ABSTRACT
The 2019 new coronavirus epidemic potentially induced by wild animals has drawn tremendous attention. Wild animal meat contamination and adulteration have become increasingly serious, particularly for highly cooked wild animal meats that are difficult to be detected. In this study, a highly specific polyclonal antibody targeting the cooked rat proteins was developed. The corresponding sandwich ELISA (swELISA) was developed and found highly sensitive and specific for cooked rat meat, while there are no cross-reactions to the cooked chicken, pork and beef meats. The limit of detection (LOD) is determined to be as low as 0.01 ug/L based OD values. The coefficient variation (CV) is 5% and 8% for intra and inter assays, respectively. The recovery efficiencies are between 90% and 110%. The sandwich ELISA can detect both raw and cooked rat meat and is also suitable for Swab test of rat contamination. The results indicated a highly reliable and robust ELISA-based assay for cooked rat meat identification and contamination.
Introduction
The 2019 new coronavirus (2019-ncoV or COVID-19) epidemic started in Wuhan’s seafood market in China and has rapidly spread and caused tragic problems to the society and people’s life (Carlos, Dela Cruz, Cao, Pasnick, & Jamil, Citation2020). It is generally considered that the 2019-ncoV is associated with wild animal consumption (Malik et al., Citation2020); however, clues are still under investigation. The wild animals that potentially carry dangerous viruses are rat, pangolin, bat, gorilla, hyena and bald eagle. For example, it is found that the viral DNA sequence from pangolin has 96% identities as the 2019-ncoV (COVID-19) DNA (Ji, Wang, Zhao, Zai, & Li, Citation2020; Lu et al., Citation2020). However, people often consumed these wild animals even before epidemic incidents.
In recent years, the problem of wild animal contamination and adulteration has become increasingly serious, particularly in developing countries. There have been reported that some pangolins, foxes, otters and rat meat were processed and sold by unscrupulous traders. Bat and pangolin became usual diets in many Asian restaurants. It is well known that these wild animals are the potential intermediate hosts of many dangerous viruses (Benvenuto et al., Citation2020), and the viral contents in their bodies are much higher than those in poultry and livestock. Many viruses are found not only in their saliva, viscera and blood but also in their fur and faeces (Akimkin et al., Citation2016). Consumption of wild animals must be forbidden.
The pathogens and other dangerous substances in rats are highly contagious and harmful. People who happen to eat the meat of the rat potentially suffer from serious diseases. For example, plague is a severe infectious disease caused by Yersinia pestis in rats (Bobrov et al., Citation2015). It is an international infectious disease, which requires quarantine, and also a class A infectious disease in China, ranking first in 39 kinds of legal infectious diseases (Sebbane, Gardner, Long, Gowen, & Hinnebusch, Citation2005). Rat meat gradually walked into the daily table due to its high protein content and cheap resources. However, many consumers are unaware of it.
In response to the adulteration of wild animal meat products, many qualitative and quantitative detection technologies have been established, for example, the protein-based immunology (Lei, Xu, Song, Liu, & Kuang, Citation2018; Lin et al., Citation2018), the non-destructive near-infrared spectroscopy (Kartakoullis, Comaposada, Cruz-Carrion, Serra, & Gou, Citation2019; Pieszczek, Czarnik-Matusewicz, & Daszykowski, Citation2018), electronic nose and electronic tongue (Mabuchi, Ishimaru, Tanaka, Kawaguchi, & Tanimoto, Citation2018) and nucleic acid-based PCR (Chen, Lu, Xiong, Xiong, & Liu, Citation2020; Kim et al., Citation2020). These physical and chemical tools have both advantages and disadvantages for the identification of meat types. Generally, immunochemical detection methods have proven to be simple, cost-effective and rapid (Pang et al., Citation2019; Song et al., Citation2018). The enzyme-linked immunosorbent assay (ELISA) is a rapid detection method that utilizes the specific reaction of an antigen with an antibody (Shen et al., Citation2019; Ye, Wu, Xu, Zheng, & Kuang, Citation2018). In addition, immunoassay kits that now widely utilized in food analysis are portable and can be used for routine in a processing facility or retail outlet. The ELISA method has been demonstrated high precision and good accuracy for the detection of food antigens (Lan et al., Citation2019; Zhou, Kuang, & Liu, Citation2020).
In our previous study, the MIP-QWM system was developed for the detection of cooked rat meat (Xin et al., Citation2020). It is label free and highly sensitive, although the mechanism is somewhat complicated. In this study, a sandwich ELISA (swELISA) method is developed for the detection of cooked rat meat and is compared to the MIP-QWM system.
Materials and methods
Reagents and solutions
Phosphate-buffered solution (PBS; pH 7.4), Tween 20, bovine serum albumin (BSA), Freund’s complete adjuvant, Freund’s incomplete adjuvant and horseradish peroxidase (HRP) were purchased from Sigma-Aldrich (Shanghai, China). PBST was purchased from Phygene Scientific (Fuzhou, Fujian). Deionized water was prepared on an ultra-pure water system by Barnstead GenPure Pro (Thermo Scientific, USA) and used for all buffers. Ninety six well plates, TMB substrate solution and stop solution, HRP-labelling kit, plate washing machine and plate reader were purchased from Thermo Fisher (Scientific, Guangzhou). Wild rat was caught outside Foshan University. Meats of three animals (pork, beef and chicken) were purchased from a local market.
Washing buffer was 0.01M PBS buffer containing 0.05% v/v tween 20. Antibody and meat antigen dilution buffer was 0.01 M PBS buffer containing 0.05% v/v tween 20 and 0.1% BSA. The coating buffer was 0.05 M carbonate buffer (pH 10.0). All other solutions were prepared from the materials purchased from Macklin Co (Shanghai).
Cooked meat sample preparation
The wild rat was handled carefully with gloves and masks. The whole rat was cooked completely in salted water. The cooked rat was chopped with an electric meat grinder and soaked in hexane to remove fats. After removing hexane, 1 g of the chopped rat was taken in a 15-ml tube, mixed in a water bath at 55°C for 1 h, adjusted the pH to 9–9.5, with 1N NaOH, continue to mix to another 1 h. The mixture was then centrifuged at 3500 r/min for 20 min, and the supernatant was collected and precipitated further with ammonium sulphate, reaching a final concentration of 60%. The precipitate was resuspended with 0.01 M PBS buffer and dialyzed against 0.01 M PBS for 2 days. Following dialysis, the crude cooked rat extract was purified further using gel filtration chromatography. The total protein content was determined by the Bradford assay, and the protein was confirmed by SDS-PAGE. The major band on SDS-PAGE was cut for peptide and mass spectrum analysis.
The meats of pork, beef and chicken were separately cooked and cut into pieces with an electric meat grinder. The apparatus was cleaned carefully between samples to prevent cross-contamination. The cooked meats were then subjected to protein extraction similar to that followed for the cooked rat protein.
Polyclonal antibody development and labelling
The polyclonal antibody was custom developed by Xema Medica Co Ltd. Briefly, the aforementioned cooked antigen was immunonized into the rabbit. The antigen was injected with adjunct reagents every week for three times. The rabbit bleed was collected after 10 days of first injection, and the antibody titre was determined by ELISA. Then, the collection of production bleeds begins when the screening data indicate that the animal has produced a high enough titre for the end application about month 4. Production bleed of 15 mL from each rabbit was collected until the required amount of antiserum is achieved. Production bleed is taken at days 10 and 17 after immunisations. Purification of the antibody was done by protein A specific affinity columns.
The HRP-labelled cooked rat polyclonal antibody was performed using the HRP-labelling kit by following the kit instruction.
Sandwich ELISA development
An ELISA was developed based on the polyclonal antibody raised against the cooked rat antigen. The antibody coated in microwells captures the target antigens from the meat sample. The same antibody was conjugated to HRP targeting other epitopes of the same antigens and was subsequently used for colour development. The optical density (OD) in the microwell is directly related to the quantity of the target antigens in the sample. Six standard solutions (0, 2, 10, 50, 200 and 1000 μg/ml) of cooked rat antigens extracted from the cooked rat were used for the standard curve. The incubation time of the plate was optimized as 30 min at room temperature. The wash steps were carried out with 250 μl PBST, five times, using a programmable automatic well wash machine. The colour development was conducted by adding 50 μl of HRP-conjugated antibody, followed by washing for another five times. Finally, 100 μl of TMB substrate solution was pipetted, and the colour developed by 15 min at room temperature. The enzyme reaction was stopped at the end of 5 min by the addition of 100 μl stop solution, and the absorbances were measured at 450 nm.
Cross-reactivity and recovery efficiency
The cross-reactivity was measured by the swELISA to check the specificity of the cooked rat polyclonal antibody. The same dilutions of cooked rat, pork, beef and chicken meat solutions were measure by swELISA.
The recovery efficiency was measured by swELISA by spiking raw rat meat into raw pork, beef and chicken meat and then cooked, and their proteins were extracted as described earlier.
Raw rat meat detection and swab tests for rat contamination
The raw rat meat detection was checked by swELISA and compared to the cooked rat meat under the same condition.
The swab tests were performed with swELISA to examine rat contamination. The kitchen-type wood surfaces contaminated by raw rat meat were swabbed by cotton balls and examined by swELISA.
Data analysis
The obtained data were analyzed by Microsoft Excel 2007 for the variances among all experimental groups. All data were obtained in triplicate, and the experiment was repeated twice. Significance was accepted at P < 0.05.
Results and discussion
Components of the targeting cooked antigen
The cooked rat antigen was prepared by cooking a whole rat, and the resulted protein was then purified. The result of gel electrophoresis of this cooked rat protein in our previous study was 30–45 kDa. Further protein components of this antigen were analyzed. The results indicated that several heat-resistant protein fragments including heat shock protein and myosin were present in the protein complex ().
Table 1. The major components of cooked rat protein complex checked by peptide and mass analysis.
Localization of target antigen
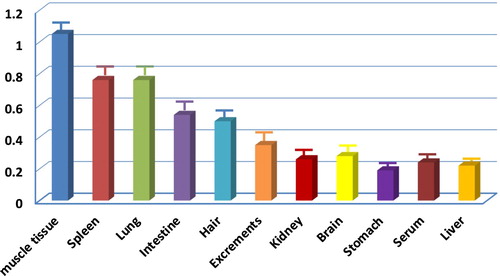
This antigen was prepared from the whole cooked rat, and the polyclonal antibody was developed thereof. It is necessary to check where those heat-resistant rat proteins were located in the whole rat body. Different parts from a rat body were separated, and the cooked protein was prepared. A swELISA was used with this polyclonal antibody to the abundance of cooked protein localization in the rat body. The semiquantitative antigen contents of cooked rat organs and excreta after total protein adjustment were presented (). It is shown that the muscle tissue has the highest antigen content, the spleen is second, the lung and the intestine have successively decreased content, and the liver, serum, kidney, brain, stomach and excrements are not much different. It is interesting to find that the rat hair and excrements contain relatively high-level heat-resistant proteins, so that the developed assay will be useful for any trace rat contaminations in the environment.
Standard curve of the two-site sandwich ELISA for cooked rat meat detection
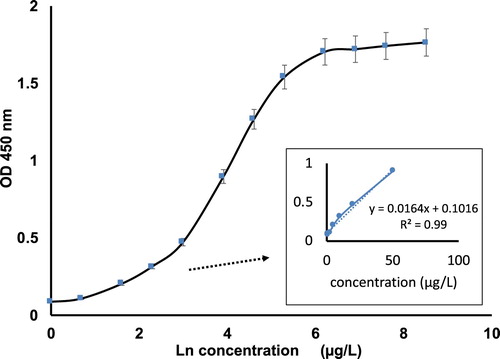
The standard curve of the two-site swELISA for cooked rat meat detection was conducted by the optimized condition described earlier. The OD in the microwell is directly related to the number of target antigens in the sample. The six standard samples were further diluted to a total of 12 samples for the standard curve generation, and the curve was presented in .
A good linear relationship was obtained between 0 and 50 µg/L as shown in . The calculated limit of detection (LOD) was three times the standard deviation of the negative control plus its mean absorbance for the antigen as calculated by the linear equation is 0.01 µg/L, which indicated that this swELISA is very sensitive for cooked rat protein detection. The intra CV of this swELISA was determined by repeating the procedure three times at 1, 2, 5, 10, 20 and 50 µg/L concentration in the same plate, calculating the average and SD for each concentration followed by CV for each point and finally average the CV of all six CVs as within 5%. The inter CV of this swELISA was determined by averaging total of 12 CVs on two different plates as within 8%. Washing five times during swELISA reduced the CVs for all the assays. The dynamic range of the swELISA for cooked rat protein detection is 1–1000 µg/L, although apparent hook effect was observed thereafter. These results demonstrated that this swELISA assay is highly robust and reliable for cooked rat meat detection.
The sensitivity of this swELISA was determined with pure cooked rat, which might be slightly affected in the presence of pork, beef and chicken meat. However, the matrix effect of this swELISA for cooked rat meat was almost negligible, as demonstrated in the late experimental data.
Cross-reaction analysis
The cross-reaction of cooked pork, beef, chicken and rat in swELISA was checked by analyzing cooked rat, chicken, beef and pork meat protein with the standard condition. The results were shown in .
Figure 3. The cross-reaction of cooked pork, beef and chicken against cooked rat checked by the swELISA.
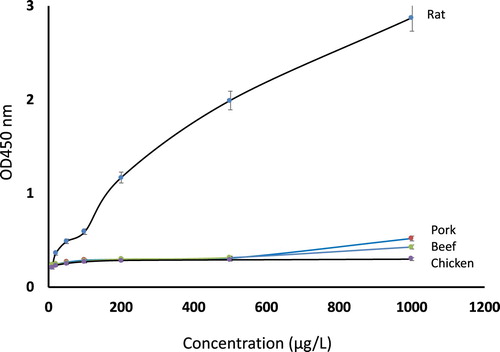
The results indicated that the swELISA is highly specific for cooked rat protein detection, while pork, beef and chicken were negligible in the assay, even at the very high protein concentrations of these meats.
Spiked sample analysis
Three levels of spikes samples (0.05, 0.1 and 0.2 ng/g) were prepared, and their protein extracts were collected, followed by the swELISA assay. The obtained results by swELISA were compared with the actual concentration ().
Table 2. The spiked sample assay results.
As given in , the recovery efficiency for all intra and inter assays of the spikes sample is between 80 and 110%. The results indicated that this swELISA is accurate in the detection of cooked rat meat in meat mixtures.
Cooked meat vs. raw meat
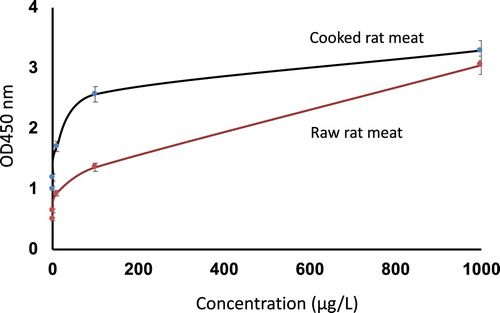
The sensitivity of swELISA for both cooked and raw rat meat was checked, and the result is shown in .
The results indicated that swELISA can be used for the detection of both raw and cooked rat meat protein. Nevertheless, the detection of cooked rat meat by swELISA is more sensitive than that of raw rat meat, particularly at the low concentrations of rat proteins.
The swab test results of rat contamination
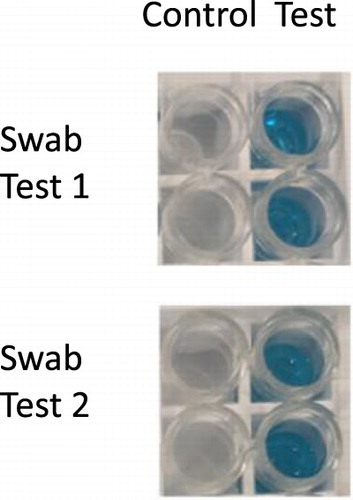
The swab tests were performed by contaminating wood surfaces with raw rat meat. Then, a cotton ball soaked in PBST was used to swab the whole surface (approximate 10 × 10 cm2). The cotton ball was then squeezed in swELISA well to afford about 50 ul solution, followed by the swELISA assay. Meanwhile, a wood surface with no contamination was performed by the same method. The results are shown in .
As the results indicated, the wells in the swELISA can be applied for swab tests for checking rat meat contamination in kitchen-type surfaces.
Commercial meat products checked by rat swELISA
Several commercial meat products from local markets were purchased. Their meat proteins were extracted and subjected for the cooked rat meat swELISA analysis. Each sample assay was repeated three times. At the same time, a positive control of cooked rat meat and a negative control of PBST were used. The results are presented in .
Table 3. The swELISA assay for some commercial meat products.
The results indicated that all of those commercial meat products had no rat meat components.
Discussion
The 2019-ncoV (COVID-19) epidemic alerts that eating wild animals would be punishable or even cause devastating social and health tragedies. As a vector or intermediate hosts, rats and other wild animals can spread a variety of viruses and industrial pollutants. Eating wild animal meats should be forbidden worldwide including China. Development of the related detection methods for wild animal meat is merging as an urgent task.
Rats are reservoirs of many diseases, including plague, leptospirosis (Theuerkauf et al., Citation2013), nephrotic syndrome (Zhu et al., Citation2018), blood fever (Low, Citation1924) and anthrax (Newman et al., Citation2010). Rat meat adulteration is becoming a serious problem in the food industry, particularly for cooked rat meat that is hardly detectable. In this paper, a highly sensitive ELISA method has been developed for the detection of cooked rat meat. The detection limit is as low as 0.01 ug/L, equal to a small rat mixed in 50 tons of various food products. The method is more sensitive for cooked rat meat than raw rat meat. Therefore, this method will be sensitive enough to detect any traces of rat meat adulteration.
In addition, the ELISA method is highly specific for rat meat detection and almost nondetectable for other common meats such as pork, beef and chicken. This may be due to the highly specific rat cooked antigens collected. Many heat-resistant proteins in the rat antigen complex are totally different among other animal meats. This indicated that a similar strategy can be applied for other wild animal meat detection, which is currently under development in our lab.
Compared to other biosensor-based detection methods, the ELISA method is quick and portable (Yuan, Liu, Yin, & Xu, Citation2019; Zeng et al., Citation2019; Zhou et al., Citation2020). The corresponding colloid gold strip is also useful for onsite detection. They will become powerful tools for meat adulteration in the food industry. We previously published (in press) a molecular-imprinted polymer-conjugated quantum weak measurement (QWM) method for this cooked rat protein detection. The results of these two methods are compared in .
Table 4. The comparison of swELISA and MIP-QWM for the detection of cooked rat meat.
In conclusion, wild animal meat contamination and adulteration (i.e. rat) can be rapidly detected by the ELISA method, particularly for any cooked meat rather than raw meat. Further efforts are made to develop similar methods for other wild animals possessing dangerous viruses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akimkin, V., Beer, M., Blome, S., Hanke, D., Hoper, D., Jenckel, M., & Pohlmann, A. (2016). New chimeric porcine coronavirus in swine feces, Germany, 2012. Emerging Infectious Diseases, 22(7), 1314–1315. doi: 10.3201/eid2207.160179
- Benvenuto, D., Giovanetti, M., Ciccozzi, A., Spoto, S., Angeletti, S., & Ciccozzi, M. (2020). The 2019-new coronavirus epidemic: Evidence for virus evolution. Journal of Medical Virology, 92(4), 455–459. doi: 10.1002/jmv.25688
- Bobrov, A. G., Kirillina, O., Vadyvaloo, V., Koestler, B. J., Hinz, A. K., Mack, D., … Perry, R. D. (2015). The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environmental Microbiology, 17(4), 947–959. doi: 10.1111/1462-2920.12419
- Carlos, W. G., Dela Cruz, C. S., Cao, B., Pasnick, S., & Jamil, S. (2020). Novel Wuhan (2019-nCoV) coronavirus. American Journal of Respiratory and Critical Care Medicine, 201(4), p7–p8. doi: 10.1164/rccm.2014P7
- Chen, X., Lu, L., Xiong, X., Xiong, X., & Liu, Y. (2020). Development of a real-time PCR assay for the identification and quantification of bovine ingredient in processed meat products. Scientific Reports, 10(1), 2052. doi: 10.1038/s41598-020-59010-6
- Ji, W., Wang, W., Zhao, X., Zai, J., & Li, X. (2020). Cross-species transmission of the newly identified coronavirus 2019-nCoV. Journal of Medical Virology, 92(4), 433–440. doi: 10.1002/jmv.25682
- Kartakoullis, A., Comaposada, J., Cruz-Carrion, A., Serra, X., & Gou, P. (2019). Feasibility study of smartphone-based Near Infrared Spectroscopy (NIRS) for salted minced meat composition diagnostics at different temperatures. Food Chemistry, 278, 314–321. doi: 10.1016/j.foodchem.2018.11.054
- Kim, M. J., Suh, S. M., Kim, S. Y., Qin, P., Kim, H. R., & Kim, H. Y. (2020). Development of a real-time PCR assay for the detection of donkey (Equus asinus) meat in meat mixtures treated under different processing conditions. Foods, 9(2), pii E130. doi: 10.3390/foods9020130
- Lan, J., Wang, M., Ding, S., Fan, Y., Diao, X., Li, Q. X., & Zhao, H. (2019). Simultaneous detection of carbofuran and 3-hydroxy-carbofuran in vegetables and fruits by broad-specific monoclonal antibody-based ELISA. Food and Agricultural Immunology, 30(1), 1085–1096. doi: 10.1080/09540105.2019.1664997
- Lei, X., Xu, L., Song, S., Liu, L., & Kuang, H. (2018). Development of an ultrasensitive IC-ELISA and immunochromatographic strip assay for the simultaneous detection of florfenicol and thiamphenicol in eggs. Food and Agricultural Immunology, 29(1), 254–266. doi: 10.1080/09540105.2017.1371114
- Lin, L., Jiang, W., Xu, L., Liu, L., Song, S., & Kuang, H. (2018). Development of IC-ELISA and immunochromatographic strip assay for the detection of flunixin meglumine in milk. Food and Agricultural Immunology, 29(1), 193–203. doi: 10.1080/09540105.2017.1364710
- Low, G. C. (1924). A case of rat-bite fever in England: Recovery of the Spirochaete from the blood. British Medical Journal, 1(3293), 236. doi: 10.1136/bmj.1.3293.236
- Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., & Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet, 395(10224), 565–574. doi: 10.1016/S0140-6736(20)30251-8
- Mabuchi, R., Ishimaru, A., Tanaka, M., Kawaguchi, O., & Tanimoto, S. (2018). Metabolic profiling of fish meat by GC-MS analysis, and correlations with taste attributes obtained using an electronic tongue. Metabolites, 9(1), 1. doi: 10.3390/metabo9010001
- Malik, Y. S., Sircar, S., Bhat, S., Sharun, K., Dhama, K., Dadar, M., & Chaicumpa, W. (2020). Emerging novel coronavirus (2019-nCoV) – Current scenario, evolutionary perspective based on genome analysis and recent developments. Veterinary Quarterly, 40(1), 68–71. doi: 10.1080/01652176.2020.1727993
- Newman, Z. L., Printz, M. P., Liu, S., Crown, D., Breen, L., Miller-Randolph, S., … Moayeri, M. (2010). Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathogens, 6(5), e1000906. doi: 10.1371/journal.ppat.1000906
- Pang, L., Quan, H., Sun, Y., Wang, P., Ma, D., Mu, P., … Hammock, B. D. (2019). A rapid competitive ELISA assay of Okadaic acid level based on epoxy-functionalized magnetic beads. Food and Agricultural Immunology, 30(1), 1286–1302. doi: 10.1080/09540105.2019.1689231
- Pieszczek, L., Czarnik-Matusewicz, H., & Daszykowski, M. (2018). Identification of ground meat species using near-infrared spectroscopy and class modeling techniques – Aspects of optimization and validation using a one-class classification model. Meat Science, 139, 15–24. doi: 10.1016/j.meatsci.2018.01.009
- Sebbane, F., Gardner, D., Long, D., Gowen, B. B., & Hinnebusch, B. J. (2005). Kinetics of disease progression and host response in a rat model of bubonic plague. The American Journal of Pathology, 166(5), 1427–1439. doi: 10.1016/S0002-9440(10)62360-7
- Shen, X., Liu, L., Xu, L., Ma, W., Wu, X., Cui, G., & Kuang, H. (2019). Rapid detection of praziquantel using monoclonal antibody-based ic-ELISA and immunochromatographic strips. Food and Agricultural Immunology, 30(1), 913–923. doi: 10.1080/09540105.2019.1641068
- Song, M., Xiao, Z., Xue, Y., Zhang, X., Ding, S., & Li, J. (2018). Development of an indirect competitive ELISA based on immunomagnetic beads’ clean-up for detection of maduramicin in three chicken tissues. Food and Agricultural Immunology, 29(1), 590–599. doi: 10.1080/09540105.2017.1418842
- Theuerkauf, J., Perez, J., Taugamoa, A., Niutoua, I., Labrousse, D., Gula, R., … Goarant, C. (2013). Leptospirosis risk increases with changes in species composition of rat populations. Naturwissenschaften, 100(4), 385–388. doi: 10.1007/s00114-013-1033-6
- Xin, M., Zeng, L., Ran, D., Chen, X., Xu, Y., Shi, D., … Zhong, S. (2020). Label-free rapid identification of cooked meat using MIP-quantum weak measurement. Food and Agricultural Immunology, 31(1), 317–328. doi: 10.1080/09540105.2020.1726879
- Ye, L., Wu, X., Xu, L., Zheng, Q., & Kuang, H. (2018). Preparation of an anti-thiamethoxam monoclonal antibody for development of an indirect competitive enzyme-linked immunosorbent assay and a colloidal gold immunoassay. Food and Agricultural Immunology, 29(1), 1173–1183. doi: 10.1080/09540105.2018.1523373
- Yuan, L., Liu, B., Yin, K., & Xu, Z. L. (2019). Development of an enzyme-linked immunosorbent assay for quantification of estriol in milk. Food and Agricultural Immunology, 30(1), 817–828. doi: 10.1080/09540105.2019.1637824
- Zeng, L., Song, S., Zheng, Q., Luo, P., Wu, X., & Kuang, H. (2019). Development of a sandwich ELISA and immunochromatographic strip for the detection of shrimp tropomyosin. Food and Agricultural Immunology, 30(1), 606–619. doi: 10.1080/09540105.2019.1609912
- Zhou, S., Kuang, H., & Liu, L. (2020). Development of an ic-ELISA and colloidal gold strip for the detection of the beta-blocker carazolol. Food and Agricultural Immunology, 31(1), 217–230. doi: 10.1080/09540105.2019.1710113
- Zhu, Y., Zuo, N., Li, B., Xiong, Y., Chen, H., He, H., … Wang, H. (2018). The expressional disorder of the renal RAS mediates nephrotic syndrome of male rat offspring induced by prenatal ethanol exposure. Toxicology, 400-401, 9–19. doi: 10.1016/j.tox.2018.03.004