?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The medicinal properties and toxicity of aconitine in traditional Chinese medicine have caused widespread concern. In this study, we screened a highly specific and sensitive monoclonal antibody with an IC50 of 3.65 ng/mL generated by immunizing mice and cell fusion, and the limits of detection of the ic-ELISA were 1.13 and 11.76 ng/mL. We then developed a colloidal gold test strip based on the antibody. For rapid detection of aconitine in herbs, the visual limit and detection limit of the test strip are 10 and 25 ng/mL, respectively; the results are obtained within 5 min and are visible to the naked eye. Therefore, the colloidal gold test strip is very suitable for on-site detection and quality screening of aconitine in Chinese herbal medicine.
Introduction
Aconitine is a component of plants of the genus Aconitum, which contains approximately 400 species distributed around the world, including 211 in China (Ma et al., Citation2015). Aconitine is mainly found in plants such as Chuanwu, Caowu and Fuzi (Zhou et al., Citation2019). On the one hand, these herbs have been used for the treatment of rheumatic fever, knee pain, chronic diarrhea, cough, edema, bronchial asthma, herpes zoster, and some endocrinal disorders for over 2000 years (Xiaodong Li, Gu, Yang, Zhang, & Shen, Citation2017; Tarbe et al., Citation2017; Yang et al., Citation2019; Zhao et al., Citation2018). In China, some herbs containing aconitine are often used in pharmaceutical wines. In addition, because of its short half-life and easy decomposition, aconitine can also be used as a pesticide (Liu et al., Citation2019; Wei, Zhang, Li, Ji, & Shao, Citation2019). On the other hand, aconitine is also a lethal toxin (Lou et al., Citation2018), which can cause arrhythmia, dizziness, numbness, hypotension and even death (Gao et al., Citation2017; Yang et al., Citation2019; Zhang, Cai, Zhang, Qi, & Lu, Citation2018), and the treatment for aconitine poisoning is still limited to supportive treatments such as gastric lavage, charcoal perfusion, injection of magnesium sulfate, etc. (Clara, Rauch, Überbacher, Felgenhauer, & Drüge, Citation2015; Zong et al., Citation2019). If a new treatment based on aconitine-containing Chinese medicine is developed (Xiaodong Li et al., Citation2017; Xiumei Li et al., Citation2018), the content of aconitine in Chinese medicine must be strictly tested to avoid patient poisoning.
At present, reports on the detection of aconitine mainly rely on instrumental methods, such as HPLC, HPLC-UV, CE, GC-MS and LC-MS-MS (Jablonski, Schlesser, & Mariappagoudar, Citation2006; Wada, Bando, Kawahara, Mori, & Murayama, Citation1994; Wang, Yi, & Zhang, Citation1987; Yin, Guo, Du, & Wang, Citation2006). However, these methods require large instruments, which are extremely expensive, and the sample preparation and analysis is time consuming. We established a rapid, sensitive method for the detection of aconitine, which is based on a highly specific monoclonal antibody against aconitine, and designed a test strip for visual detection of aconitine, which is inexpensive, provides rapid detection, and does not require any large instruments.
Materials and methods
Chemicals and instruments
Aconitine, ovalbumin (OVA), keyhole limpet hemocyanin (KLH), enzyme immunoassay-grade horseradish peroxidase-labeled goat anti-mouse immunoglobulin, 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide (EDC), 1-hydroxypyrrolidine-2,5-dione (NHS), succinic anhydride, and horseradish peroxidase were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents and chemicals used were acquired from the National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China). Nitrocellulose (NC) high-flow-plus membrane (Pura-bind RP) was obtained from Whatman-Xinhua Filter Paper Co. (Hangzhou, China). Sample pad (glass fibre membrane, GL-b01), polyvinylchloride (PVC) backing card, and absorption pad (SX18) were supplied by GoldBio Tech Co. (Shanghai, China).
The instruments used in this study included a UV–Vis scanner (Bokin instruments, Tsushima, Japan), a vortex machine (Shanghai Huxi Analysis instrument Factory Co., Ltd. Shanghai, China), and a Multiskan MKS microplate reader (Thermo LabSystems Inc. Beijing, China). A Bio Dot TSR3000 Membrane Strip Reader was acquired from Gene Co., Ltd. Shanghai Branch (Shanghai, China).
The buffer solutions used in this study were prepared with ultrapure water (Milli-Q purification system, Millipore Co., Bedford, MA, USA).
Antigen preparation
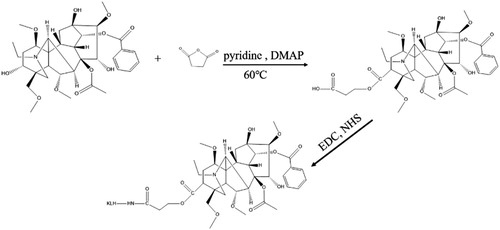
Aconitine (ACO) is a small molecule containing hydroxyl groups, thus to obtain mAb against ACO, it should be conjugated to the carrier proteins BSA/OVA via the hydroxyl group. However, the success rate of hydroxyl coupling to proteins is low, so we used the active ester method to convert the hydroxyl group into a carboxyl group, and used the carboxyl group to couple the ACO to protein (Xu et al., Citation2010), as shown in . Briefly, ACO, succinic anhydride and 4-dimethylaminopyridine (DMAP) were dissolved in pyridine at a concentration of 20 mg/mL. ACO (500 μL), succinic anhydride (1.94 mL), and DMAP (3.94 mL) were allowed to react at 60°C for 12 h under stirring. Then ice water was added to the mixture to stop the reaction, and the mixture was dried under nitrogen. Next, water and chloroform were added to extract the mixture, the extraction was repeated three times, and the combined extracts were dried. To prepare ACO-EDC-KLH, the mixture was dissolved in DMF at 2 mg/mL, and EDC (0.64 mg), NHS (0.38 mg), and ACO solution (360 μL) were stirred at room temperature for 6 h, then the mixture was added dropwise to 5 mg KLH and reacted for 12 h at room temperature. Subsequently, the mixture was dialyzed against 0.01 M PBS for 3 days. Finally, we obtained the immunogen ACO-EDC-KLH, and synthesized the antigens ACO-EDC-OVA and ACO-EDC-BSA by the same method.
All the antigens produced were characterized by gel electrophoresis, and stored at −20°C.
MAb preparation
The immunization process in mice was the standard method carried out in our laboratory (Kong, Liu, Song, Kuang, & Xu, Citation2017; Li, Liu, Kuang, & Xu, Citation2019). Briefly, the ACO-EDC-KLH antigen was used as the immunogen in healthy female BALB/c mice (6–8 weeks old). The mice were immunized by subcutaneous multipoint injection, using 100 μL per mouse. For the first immunization, Freund’s complete adjuvant was used, and for booster immunizations, Freund’s incomplete adjuvant was used. Each mouse received 100 μg of ACO-EDC-KLH for the first immunization and then 50 μg of ACO-EDC-KLH at intervals of three weeks. Blood samples were collected one week apart to analyze the serum titre and inhibition. Ic-ELISA was used to screen mice and the mouse with the highest serum antibody titre and the best inhibition was selected, and after sacrifice its spleen was used for cell fusion.
Cell fusion
The cell fusion process was as described previously (Lei et al., Citation2018; Wang et al., Citation2019). Three days after the final immunization, the mouse spleen cells and myeloma cells were fused by the PEG 4000 method, then the hybridoma cells were selected using selective medium (HAT medium), and cell culture was carried out using HT medium. After one week of fusion, the wells containing positive cell were detected by ic-ELISA, and the inhibitory effect of the positive cell wells was further determined by ic-ELISA. The well-inhibited positive cell wells were subcloned by the limiting dilution method, and then detected and picked again after one week. After three rounds of subcloning, a monoclonal hybridoma cell line 5D1 capable of secreting ACO-specific antibodies was obtained. The ic-ELISA were performed as previously described (Chen et al., Citation2019).
Specificity of the mAb
Monoclonal antibody specificity was assessed by measuring cross-reactivity (CR) with Indaconitine, Lappaconitine, Aconitumtalassicum, Bulleyaconitine A, and Aconitine 3-acetate, and analyzed using ic-ELISA. The CR value was calculated using the following formula (Berlina, Zherdev, Xu, Eremin, & Dzantiev, Citation2017; Isanga et al., Citation2017):
Preparation of colloidal gold nanoparticles
We prepared colloidal gold particles for antibody labelling. Briefly, 25 mL of 0.1 g/L chloroauric acid was heated to boiling under stirring, and 1% (w/v) trisodium citrate (1 mL) was added, stirred for 10 min until the colour of the solution turned to wine red, then cooled to room temperature under constant stirring, and stored at 4°C. Transmission electron microscopy (TEM) examinations revealed that the gold nanoparticles had a uniform particle size of 20 nm. All solvents were prepared in ultrapure water and filtered through a transfer membrane.
Preparation of gold nanoparticle-labeled mAb
The ACO mAb was labelled with gold nanoparticles in our laboratory according to the method previously described (Lu, Wei, Liu, Song, & Hua, Citation2018; Yue, Liu, Song, & Hua, Citation2017). Briefly, the colloidal gold solution was adjusted to pH 7 with 0.1 M K2CO3, and 10 μg of the ACO mAb was added to 1 mL colloidal gold solution. The mixture was incubated for 30 min at room temperature, then 50 μL of 10% (w/v) BSA was added and mixed at room temperature for 2 h. Finally, the product was centrifuged for 45 min at 7000 rpm, and the precipitate was washed three times with 0.02 M PBS (pH 7.4) containing 5% sucrose, 1% BSA, and 0.5% PEG 6000. The conjugated product was dissolved in 5 mL of 0.02 M PBS containing 0.02% NaN3, 0.1% PEG, and 0.1% Tween, then stored at 4°C until further use.
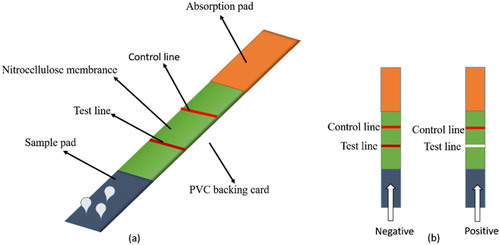
Preparation of the lateral-flow ICA test strip
The detection antigen and goat anti-mouse IgG were sprayed onto the NC membrane to create the test line and quality control line, respectively. The films were then dried at 37°C for 12 h. The sample pad, NC membrane and absorption pad were assembled on the PVC backing card as shown in (a). When the sample did not contain ACO, the test line appeared red. When the ACO content in the sample was greater than 25 ng/mL, the test line appeared colourless ((b)). The control line should always show red regardless of the presence of ACO in the sample.
Detection of ACO in samples
Aconitine is mainly found in Aconitum plants of the Ranunculaceae family. We chose to use Chuan Mutong, which belongs to the Ranunculaceae family but is a member of the genus Clematis, as a negative sample (Gao et al., Citation2016). First, the Chinese herbal medicine Chuan Mutong was crushed, and eight samples of 2 g of powder were weighed into centrifuge tubes, then ACO standards were added to these centrifuge tubes at 0.01, 0.025, 0.05, 0.1, 0.25, and 0.5 μg/g. The sample without ACO was used as the negative control. The extraction process was as follows: 5 mL of 1 M HCl was added to the centrifuge tube, sonicated for 15 min, centrifuged at 8000 rpm for 10 min, then the supernatant was removed and extraction was repeated once again, and the supernatants were combined and mixed. An aliquot of 1 mL solution was removed, the pH was adjusted to 7 with 2 M NaOH, and diluted to 2 mL.
Taken an appropriate amount of Fuzi Lizhong Pills, broken to powder, and weighed 3 g sample into centrifuge tubes, added 2 mL ammonium hydroxide, mixed well, let it stood for 2 h, then added 10 mL ether, shaken for 1 h, taken the supernatant, repeated the extraction twice, combine the supernatants to dry, reconstituted 3 ml of methanol, and passed through 0.22 μm filter membrane, and measured on the HPLC-QTOF and test strip.
Results and discussion
Characterization of antigen and mAb
We derivatized the hydroxyl group of ACO into a carboxyl group by the reactive ester method, and coupled it to a carrier protein through the carboxyl group.
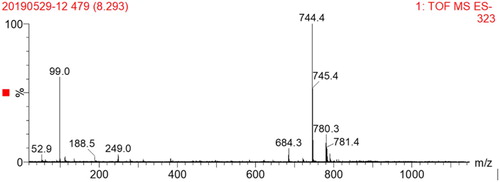
The hapten was identified by UPLC-TOF (), and the molecular weight of ACO was 645.74 g/mol; when the hydroxyl group was derivatized to a carboxyl group, the molecular weight became 745.82 g/mol. This demonstrated that the hapten ACO-COOH was successfully synthesized.
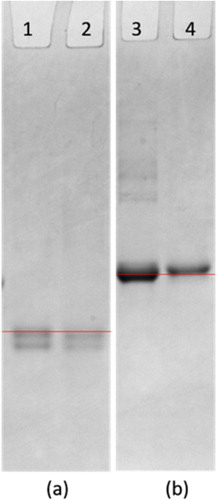
We characterized the antigens ACO-EDC-OVA and ACO-EDC-BSA by gel electrophoresis. As shown in , the band of ACO-EDC-OVA obviously lagged behind the band of OVA, while the band of ACO-EDC-BSA obviously lagged behind the band of BSA. These results confirmed the successful coupling of the coating antigens. The molecular weight of KLH is too large to be identified by gel electrophoresis, but since we used the same coupling method as for the coating antigens, we believe that it was also coupled successfully.
Mab characterization
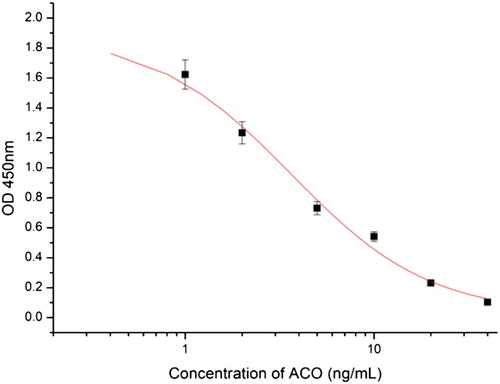
After cell fusion and screening, we selected mAb 5D1, and we used ic-ELISA to assess the IC50, detection limit (LOD) and CR values of mAb 5D1. We optimized the content of NaCl, PH, coating time, and blocking time, and obtained the best conditions were NaCl 0.8%, pH 7.5, coating time 2 h, and blocking time 2.5 h, on this basis the standard curve (R2 = 0.987; ) shows the IC50 and linear range as 3.65 ng/mL and 1.13–11.76 ng/mL, respectively. shows the CR values of mAb 5D1 with its structural analogs, confirming that this antibody has good specificity.
Table 1. The CR value of mAb against ACO by the ic-ELISA method.
Optimization of the test strip
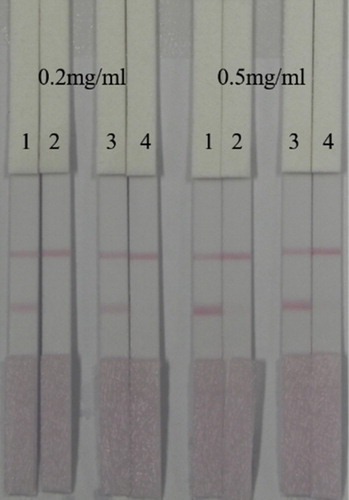
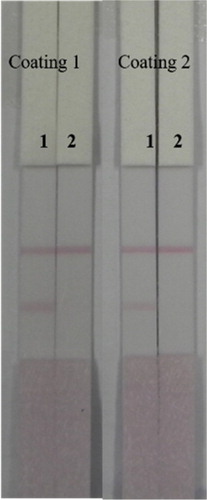
Two coating antigens (coating 1: ACO-EDC-BSA; coating 2: ACO-EDC-OVA) were evaluated. The coating concentration, as shown in , was 0.2 mg/mL, and 0 and 50 ng/mL ACO standard was added to Pad 1 and Pad 2, respectively. Since the T line of coating 2 was too faint, we chose to use coating 1 for subsequent experiments.
Figure 6. The optimization of ACO lateral-flow ICA strip (Coating 1: ACO-EDC-BSA, Coating 2: ACO-EDC-OVA). Strip 1: ACO negative sample (0 ng/mL); Strip 2: ACO positive sample (50 ng/mL).
In addition, we also optimized the amount and concentration of gold-labeled antibody. The amount of gold-labeled antibody we used was 1 mL of gold nanoparticles with 4 µL K2CO3 and 8 or 10 µg/mL antibody. Coating concentration was 0.2 or 0.5 mg/mL. The amount of gold-labeled antibody used for Pad 1 and Pad 2 was 8 µg/mL, while for Pad 3 and Pad 4 it was 10 µg/mL. As shown in , to Pad 1 and Pad 3 we added 0 ng/mL ACO standard, while to Pad 2 and Pad 4 we added 25 ng/mL ACO standard. When the amount of gold-labeled antibody was 8 µg/mL, the T-line was clearer, so we chose to use 1 mL of gold nanoparticles with 4 µL K2CO3 and 8 µg/mL antibody. When the coating antigen concentration was 0.5 mg/mL, the colour of the T line was too dark, which affected the sensitivity, so we chose to use a coating concentration of 0.2 mg/mL for the sample test experiment.
Sensitivity of the immunochromatographic strip
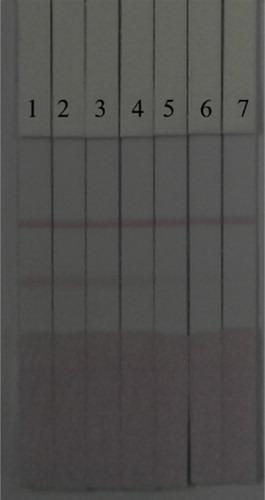
Under optimal conditions, the sensitivity of the immunochromatographic strip was evaluated by diluting different concentrations of ACO standard with 0.01 M PBS (). As the ACO concentration increased, the colour of the T line became weaker. The lowest detectable line visible to the naked eye was 5 ng/mL. When the concentration was 10 ng/mL, the T line was colourless.
Detection of ACO in herbal preparations
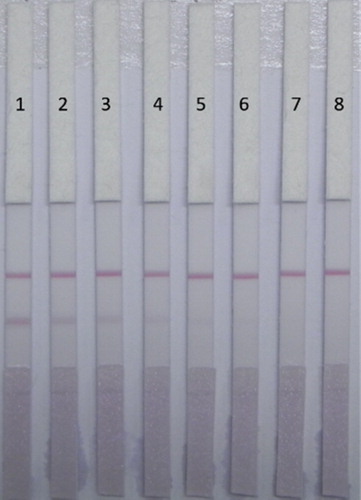
Under optimal conditions, seven samples of Chuan Mutong were tested for ACO using ICA test strips, and samples without ACO were used as negative controls to observe the colour on the T line (). As the ACO concentration increased, the colour of the T-line became lighter, until when the concentration of ACO was 25 ng/mL, the T line disappeared. The visual LOD and cutoff values of the test strip were 10 and 25 ng/mL, respectively. These results indicated that the antibody strip based on antibody 5D1 could be used for the detection of ACO in Chinese herbal medicine. Because the ICA test strip we developed was sensitive, accurate, fast, and very easy to use, the test strip is ideal for on-site screening of samples, and is especially suitable for the detection of ACO in a large number of samples.
Figure 9. ACO detection by lateral-flow ICA strip in Chuan Mutong samples (n = 7). Pad 1 = 0 ng/mL, Pad 2 = 1 ng/mL, Pad 3 = 2.5 ng/mL, Pad 4 = 5 ng/mL, Pad 5 = 10 ng/mL, Pad 6 = 25 ng/mL, and Pad 7 = 50 ng/mL.

The content of aconitine in Fuzi lizhong pills was determined by HPLC-QTOF to be 0.54, 0.52, 0.51 µg/g, the average value was 0.523 µg/g. The extract was diluted to 50, 100, 150, 200, 250, 300, and 400 ng/mL by PBS. We tested these diluted samples with ICA test strip, the test results were shown in . In the pad 6, the test line completely disappeared, and the test line in pad 3 started to lighten compared to the colour in pad 1. So the lowest detection line in the Fuzi lizhong pills was100 ng/mL, and the visual LOD and cutoff values of the test strip were 100 and 250 ng/mL, respectively. We found the sensitivity was worse compared to the added sample, because the matrix interference of the proprietary Chinese medicine was greater than that of a single traditional Chinese medicine.
Conclusions
In this study, the highly specific and sensitive monoclonal antibody 5D1 was screened by ic-ELISA and found to have an IC50 of 3.65 ng/mL. Based on this antibody, a method for detecting ACO using an ICA test strip was established. The sensitivity of the test strip was 5 ng/mL by the naked eye. In herbal samples, the cutoff value of the ICA strip was 10 ng/mL, and in Fuzi lizhong pills, the cutoff value was 100 ng/mL. This test strip is effective for screening simple and complex samples. Our results show that the mAb-based test strips we developed are effective for on-site testing of herbal medicines and screening of large numbers of samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Berlina, A. N., Zherdev, A. V., Xu, C., Eremin, S. A., & Dzantiev, B. B. (2017). Development of lateral flow immunoassay for rapid control and quantification of the presence of the colorant Sudan I in spices and seafood. Food Control, 73, 247–253. doi: 10.1016/j.foodcont.2016.08.011
- Chen, Y., Liu, L., Cui, G., Wu, X., Kuang, H., & Xu, C. (2019). Development of an immunochromatographic strip for the detection of rosiglitazone in functional foods based on monoclonal antibodies. Analytical Methods, 11(38), 4910–4916. doi: 10.1039/C9AY01534E
- Clara, A., Rauch, S., Überbacher, C. A., Felgenhauer, N., & Drüge, G. (2015). High-dose magnesium sulfate in the treatment of aconite poisoning. Anaesthesist, 64(5), 381–384. doi: 10.1007/s00101-015-0013-y
- Gao, S. S., Li, J. R., Wu, F. B., Wei, X. M., Bian, J. H., Chen, J. B., Cheng, S. Q., Sun, S. Q., & Lv, G. H. (2016). [Development of FTIR fingerprint for identification of armand clematis stem (Chuanmutong) and related herbs]. China Journal of Chinese Materia Medica, 153(3), 737–743.
- Gao, X., Zhang, X., Hu, J., Xu, X., Zuo, Y., Wang, Y., Ding, J., Xu, H., & Zhu, S. (2017). Aconitine induces apoptosis in H9c2 cardiac cells via mitochondria-mediated pathway. Molecular Medicine Reports, 17(1), 284–292.
- Isanga, J., Mukunzi, D., Chen, Y., Suryoprabowo, S., Liu, L., Hua, K., & Xu, C. (2017). Development of a monoclonal antibody assay and a lateral flow strip test for the detection of paromomycin residues in food matrices. Food & Agricultural Immunology, 28(3), 355–373. doi: 10.1080/09540105.2016.1272551
- Jablonski, J. E., Schlesser, J. E., & Mariappagoudar, P. (2006). HPLC-UV Method for Nicotine, Strychnine, and aconitine in Dairy Products. Journal of Agricultural and Food Chemistry, 54(20), 7460–7465. doi: 10.1021/jf061115a
- Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of sensitive, rapid, and effective immunoassays for the detection of vitamin B12 in fortified food and nutritional supplements. Food Analytical Methods, 10(1), 10–18. doi: 10.1007/s12161-016-0543-1
- Lei, X., Song, S., Hong, T., Liu, L., Zheng, Q., Xu, C., & Hua, K. (2018). Development of indirect competitive enzyme-linked immunosorbent and immunochromatographic strip assays for tiamulin detection in chicken. ACS Omega, 3(3), 3581–3586. doi: 10.1021/acsomega.8b00289
- Li, X., Gu, L., Yang, L., Zhang, D., & Shen, J. (2017). Aconitine: A potential novel treatment for systemic lupus erythematosus. Journal of Pharmacological Sciences, 133(3), 115–121. doi: 10.1016/j.jphs.2017.01.007
- Li, X., Liu, J., Pan, F., Shi, D., Wen, Z., & Yang, P. (2018). Quercetin and aconitine synergistically induces the human cervical carcinoma HeLa cell apoptosis via endoplasmic reticulum (ER) stress pathway. PLOS ONE, 13(1), e0191062. doi: 10.1371/journal.pone.0191062
- Li, Y., Liu, L., Kuang, H., & Xu, C. (2019). Development of a lateral flow immunoassay for the simultaneous detection of four dipyrone metabolites in milk. Analytical Methods, 11(23), 3041–3052. doi: 10.1039/C9AY00774A
- Liu, Y., Zhao, W. W., Ding, K. N., Jin, Z. J. Y., Li, Z. X., & Li, F. Z. (2019). Synthesis of “lotus root”-like mesoporous titanium dioxide and its effects on UV response to aconitine release. Journal of Alloys and Compounds, 777, 285–293. doi: 10.1016/j.jallcom.2018.10.388
- Lou, J., Wu, H., Wang, L., Zhao, L., Li, X., Kang, Y., Wen, K., & Yin, Y. (2018). Taurine-magnesium coordination compound, a potential anti-arrhythmic complex, improves aconitine-induced arrhythmias through regulation of multiple ion channels. Toxicology and Applied Pharmacology, 356, 182–190. doi: 10.1016/j.taap.2018.08.008
- Lu, Z., Wei, J., Liu, L., Song, S., & Hua, K. (2018). Development of ic-ELISA and lateral-flow immunochromatographic strip for detection of vitamin B2 in an energy drink and vitamin tablets. Food & Agricultural Immunology, 28(1), 121–132.
- Ma, L., Gu, R., Tang, L., Chen, Z. E., Di, R., & Long, C. (2015). Important poisonous plants in tibetan ethnomedicine. Toxins, 7(1), 138–155. doi: 10.3390/toxins7010138
- Tarbe, M., De Pomyers, H., Mugnier, L., Bertin, D., Ibragimov, T., Gigmes, D., & Mabrouk, K. (2017). Gram-scale purification of aconitine and identification of lappaconitine in Aconitum karacolicum. Fitoterapia, 120, 85–92. doi: 10.1016/j.fitote.2017.05.008
- Wada, K., Bando, H., Kawahara, N., Mori, T., & Murayama, M. (1994). Determination and quantitative analysis of alkaloids in Aconitum japonicum by liquid chromatography atmospheric pressure chemical ionization mass spectrometry. Biological Mass Spectrometry, 23(2), 97–102. doi: 10.1002/bms.1200230209
- Wang, Z. H., He, Y., Zhang, J. Z., Hu, C. H., & Guo, D. A. (1987). Detection of aconitine in biological samples by GC/MS. Journal of Forensic Medicine, 3, 25–30.
- Wang, Z., Wu, X., Liu, L., Xu, L., Kuang, H., & Xu, C. (2019). An immunochromatographic strip sensor for sildenafil and its analogues. Journal of Materials Chemistry B, 7(14), 6383–6389. doi: 10.1039/C9TB00280D
- Wei, S., Zhang, H., Li, B., Ji, J., & Shao, X. (2019). Insecticidal effect of aconitine on the rice brown planthoppers. PLOS ONE, 14(8), e0221090. doi: 10.1371/journal.pone.0221090
- Xu, Y., Huang, Z. B., He, Q. H., Deng, S. Z., Li, L. S., & Li, Y. P. (2010). Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chemistry, 119(2), 834–839. doi: 10.1016/j.foodchem.2009.08.049
- Yang, H., Wang, H., Liu, Y., Yang, L., Sun, L., Tian, Y., Zhao, B., & Lu, H. (2019). The PI3K/Akt/mTOR signaling pathway plays a role in regulating aconitine-induced autophagy in mouse liver. Research in Veterinary Science, 124, 317–320. doi: 10.1016/j.rvsc.2019.04.016
- Yin, J., Guo, W., Du, Y., & Wang, E. (2006). Facile separation and determination of aconitine alkaloids in traditional Chinese medicines by CE with tris(2,2′-bipyridyl) ruthenium(II)-based electrochemiluminescence detection. Electrophoresis, 27(23), 4836–4841. doi: 10.1002/elps.200600288
- Yue, L., Liu, L., Song, S., & Hua, K. (2017). Development of a gold nanoparticle immunochromatographic assay for the on-site analysis of 6-benzylaminopurine residues in bean sprouts. Food & Agricultural Immunology, 29(1), 14–26.
- Zhang, F., Cai, L., Zhang, J., Qi, X., & Lu, C. (2018). Aconitine-induced cardiac arrhythmia in human induced pluripotent stem cell-derived cardiomyocytes. Experimental and Therapeutic Medicine, 16(4), 3497–3503.
- Zhao, D., Yong, S., Shi, Y., Shi, X., Qin, Q., Zi, S., Zhao, E., Yu, D., & Kennelly, E. J. (2018). Probing the transcriptome of Aconitum carmichaelii reveals the candidate genes associated with the biosynthesis of the toxic aconitine-type C 19 -diterpenoid alkaloids. Phytochemistry, 152, 113–124. doi: 10.1016/j.phytochem.2018.04.022
- Zhou, R., Dai, G., Zhou, X., Zhang, M., Wu, P., Zhang, D., Song, H., Liu, X., & Qin, Y. (2019). Progress towards the synthesis of aconitine: Construction of the AE fragment and attempts to access the pentacyclic core. Organic Chemistry Frontiers, 6(3), 377–382. doi: 10.1039/C8QO01228H
- Zong, X., Yan, X., Wu, J., Liu, Z., Zhou, H., Li, N., & Liu, L. (2019). Potentially cardiotoxic diterpenoid alkaloids from the roots of aconitum carmichaelii. Journal of Natural Products, 82(4), 980–989. doi: 10.1021/acs.jnatprod.8b01039