important Chinese medicinal herb, has a long history for the treatment of rheumatic diseases. In this study, the antitumour activities of D. orientalis leaf polyphenols (DOLPs) against human cervical cancer Hela cells were investigated in vitro and in vivo. Hela cell proliferation, cell cycle, and apoptosis were investigated, the results revealed that DOLPs could inhibit Hela cell proliferation by blocking cell-cycle progression, inducing apoptosis and cell-cycle arrest. Moreover, Hela cells treated with DOLPs also showed an overproduction of reactive oxygen species (ROS) and a decrease of mitochondrial membrane potential. These results suggesting that the apoptotic effect of DOLPs on Hela cells was associated with an increased level of ROS and ROS production mediate apoptosis via the mitochondrial pathway. This article not only provides a basis for the functional mechanism and future application of DOLPs, but also promotes the high value utilization of D. orientalis leaf.
1. Introduction
According to statistics, about 200,000 women die of cervical cancer and more than 500,000 women were diagnosed with cervical cancer each year (Chaturvedi et al., Citation2007; Hu et al., Citation2010). Human papillomavirus is considered to be the greatest risk factor for cervical cancer (Gorin, Glenn, & Perkins, Citation2011). Although the human papillomavirus vaccine has been successfully developed and licensed, it has been used in several areas, including the United States, Europe, Canada and Australia (Lowy & Schiller, Citation2006). But vaccines are only effective for some types of human papillomavirus and have not been widely used in developing countries, which leading to the incidence of human papillomavirus infection-related cervical cancer has not been eliminated (Zhu, Wang, Yang, Han, & Li, Citation2013). After being diagnosed with cervical cancer, surgery is the first choice for patients with early-stage cervical cancer, while radiotherapy and chemotherapy are the effective treatments for advanced patients. With the development of various new chemotherapeutic drugs such as cisplatin and the combination use of various chemotherapeutic drugs, chemotherapy is becoming more and more important in the treatment of patients with advanced cervical cancer (Shimada et al., Citation2016). However, the side effects of these antitumour drugs are awful and most common side effects associated with platinum agents are nephrotoxicity, myelosuppression, nausea and vomiting, and hypersensitivity reactions (Holman, Ren, & Westin, Citation2015; Marel, Lizard, Izard, Latruffe, & Delmas, Citation2008), which brings patients more pain and leads to a low quality of life. Therefore, hunt for an effective clinical approaches to treat this cancer is still a challenge.
According to Traditional Chinese Medicine theory, the accumulation of heat and toxins plays a key role in the occurrence and development of cancers. In this regard, Chinese medicinal herbs and the person-based formulas contain hundreds even thousands of active ingredients, which may regulate the activities or expression of a broad spectrum of proteins (Zhang, Liang, & He, Citation2017). Therefore, Chinese herbal medicine might be a promising approach for the management of cancers. However, huge efforts still need to be deployed in this field to bring the most potential of medicinal herbs for cancer treatment. Debregeasia orientalis is a wild medicinal plant resources since ancient times in China. D. orientalis is widely distributed in the subtropical and tropical area of eastern Asia (Wang, Citation2011). Its leaves, stems and roots have been used extensively as one of the traditional Chinese folk herbs to treat various diseases, such as eliminating dampness, detoxification and otitis media (Liu, Citation2013). D. orientalis is rich in functional ingredients, such as polyphenols and flavonoids, and possesses great activity on anti-cancer and antibacterial (Liu, Gu, & Han, Citation2004; Wang, Citation2011). D. orientalis fruit could be edible and contains a variety of essential amino acids, high in vitamins and minerals. In addition, D. orientalis fruit is rich in polyphenols and possesses anticancer activity. However, this wild plant resources has not got enough attention and development it deserved. Besides, there are little research about it. As a kind of plant resource with long medicinal history, its potential functional value should be further developed. That would be great significance for develop wild plant resources.
2. Materials and methods
2.1. Materials and reagents
Human cervical cancer cell Hela: purchased from the Experimental Animal Center of Fourth Military Medical University cell bank. The Roswell Park Memorial Institute-1640 medium was purchased from Life Technologies (Carlsbad, CA, USA). Fetal bovine serum was purchased from Zhejiang Tianhang Biological Technology Co. (Zhejiang, China). Hochest33258 staining, propidium iodide (PI), Dimethyl sulfoxide, Rhoamine-123,3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and RNase-A were purchased from Sigma (St. Louis, MO, USA). Annexin V-FITC (fluorescein isothiocyanate)/PI (propidium iodide) kit and DCFH-DA (2’,7’-Dichlorodihydrofluorescein diacetate) were purchased from Nanjing Jiancheng biology Co. (Nanjing, China). All the other cell culture reagents were purchased from Sinopharm (Beijing, China). D. orientalis leaves were collected in Shaanxi Province, China.
2.2. D. orientalis leaf polyphenols (DOLPs) extraction
Ultrasonic extraction was used for D. orientalis leaves polyphenols extraction. Briefly, 20.0 g of dried D. orientalis leaf powder was weighed and extracted under the condition 70% methanol (material to liquid ratio was 1:30), 60°C, 100 W and 40 min extraction. The extract solution was centrifuged at 3500g for 20 min. The supernatant was collected and concentrated by a rotary evaporator at 40°C under reduced pressure. The resin (50 g) and extracting solution (1.5 mg/mL total polyphenol) were blended in a conical flask and then oscillated for 10 h. After adsorbed, the supernatant was discarded and the remaining fraction was eluted five times with distilled water. The resin was loaded into a resin column, wet-loaded, eluted by 70% ethanol at a flow rate of 3 bed volume/h. The third column volume effluent was collected and evaporated at 40°C, then dried by a vacuum freeze dryer to obtain the refined extracts of D. orientalis leaves. The sample was stored at −20°C until needed. The total polyphenol content in samples was determined according to the protocol of Eom, Park, Dong, Kim, and Dong (Citation2009).
LC-20AR HPLC system (Shimadzu Corp, Kyoto, Japan) was used to identify and quantify the polyphenol compounds of D. orientalis leaves extracts. The chromatographic separation was carried out on an InertSustain C18 column (4.6 mm i.d. × 250 mm, 5 mm, Shimadzu, Japan). With 0.1% trifluoroacetic acid and 20% methanol as mobile phase A and acetonitrile as mobile phase B at a flow rate of 0.8 mL/min. The gradient programme was set as follows: 0–30 min, 0–3% B; 30–60 min, 3–5% B; 60–80 min, 5–7% B, 80–90 min, 7–10% B. The chromatogram was detected at 278 nm. The injection volume of each sample and standard solution was 10 μL and the column temperature was maintained at 30°C.
2.3. Cell culture and grouping
Hela cells were cultured in the Roswell Park Memorial Institute-1640 medium supplemented with 10% (v/v) fetal bovine serum (Gibco, USA), 100 μg/mL streptomycin and 100 unit/mL penicillin, and then incubated at 37°C with 5% CO2 supplied in the incubator, using the cells in logarithmic growth phase to experiment. Three sets of experiments were performed as follows: the test substance treatment group with the concentration of 100, 200, and 400 μg/mL of DOLPs respectively, the control group and 5‰ dimethyl sulfoxide group. The test compounds were dissolved in dimethyl sulfoxide and diluted with the serum-free culture medium. All the experiments were repeated three times independently.
2.4. Cell proliferation and inhibition assay
The inhibitory effects of DOLPs on Hela cells were measured by MTT assay (Koyama et al., Citation2010). Cells were cultured in a 96-well culture plate (5 × 103 cells/well). After 24 h, replace fresh medium and treated with different concentrations of DOLPs to each group, designed five replicate holes for each treatment group and the blank control group. After incubating for 24 h, medium was discarded and washed three times with PBS, added 10 μL with 5 mg/mL of MTT and 150 μL serum-free medium into every well, and then incubated at the incubator for 4 h, discarded the supernatant and added 150 μL dimethyl sulfoxide at each well, shaking gently for 10 min. Measuring the optical density of the light at 490 nm with the microplate reader. Cell viability was calculated by the following formula:
2.5. Cell morphology analysis
Hoechst 33258 staining assay was used to observe the change of morphology of cells (Liu, Xiao, Xiong, Wei, & Ruan, Citation2011). Hela cells were cultured in a six well plate (4 × 105 cells/well) for 24 h and then treated with different concentrations of DOLPs for 24 h. Discard the medium and wash each well twice with PBS, 1 mL of paraformaldehyde at a concentration of 4% was added and placed at 4°C for l5 min. The cells were washed again with PBS for three times and added 1 mL of Hoechst 33258 at the concentration of 5 mg/mL, then place in the dark at room temperature for 10 min. Re-washing the cells three times with PBS, then put it under the Inverted fluorescent microscope and took pictures.
2.6. Cell cycle analysis by flow cytometry
The cell cycle distribution was detected by flow cytometry (Millipore Corporation, Billerica, MA, USA) using propidium iodide (Marel et al., Citation2008). After incubated 24 h, Hela cells were treated with different concentrations of DOLPs for 24 h. Collecting all the suspension cells and adherent cells, suspending in cold PBS and then centrifugal. Discard the supernatant, added 1 mL of 70% ethanol slowly, fixed cells in −20°C for 24 h. Subsequently, the fixed cells were washed three times with cold PBS and treated with the binding buffer containing RNase (1 mg/mL) and propidium iodide (400 mg/mL), then incubated at 37°C avoid light for 30 min. Finally, the stained cells were analysed by flow cytometry.
2.7. Apoptotic cells analysis
The apoptotic cells were quantified by Annexin V-FITC/PI double staining assay using Annexin V-FITC detection kit (Zhou, Wei, Cao, & Ruan, Citation2013). Hela cells were cultured in a six well plate (4 × 105 cells/well) for 24 h and then treated with different concentrations of DOLPs. Harvested all the cells after 24 h, washed and resuspended in 0.6 mL binding buffer, added 5 μL Annexin V-FITC and 10 μL propidium iodide, then placed at room temperature and avoid light to staining 10 min. The number of apoptosis of Hela cells were detected by flow cytometry, and defined as follow: lower left quadrant, living cells (Annexin V+/ propidium iodide +); lower right quadrant, early apoptotic cells (Annexin V+/ propidium iodide +); upper right quadrant, late apoptotic cells (Annexin V+/ propidium iodide +); upper left quadrant, primary necrotic cells (Annexin V+/ propidium iodide +).
2.8. Intracellular ROS measurement
The intracellular ROS of Hela cells was detected by DCFH-DA probe and flow cytometry (Raza, John, & Benedict, Citation2011). Hela cells were cultured in a six well plate (4 × 105 cells/well) for 24 h and then treated with different concentrations of DOLPs for 10 h. Later, discarded the liquid waste, washed the cells three times with PBS and collect cells by digestion and centrifugation. Then resuspended the cells in 0.6 mL serum-free medium, add DCFH-DA (4 μM) and incubated at incubator for 30 min. Detect the fluorescence intensity of cells on each well with flow cytometry and analyse the changes of ROS in Hela cells.
2.9. Δψm analysis
The effect of DOLPs on the loss of mitochondrial transmembrane potential (ΔΨm) was measured by flow cytometry using a rhodamine-123 fluorescent probe (Tian, Liu, Yang, & Wang, Citation2016). Rhodamine-123 relies on mitochondrial transmembrane potential into the mitochondrial matrix in normal cells, which leads to fluorescence intensity diminish or disappear. When apoptosis occurs, the integrity of the mitochondrial membrane is destroyed, and the mitochondrial membrane permeability transport pore is opened, which causes the breakdown of the ΔΨm. Rhodamine-123 re-released into the cytoplasmic matrix and exhibits a dependent accumulation in cytoplasmic matrix indicated by a fluorescence emission shift from 488 to 505 nm. Therefore, by detecting the changes of fluorescence intensity in intracellular can reflect the changes in mitochondrial membrane potential and apoptosis. In brief, Hela cells were cultured in a six well plate (4 × 105 cells/well) for 24 h and then treated with different concentrations of DOLPs for 10 h. The cells were harvested, washed twice with PBS and 0.6 mL buffer and 10 μL Rhodamine-123 dye was added then incubated for 10 min. The single-cell suspension was analysed by flow cytometry and the change of Δψm of Hela cells were reflected by the blue fluorescence intensity.
2.10. Western blot for protein expression detection
Hela cells (4 × 105 cells/well) were cultured in a six well plate for 24 h and then treated with different concentrations of DOLPs for 24 h. collecting the cells of control group and DOLPs-treated group and lysed the cells with protein extraction reagent and proteinase inhibitors. The lysates were centrifuged at 15,000g for 30 min, draw the supernatant and determined the protein concentration by using the Bradford assay (Li et al., Citation2013). The proteins were separated by 15% SDS-PAGE, then transferred to PVDF membranes and blocked with 5% fat-free dry milk for 2 h. Closed overnight with one antibody (1:500) at 4°C. Later, two antibody (1:2000), p53, Bcl-2, Bax, Cyt-c, PARP, caspase-3, caspase-9 and β-Actin are incubated under the condition of 37°C for 2 h. Colouring with alkaline phosphatase colour kit, then take and preserve pictures.
2.11. Anti-tumour experiment in animals
To investigate the antitumour effects of DOLPs in vivo, 30 athymic female BLAB/c-nude mice (aged 5–6 weeks) were used for experiments which purchased from Shanghai Slac Animal Center (Shanghai, China). Animals were housed within a dedicated SPF facility with alternating 12 h periods of light and darkness at 23 ± 5°C with 60% ± 5% relative humidity. All of the animals were allowed free access to tap water and rodent chow. Hela cells were injected subcutaneously (1 × 106 cells/mL) into the right inguinal area of the mice using a syringe. After five days, 30 mice bearing cervical xenografts were divided randomly into three groups (control, 100 mg/kg of DOLPs, and 200 mg/kg of DOLPs) and treatment was initiated. Each group was composed of 10 mice. DOLPs was orally administered five times per week at a dose of 100 or 200 mg/kg body weight, while vehicle-treated mice were administered orally the PBS. Mice weight and tumour volume were surveyed twice per week. The volumes of tumours were measured using a vernier caliper. Mice were sacrificed 21 days after administration and tumours were excised to measure tumour volume. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee following the guidelines of Fourth Military Medical University, China (XJYYLL-2015689).
3. Result and analysis
3.1. HPLC analysis for DOLPs
The extracts DOLPs were determined by HPLC at 278 nm and the results were shown in (A,B) and . The total phenols of the extracts was 75.36%, ten polyphenol compounds in DOLPs were identified in turn as follows: (1), Gallic acid; (2), protocatechuate; (3), Epigallocatechin; (4), (+)− catechins; (5), caffeine; (6), Chlorogenic acid; (7), Epigallocatechin gallate; (8), Epicatechin; (9), Gallocatechin gallate; (10), Epicatechin gallate. Among of them, Chlorogenic acid, Caffeine, Protocatechuate, Epigallocatechin, Epicatechin and (+)− Catechins are the major components of DOLPs, their content were 22.67, 15.80, 10.13, 7.60, 6.04, 5.54 mg/100 mg respectively, and the contents of others were Gallic acid, Epigallocatechin gallate, Gallocatechin gallate, Epicatechin gallate were 0.06, 1.97, 1.38, 1.45 mg/100 mg respectively.
Figure 1. HPLC chromatogram of phenolic compounds present in the extract of Debregeasia orientalis leaves (A) The extract of Debregeasia orientalis leaves; (B) Standard substances.
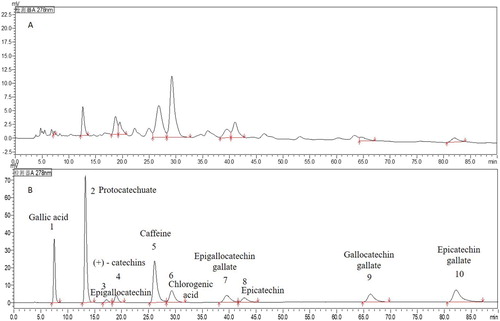
Table 1. Phenolic acid composition of DOLPs.
3.2. DOLPs inhibited proliferation of human cervical cancer cells
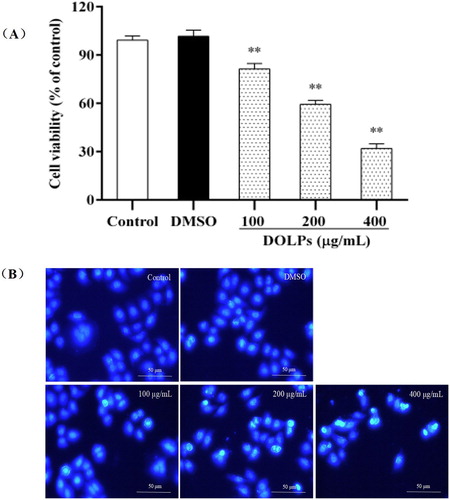
As shown in , DOLPs at 100, 200 and 400 μg/mL (Li, He, Tian, Shi, & Yang, Citation2016; Song, Li, & Li, Citation2016), significantly inhibited cellular proliferation of Hela cells for 24 h in a dose-dependent manner, when compared with the control group ((A)). Interestingly, as observed under an inverted fluorescence microscope ((B)), compared with the control group, the cells in the treatment group were deformed and deeply stained, punctate fluorescence was increased and enhanced, the nuclear shrinkage, chromatin margination and even some of the disorganization. With an increase in the concentration of the test substance, the cell deformation and hyperchromatic phenomenon became more obvious. Besides, some of them lost their ability to adhere to the plate surface, and detached from the bottom, aggregated, and floated in the medium, leading to a decrease in the density of DOLPs-treated cells. The extent of the changes in cell morphology and density depended on the concentrations of DOLPs.
3.3. DOLPs arrested the cell-cycle and upregulated the transcriptional factor p53
To determine the cellular mechanism of growth inhibitory effects of DOLPs on Hela cells, we investigated cell-cycle progression after DOLPs treatment. As shown in , In contrast with the control group, DOLPs caused 2.55%, 17.59% and 20.71% increases in S phase after treated with 100, 200 and 400 μg/mL, and accompanied by a decrease in G0/G1 phase from 67.75% to 63.15%, 52.02% and 46.07%, respectively. These suggesting that the treated cervical cancer cells were subjected to a blockage at the S phase of the cell cycle.
Figure 3. Regulative effects of DOLPs on cell-cycle arrest and the expression of correlative proteins in Hela cells. (A) The distributions of cell number and DNA content of Hela cells are shown as histogram trellis plots; (B) The distribution of cell cycles in DOLPs-treated cells, the protein expression of p53 was determined in Hela cells; (C) with or without DOLPs (control, 100, 200, and 400 μg/mL) for 24 h by western blot assay; (D) Column bar graph of p53 protein expression. *p < 0.05, **p < 0.01.
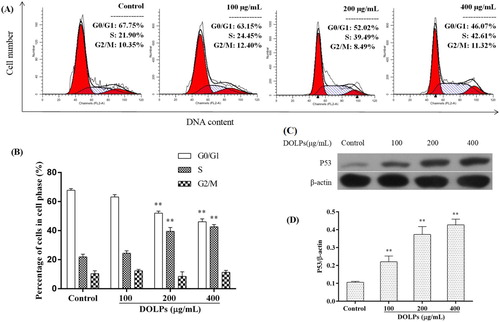
To further test whether DOLPs-induced protein expression was associated with S phase blockage in Hela cells, the levels of cell-cycle-regulating proteins p53 was determined by immunoblotting. As shown in (C,D), we found that p53 levels were significantly upregulated after treated with DOLPs, suggesting that cervical cancer cells cycle blocking may be related to the high expression of p53 caused by DOLPs.
3.4. DOLPs induced mitochondria-mediated intrinsic apoptosis
To clarify whether DOLPs-induced cell death in Hela cells was associated with apoptosis, DOLPs-induced apoptosis was analysed by flow cytometry using a combination of annexin V and propidium iodide staining ((A)). The results showed that after the DOLPs treatment, early apoptosis rate and the late apoptosis rate were all increased in a dose-dependent manner in Hela cells, the early and the late apoptosis were 13.84%, 31.59% and 50.98% respectively ((B)).
Figure 4. DOLPs induced apoptosis through the initiation of the mitochondrial pathway. (A) Dot plots of annexin V/PI staining; (B) Column bar graph of apoptotic cells; (C, E) The expression of Bcl-2 family protein treated with DOLPs; (D, F) The expression of caspase-9 and caspase-3 protein. * p < 0.05, ** p < 0.01.
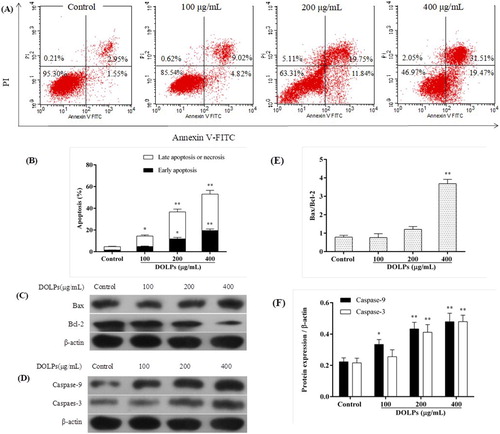
The expression of Bax and Bcl-2 are meanings promoting or inhibiting apoptosis respectively, the Bax/Bcl-2 ratio and caspases is an important indicators for apoptosis (Li et al., Citation2016; Yamashita et al., Citation1998). The Immunoblot results were shown in (C), it showed that the DOLPs could increase Bax protein levels and decreased Bcl-2 levels, the Bax/Bcl-2 ratio shows a sharp increase in this experiment ((E)).
To further examine the involvement of caspases in apoptosis induction of DOLPs, the activities of caspase-3 and caspase-9 were determined subsequently, as shown in (D). The results showed that both caspase-3 and caspase-9 were activated significantly after DOLPs treatment at the concentration of 200 and 400 μg/mL ((F)) compared to the control group. This indicates that the mitochondrial pathway is involved in DOLPs-induced apoptosis.
3.5. DOLPs causing intracellular ROS over-production and enhancing intracellular oxidative stress leading to cervical cancer cell apoptosis
The flow cytometry was employed to detect the mitochondrial transmembrane (ΔΨm) and the DCFH-DA fluorescence intensity was used to measure the ROS changes in Hela cells exposed to DOLPs (100, 200 and 400 μg/mL) for 10 h. As shown in (A,B), the treatment with DOLPs increased the DCFH-DA fluorescence intensity and the RHO-123 fluorescence intensity in Hela cells in a dose-dependent manner, indicating that DOLPs caused the accumulation of intracellular ROS and induced opening of permeability transition pores in the inner membrane, thereby releasing the RHO-123 from mitochondrial into the cytoplasm. When the pores remain open for a long time, mitochondrial swelling and disruption of the outer membrane are caused.
Figure 5. DOLPs-mediated accumulation of intracellular ROS in Hela cells. (A) The intracellular ROS level measured by a DCFH-DA probe; (B) The ΔΨm measured by rhodamine-123; (C, D) The protein expression of Cyt-c and PARP. * p < 0.05, ** p < 0.01.
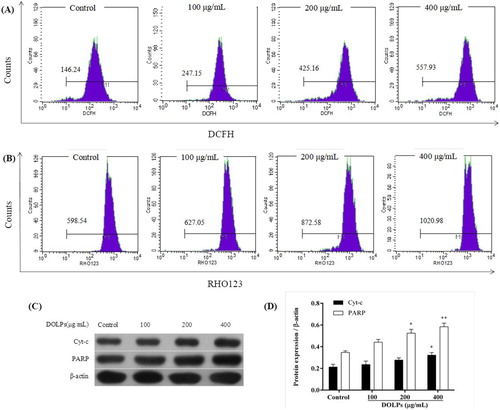
The release of Cyt-c from mitochondrial into the cytoplasm and the change in mitochondrial membrane permeability are closely related events, and the increase in the release of Cyt-c means that the cells undergo irreversible apoptosis (Goldstein et al., Citation2005). Besides, the accumulation of intracellular ROS leads to DNA damage, which in turn activates the PARP enzyme system (Davalli, Marverti, Lauriola, & D’Arca, Citation2018). The present study measured the change of the release of the Cyt-c protein and the activation of PARP in Hela cells. Immunoblot analysis showed that the treatment of Hela cells with DOLPs at 100, 200 and 400 μg/mL increased Cyt-c protein and PARP protein levels after 24 h of exposure (p < 0.05, (C,D)). These results suggest that DOLPs induces growth inhibition and apoptosis through enhancing intracellular oxidative stress of cervical cancer Hela cells.
3.6. Effects of DOLPs on tumour growth in nude mice
Based on the in vitro findings of the anticancer potential of DOLPs through reactive oxygen species-dependent mitochondria pathway in cervical cancer Hela cells, and then, the tumour-bearing animal trial were carried out, we measured the in vivo effects of DOLPs on cervical tumour growth using Hela cervical cancer xenograft models. None of the doses had any detectable toxicity reaction, and there were no statistically significant effects on the behaviour or appearance of the mice (data showed in supplementary materials). In addition, there was not a significant alteration in body weight ((B,C)). However, we can see from (D), the Hela tumour volume significantly reduced in mice treated with 100 or 200 mg/kg DOLPs as compared with control. A significant reduction in the Hela tumour size on day 13 was observed in the group treated with DOLPs compared with the control. These trends persisted over time, and the reduction was greatest on day 21. On day 21, the mice were sacrificed and the tumours resected, compared with the control, the average tumour size was very significantly reduced by the DOLPs treatment ((E)), the Hela mice treated with DOLPs showed a 43% reduction in tumour size in the 100 mg/kg group and a 59% reduction in the 200 mg/kg group on day 21 compared with the control group (). These findings suggest that DOLPs could strongly inhibit the growth of cervical cancer Hela tumours by inducing apoptosis of tumour cells.
Figure 6. Effects of DOLPs on inhibition of Hela cervical cancer growth and induction of apoptosis. (A) Tumours at the end of the study; (B) Body weight of Hela tumour-bearing mice; (C) Tumour volume of Hela tumour-bearing mice; (D) Average body weight at the end of the study; (E) Average tumour sizes at the end of the study. * p < 0.05, **p < 0.01.
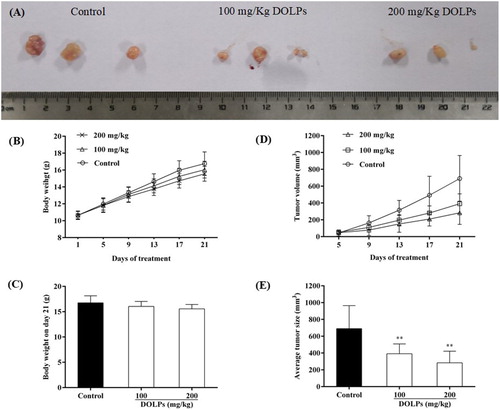
Table 2. Tumour inhibition rate of mice implanted with MDA-MB-231 breast cancer cells treated with DOLPs.
4. Conclusion and discussion
This study shows that the extracts of D. orientalis leaf polyphenols (DOLPs) has a good anti-cancer effect on human cervical cancer in vitro and in vivo. The inhibitory effect to cervical cancer cells in vitro showed that it could significantly anti-proliferation and inducing cancer cells apoptosis, it could been arrested the cancer cell cycle at S phase. In addition, the series changes of apoptosis-related proteins indicated that this apoptosis process could be involved in the intracellular mitochondrial apoptosis pathway. And what is more, this anti-cancer cells effect of DOLPs in vitro has also been confirmed in the tumour-bearing animal experiments in vivo. The anti-cancer effects of this extracts may be mainly due to the contribution of D. orientalis leaf polyphenols. So, we could conclude that DOLPs may be a promising polyphenolic fraction for the development of novel effective cervical cancer preventive or therapeutic agents.
Although human papillomavirus vaccines have been licensed in some areas, such as the USA, Europe, Canada, and Australia, the incidence of human papillomavirus infection-related cervical cancer remains high (Lowy & Schiller, Citation2006; Roden & Stern, Citation2018). This is because these vaccines are effective only against certain types of human papillomavirus, and they have not been promoted in developing countries (Roden & Stern, Citation2018). Although great progress have been made in conventional treatments such as surgery, chemotherapy and radiotherapy, the prognosis for survival from cervical cancer remains unsatisfactory (Rischpler et al., Citation2016). Besides, the high doses of chemotherapeutics required for treatments usually caused serious cytotoxic effects on the normal organs, tissues or cells of the patients (Xiao et al., Citation2018). The use of natural products to replace chemotherapeutic drugs with strong side effects has become increasingly popular in the prevention or treatment of cancer. For centuries, urticaceae plant has been used in traditional Chinese medicines. A related species, D. orientalis, is believed to hold medicinal properties as well (Almubayedh & Ahmad, Citation2018; Nisa et al., Citation2011). In this regard, DOLPs may have a high cervical carcinoma-inhibitory activity. In this study, we demonstrated that DOLPs exhibited obviously high cell growth inhibition, cell-cycle arrest, induction of apoptosis, ROS production, and mitochondrial membrane potential down regulation against human cervical cancer Hela cells in vitro, and did exert significant anticancer efficacy in nude mice bearing Hela tumours. These findings may help to the understanding of the strong positive relationship between total polyphenols content in DOLPs and antitumour activity, and makes it a promising polyphenolic fraction for the development of novel effective cancer preventive or therapeutic agents.
It is well known that cell proliferation and apoptosis are linked through cell-cycle regulators and apoptotic stimuli that affect both processes (Alenzi, Citation2004). Thus, induction of cell-cycle arrest has become a highly desirable regimen for the treatment of cancer (Li et al., Citation2011). In this study, the mechanisms of the DOLPs-induced inhibitory effect of Hela cells have been clarified by inducing S cell-cycle arrest, and consequently apoptosis in a dose-dependent manner. p53, the most frequently mutated tumour suppressor in human cancers, functions as a tumour suppressor by transcriptionally regulating numerous downstream target genes involved in cell cycle progression and cell death as a transcription factor. Whereas the induction of different gene products may mediate p53-dependent apoptosis or growth arrest, high levels of p53 expression alone are frequently insufficient to induce apoptosis, and other signals appear to be required (Atul & Tomoo, Citation2016). Thus, by knowing that p53 exerts its effects in maintaining genome stability through negatively regulate progression of the cell cycle in response to DNA damage or other cellular stressors (Reisman, Takahashi, Polson, & Boggs, Citation2012). Our results showed that the treatment of Hela cells with DOLPs resulted in a significant upregulation in the expression level of p53 protein and caused significantly increases of cells in the S phase after 24 h of exposure in comparison with untreated Hela cells, which indicate that DOLPs induce apoptosis in Hela cells by activates the p53 signalling pathway and mediates S-phase arrest. Chlorogenic acid has been shown to induce S-phase arrest in other cell lines, such as Human Colon Cancer Caco-2 Cells (Ekbatan, Li, Ghorbani, Azadi, & Kubow, Citation2018).
Selective induction of apoptosis is an appreciated trait of chemopreventive and chemotherapeutic regimens. The intrinsic mitochondrial apoptotic pathway is thought to be the major pathway for apoptosis, and thus, targeting the mitochondria is a promising strategy for the treatment of cancer (Li et al., Citation2016; Xu et al., Citation2009). Apoptosis mediated by mitochondria is highly regulated by the Bcl-2 family proteins, and the balance between the expression levels of proapoptotic (Bax and Bak) and antiapoptotic (Bcl-2 and Bcl-xL) proteins is critical for cell survival or cell death (Li et al., Citation2016; Xu et al., Citation2009). The present study found that DOLPs treatment could cause a significant increase in Bax protein expression and a decrease in Bcl-2 protein expression, indicating that the change in the ratio between proapoptotic and antiapoptotic Bcl-2 family proteins might largely contribute to the mitochondria-mediated apoptosis. Furthermore, the caspase-cascade system plays crucial roles in the process of intracellular apoptotic signals induction, transduction and amplification (Fan, Han, Cong, & Liang, Citation2005). The initiator caspase-9 is responsible for cleavage and activation of the executioner caspases within the apoptotic mitochondrial pathway, such as downstream caspase-3 (Fan et al., Citation2005; Hail, Carter, Konopleva, & Andreeff, Citation2006). In this study, we evaluated the activity of caspase-9 and its downstream cleavage caspase-3. The results clearly showed that DOLPs significantly increased the expression of caspase-9 and caspase-3, suggesting that DOLPs-induced apoptosis in human cervical carcinoma Hela cells involves the intrinsic mitochondrial pathway.
Mitochondria are both source and target of ROS during apoptosis, and the decrease of mitochondrial membrane potential leads to ROS generation and Cyt-c release, which furthermore leads to oxidative stress and apoptosis (Simon, Haj-Yehia, & Levi-Schaffer, Citation2000; Sreelatha, Jeyachitra, & Padma, Citation2011). ROS is thought to be a second messenger in series of signalling pathways and can also directly damage DNA (Davalli et al., Citation2018; Simon et al., Citation2000; Sreelatha et al., Citation2011), thereby activating the PARP enzyme system (Wang et al., Citation1997). To investigate whether the mitochondrial dysfunction and DNA damage in Hela cells treated with DOLPs was promoted by ROS production, we measured ROS levels using the cell permeable dye DCFH-DA, mitochondrial membrane potential using a rhodamine-123 fluorescent probe, and the release of Cyt-c and the expression level of PARP in Hela cells was analysed by Western-blot. As compared with the untreated control cells, the accumulation of intracellular ROS was significantly increased, the ΔΨm of Hela cells treated with DOLPs was significantly decreased, and the release of Cyt-c and the expression level of PARP was also significantly increased. These result indicated that the apoptotic effect of DOLPs on Hela cells was associated with an increased level of intracellular ROS and ROS production mediate apoptosis via the mitochondrial pathway. These findings are in consistent with the previous reports, showing that polyphenol extracts from pomegranate peel significantly inhibited cell proliferation, increased intracellular ROS generation and decrease mitochondrial membrane potential (Song et al., Citation2016). Although polyphenols are recognized as antioxidants (Scalbert, Johnson, & Saltmarsh, Citation2005), their antioxidant properties may not fully responsible for their chemopreventive functions (Sarwar et al., Citation2015; Scalbert et al., Citation2005). Polyphenols are thought to exhibit pro-oxidant properties in presence of transitional metal ions (Farhan et al., Citation2016; Yamashita et al., Citation1998). Therefore, the pro-oxidative properties of polyphenols can explain their anti-cancer properties, that is, polyphenol mediate chromatin-bound endogenous transitional metal ions to generate ROS in apoptosis processes of cancer cells (Farhan et al., Citation2016; Sarwar et al., Citation2015). Previous studies have reported that Catechin (C), epicatechin (EC), epigallocatechin (EGC) and epigallocatechin-3-gallate (EGCG) exert prooxidant actions in the presence of transition metals such as copper (Farhan et al., Citation2016). Thus, potential mechanisms for the anticancer properties of DOLPs were owing to the promotion of ROS, which in turn could result change in the ratio of Bax/Bcl-2 and activation of caspases, thereby mediate cell injury/death.
The above results show that DOLPs has an anti-cervical cancer effect in vitro. It is necessary to further evaluate its anticervical cancer effect in vivo. The in vivo anticancer efficacy of DOLPs extract was validated by our experiments in nude mice bearing Hela tumours. We found that DOLPs remarkably decreased the volume of Hela tumour xenografts in nude mice compared with the control group. These results suggest that DOLPs inhibits the growth of Hela tumour xenografts are consistent with our in vitro studies.
Our study is the first to evaluate the significant anticancer activity of the polyphenol-enriched extracts from DOLPs against human cervical cancer Hela cells. We demonstrate the effects of DOLPs in inhibiting the growth of Hela cancer cells in vitro and in vivo. The results suggest that DOLPs may be an effective ingredient for the treatment and/or management of cervical cancer. Our data also provide compelling evidence for further evaluation of DOLPs as a chemopreventive regimen for cervical cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alenzi, F. Q. (2004). Links between apoptosis, proliferation and the cell cycle. British Journal of Biomedical Science, 61, 99–102. doi: 10.1080/09674845.2004.11732652
- Almubayedh, H., & Ahmad, R. (2018). Ethnopharmacological uses, phytochemistry, biological activities of Debregeasia salicifolia: A review. Journal of Ethnopharmacology, 231, 179–186. doi: 10.1016/j.jep.2018.11.023
- Atul, R., & Tomoo, I. (2016). Non-canonical cell death induced by p53. International Journal of Molecular Sciences, 17(12), 2068. doi: 10.3390/ijms17122068
- Chaturvedi, A. K., Engels, E. A., Gilbert, E. S., Chen, B. E., Storm, H., Lynch, C. F., … Kaijser, M. (2007). Second cancers among 104,760 survivors of cervical cancer: Evaluation of long-term risk. Journal of the National Cancer Institute, 99, 1634–1643. doi: 10.1093/jnci/djm201
- Davalli, P., Marverti, G., Lauriola, A., & D’Arca, D. (2018). Targeting oxidatively induced DNA damage response in cancer: Opportunities for novel cancer therapies. Oxidative Medicine and Cellular Longevity, 1–21. doi: 10.1155/2018/2389523
- Ekbatan, S. S., Li, X.-Q., Ghorbani, M., Azadi, B., & Kubow, S. (2018). Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-phase cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. International Journal of Molecular Sciences, 19, 723. doi: 10.3390/ijms19030723
- Eom, S. H., Park, H. J., Dong, W. S., Kim, W. W., & Dong, H. C. (2009). Stimulating effects of far-infrared ray radiation on the release of antioxidative phenolics in grape berries. Food Science and Biotechnology, 18, 362–366.
- Fan, T.-J., Han, L.-H., Cong, R.-S., & Liang, J. (2005). Caspase family proteases and apoptosis. Acta Biochimica et Biophysica Sinica, 37, 719–727. doi: 10.1111/j.1745-7270.2005.00108.x
- Farhan, M., Khan, H. Y., Oves, M., Al-Harrasi, A., Rehmani, N., Arif, H., … Ahmad, A. (2016). Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins, 8, 37. doi: 10.3390/toxins8020037
- Goldstein, J., Munoz-Pinedo, C., Ricci, J., Adams, S., Kelekar, A., Schuler, M., … Green, D. R. (2005). Cytochrome c is released in a single step during apoptosis. Cell Death and Differentiation, 12, 453–462. doi: 10.1038/sj.cdd.4401596
- Gorin, S. N. S., Glenn, B. A., & Perkins, R. B. (2011). The human papillomavirus (HPV) vaccine and cervical cancer: Uptake and next steps. Advances in Therapy, 28, 615–639. doi: 10.1007/s12325-011-0045-x
- Hail, N., Carter, B., Konopleva, M., & Andreeff, M. (2006). Apoptosis effector mechanisms: A requiem performed in different keys. Apoptosis, 11, 889–904. doi: 10.1007/s10495-006-6712-8
- Holman, L. L., Ren, Y., & Westin, S. N. (2015). Status epilepticus associated with platinum chemotherapy in a patient with cervical cancer: A case report. BMC Cancer, 15, 728. doi: 10.1186/s12885-015-1755-2
- Hu, X., Schwarz, J. K., Lewis Jr., J. S., Huettner, P. C., Rader, J. S., Deasy, J. O., … & Wang, X. (2010). A microRNA expression signature for cervical cancer prognosis. Cancer Research, 70, 1441–1148. doi: 10.1158/0008-5472.CAN-09-3289
- Koyama, S., Cobb, L. J., Mehta, H. H., Seeram, N. P., Heber, D., Pantuck, A. J., & Cohen, P. (2010). Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Hormone & Igf Research, 20, 55–62. doi: 10.1016/j.ghir.2009.09.003
- Li, W., He, N., Tian, L., Shi, X., & Yang, X. (2016). Inhibitory effects of polyphenol-enriched extract from Ziyang tea against human breast cancer MCF-7 cells through reactive oxygen species-dependent mitochondria molecular mechanism. Journal of Food and Drug Analysis, 24, 527–538. doi: 10.1016/j.jfda.2016.01.005
- Li, L., Lu, N., Dai, Q., Wei, L., Zhao, Q., Li, Z., … Guo, Q. (2011). GL-V9, a newly synthetic flavonoid derivative, induces mitochondrial-mediated apoptosis and G2/M cell cycle arrest in human hepatocellular carcinoma HepG2 cells. European Journal of Pharmacology, 670, 13–21. doi: 10.1016/j.ejphar.2011.08.054
- Li, J. T., Zhang, J. L., He, H., Ma, Z. L., Nie, Z. K., Wang, Z. Z., & Xu, X. G. (2013). Apoptosis in human hepatoma HepG2 cells induced by corn peptides and its anti-tumor efficacy in H22 tumor bearing mice. Food and Chemical Toxicology, 51, 297–305. doi: 10.1016/j.fct.2012.09.038
- Liu, Y. (2013). Utility and exploitation of the plants within the family Urticaceae in Qinling Mountains. Value Engineering, 25, 261–262.
- Liu, R., Gu, Q. Q., & Han, B. (2004). Three phenolics-derived chemical constituents of Debregeasia longifolia (Burm.f.) Wedd. and their antitumor activity. Chinese Journal of Medicinal Chemistry, 14(14), 193–196.
- Liu, H., Xiao, Y., Xiong, C., Wei, A., & Ruan, J. (2011). Apoptosis induced by a new flavonoid in human hepatoma HepG2 cells involves reactive oxygen species-mediated mitochondrial dysfunction and MAPK activation. European Journal of Pharmacology, 654, 209–216. doi: 10.1016/j.ejphar.2010.12.036
- Lowy, D. R., & Schiller, J. T. (2006). Prophylactic human papillomavirus vaccines. Journal of Clinical Investigation, 116, 1167–1173. doi: 10.1172/JCI28607
- Marel, A. K., Lizard, G., Izard, J. C., Latruffe, N., & Delmas, D. (2008). Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Molecular Nutrition & Food Research, 52, 538–548. doi: 10.1002/mnfr.200700185
- Nisa, S., Bibi, Y., Waheed, A., Zia, M., Sarwar, S., Ahmed, S., & Chaudhary, M. F. (2011). Evaluation of anticancer activity of Debregeasia salicifolia extract against estrogen receptor positive cell line. African Journal of Biotechnology, 10, 990–995.
- Raza, H., John, A., & Benedict, S. (2011). Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. European Journal of Pharmacology, 668, 15–24. doi: 10.1016/j.ejphar.2011.06.016
- Reisman, D., Takahashi, P., Polson, A., & Boggs, K. (2012). Transcriptional regulation of the p53 tumor suppressor gene in S-phase of the cell-cycle and the cellular response to DNA damage. Biochemistry Research International, 808934. doi: 10.1155/2012/808934
- Rischpler, C., Okamoto, S., Meyer, P., Schwaiger, M., Maurer, T., & Eiber, M. (2016). 68Ga-PSMA-HBED-CC ligand uptake in cervical, coeliac and sacral ganglia as an important pitfall in prostate cancer PET imaging. Journal of Nuclear Medicine, 57, 517–517. doi: 10.2967/jnumed.115.165050
- Roden, R. B. S., & Stern, P. L. (2018). Opportunities and challenges for human papillomavirus vaccination in cancer. Nature Reviews Cancer, 18, 240–254. doi: 10.1038/nrc.2018.13
- Sarwar, T., Zafaryab, M., Husain, M. A., Ishqi, H. M., Rehman, S. U., Rizvi, M. M. A., & Tabish, M. (2015). Redox cycling of endogenous copper by ferulic acid leads to cellular DNA breakage and consequent cell death: A putative cancer chemotherapy mechanism. Toxicology and Applied Pharmacology, 289, 251–261. doi: 10.1016/j.taap.2015.09.018
- Scalbert, A., Johnson, I. T., & Saltmarsh, M. (2005). Polyphenols: Antioxidants and beyond. The American Journal of Clinical Nutrition, 81, 215S–217S. doi: 10.1093/ajcn/81.1.215S
- Shimada, M., Sato, S., Oishi, T., Itamochi, H., Kigawa, J., Takeshima, N., … Ochiai, K. (2016). Feasibility study on combination chemotherapy using nogitecan hydrochloride (topotecan) and cisplatin for patients with metastatic, persistent, or recurrent uterine cervical cancer. International Journal of Clinical Oncology, 21, 969–974. doi: 10.1007/s10147-016-0984-y
- Simon, H.-U., Haj-Yehia, A., & Levi-Schaffer, F. (2000). Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis, 5, 415–418. doi: 10.1023/A:1009616228304
- Song, B., Li, J., & Li, J. (2016). Pomegranate peel extract polyphenols induced apoptosis in human hepatoma cells by mitochondrial pathway. Food and Chemical Toxicology, 93, 158–166. doi: 10.1016/j.fct.2016.04.020
- Sreelatha, S., Jeyachitra, A., & Padma, P. (2011). Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food and Chemical Toxicology, 49, 1270–1275. doi: 10.1016/j.fct.2011.03.006
- Tian, H., Liu, L., Yang, T. J., & Wang, Y. Q. (2016). Gentiopicroside inhibits cancer cell growth in OVCAR-3 ovary cancer cells through the mediation of apoptosis, loss of mitochondrial transmembrane potential and NF-kB signalling pathway. Biomedical Research, 27(2), 413–418.
- Wang, J. (2011). Research status and development value of plants in Debregeasia Gaudich. Chinese Journal of Ethnomedicine and Ethnopharmacy, 20(12), 48–49.
- Wang, Z. Q., Stingl, L., Morrison, C., Jantsch, M., Los, M., Schulze-Osthoff, K., & Wagner, E. F. (1997). PARP is important for genomic stability but dispensable in apoptosis. Genes & Development, 11, 2347–2358. doi: 10.1101/gad.11.18.2347
- Xiao, L., Ma, N., He, H., Li, J., Cheng, S., Yang, Q., … Su, X. (2018). Development of a novel drug targeting delivery system for cervical cancer therapy. Nanotechnology, 30, 075604. doi: 10.1088/1361-6528/aaf3f8
- Xu, Y., Ge, R., Du, J., Xin, H., Yi, T., Sheng, J., … Ling, C. (2009). Corosolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Letters, 284, 229–237. doi: 10.1016/j.canlet.2009.04.028
- Yamashita, N., Murata, M., Inoue, S., Burkitt, M. J., Milne, L., & Kawanishi, S. (1998). α-Tocopherol induces oxidative damage to DNA in the presence of copper (II) ions. Chemical Research in Toxicology, 11, 855–862. doi: 10.1021/tx970129v
- Zhang, Y., Liang, Y., & He, C. (2017). Anticancer activities and mechanisms of heat-clearing and detoxicating traditional Chinese herbal medicine. Chinese Medicine, 12, 20. doi: 10.1186/s13020-017-0140-2
- Zhou, D., Wei, A., Cao, C., & Ruan, J. (2013). DICO, a novel nonaromatic B-ring flavonoid, induces G2/M cell cycle arrest and apoptosis in human hepatoma cells. Food and Chemical Toxicology, 57, 322–329. doi: 10.1016/j.fct.2013.03.032
- Zhu, X., Wang, J., Yang, O., Han, W., & Li, H. (2013). Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells. European Journal of Medical Research, 18, 46. doi: 10.1186/2047-783X-18-46