ABSTRACT
Nitrofurans comprise a class of synthetic broad-spectrum antibacterial agents, which had been used extensively as therapeutic drugs and feed additives in animal husbandry. Because of the potential carcinogenic and mutagenic effects of these drugs and their metabolites, most countries have banned their use in food animals. However, driven by economic interests, the illegal use of nitrofurans poses a significant threat to human health. Therefore, to safeguard health of the consumer, it is of paramount importance to develop efficient, sensitive, and reliable detection methods to monitor nitrofuran and their metabolite residues in animal-derived foods. In this paper, the progress in immunoassays for nitrofuran residues is reviewed, including enzyme-linked immunosorbent assay, fluoroimmunoassay, chemiluminescence immunoassay, immunochromatography, and immunosensor assay. Basic principles and applications of these methods were described and compared in this paper. Furthermore, the future perspective on the improvement and development of the immunoassays for the detection of nitrofurans has been discussed.
1. Introduction
Nitrofurans are a class of synthetic broad-spectrum antibacterial agents, primarily including furazolidone (FZD), furaltadone (FTD), nitrofurantoin (NFT), and nitrofurazone (NFZ). Due to their robust antibacterial activity on most Gram-positive and Gram-negative bacteria, certain species of fungi and protozoa, these drugs have been used widely to prevent and treat disease in poultry and livestock farming, beekeeping and aquaculture, and are used as feed additives and growth promoters in livestock and poultry breeding (Tang et al., Citation2016). Studies have shown the toxic effect of nitrofurans on livestock and poultry when used in large doses or for a long term, causing carcinogenic, teratogenic, and mutagenic effects. The metabolites of these drugs will harm human health after being absorbed by the body (Wang, Citation2017).
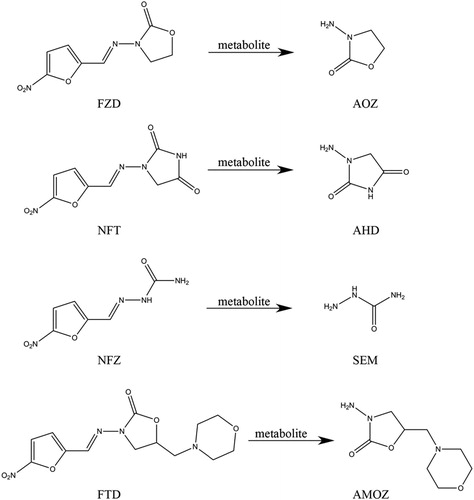
Protocols for the monitoring of nitrofurans were established by nations worldwide. The EU has banned the use of nitrofurans in livestock, poultry, and aquatic animals since 1995, and the published Commission Decision 2003/181/EC limited the presence of the nitrofuran drug or metabolite in poultry, meat, and aquatic animal products below 1.0 μg/kg (Liu et al., Citation2013). In 2004, the United States FDA listed nitrofurazone and furazolidone as prohibited drugs in the use of imported animal-sourced foods and banned the use of furazolidone and nitrofurantoin in food animals in Federal Regulation 21CFR530.41. In the Food Code released in 2002, South Korea stipulated that furazolidone should not be detected in pork (Li et al., Citation2016). In its Announcement No. 193 issued on April 2002, the Ministry of Agriculture and Rural Affairs of the People's Republic of China explicitly stated that nitrofurans should be banned as veterinary drugs. While nitrofurans are rapidly metabolized in vivo, their metabolites are stable when combined with proteins, and can remain in biological tissues for a long time (Zhou et al., Citation2016). Hence, the metabolites of nitrofurans are usually used as markers for drug residues in the detection of this class of drugs, while the detection of the parent drugs is rarely studied. The main metabolites of furazolidone, furaltadone, nitrofurantoin, and nitrofurazone are 3-amino-2-oxazolidinone (AOZ), 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ), 1-aminohydantoin (AHD), and semicarbazide (SEM), respectively. The structures of these four nitrofurans and their metabolites were shown in . In practice, the detection of these four metabolites can reflect whether the four nitrofurans have been used against the law. Nitrofuran metabolites are listed as prohibited (discontinued) compounds for detection in the National Monitoring Plan for Veterinary Drug Residues in Domestic Aquatic Products (Year 2020), and their presence is limited to 1.0 μg/kg (Announcement No.Citation783-Citation1-Citation2006; Announcement No.Citation1077-Citation2-Citation2008). For this reason, it is essential to establish sensitive, efficient, and rapid detection methods for the monitoring of nitrofuran residues.
At present, the detection of nitrofurans and their metabolites is performed by either instrumental analysis or immunoassay. Each has its strengths and weaknesses and can be complementary to each other. Instrumental analysis is highly accurate, but is also time-consuming and laborious, thus is generally used in confirmatory testing. The immunoassay, relying on the specific binding between an antigen and an antibody, does not require the use of large-scale instruments and has the advantages of high sensitivity, low detection cost, and simple operation. They are, therefore, better suited for the rapid on-site analysis of large numbers of samples and are the most widely used screening methods for veterinary drug residues at present. The low molecular weight of nitrofuran metabolites and the difficulty in preparing high-affinity antibodies for these metabolites often make it necessary to derivatize the metabolites before detection. By measuring the content of these derivatives, the presence of nitrofuran metabolites can be determined. The main derivatization reagents are nitrobenzaldehydes (NP) and carboxybenzaldehyde (CP). Whereas, there have also been reports on the direct detection of metabolites without derivatization in recent years. Common immunoassay methods include enzyme-linked immunosorbent assay, chemiluminescence immunoassay, fluoroimmunoassay, immunochromatography, and immunosensor assay. Among these, enzyme-linked immunosorbent assay (ELISA) is a classic and most commonly used type of immunoassay. With the development of detection techniques for drug residues in food, the detection of nitrofurans and their metabolites is becoming simpler, faster, and more accurate. It was discussed in this review about the application and comparison of various immunoassay methods for nitrofurans and their metabolites detection.
2. Immunoassays for nitrofuran detection
2.1. ELISA
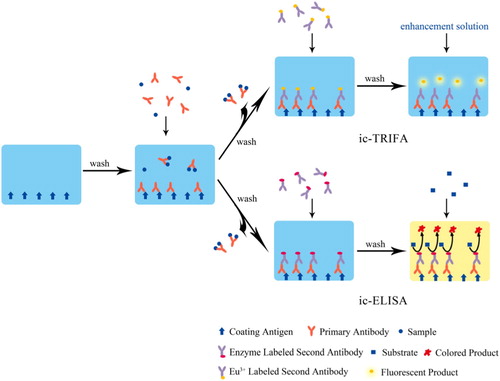
ELISA is a marker-based immunoassay that combines the highly efficient catalysis and signal amplification of the enzyme with the specific binding between an antigen and an antibody. Cooper et al. firstly reported the use of ELISA in the detection of the furazolidone metabolite (Cooper et al., Citation2004). Though several new immunoassay technologies have emerged in recent years, ELISA is still the most widely used and most successfully commercialized immunoassay method for nitrofuran drugs and their metabolites detection up to now due to its superior advantages in sensitivity, specificity, easy operation, and high-efficiency. ELISA for nitrofurans could be performed by either indirect competition (ic-ELISA), the most extensively studied method, or direct competition (dc-ELISA). The ic-ELISA works by coating the surface of the solid support (such as a microplate) with an antigen (coating antigen), followed by the simultaneous addition of the sample and primary antibody. After the competitive reaction, the enzyme-labelled secondary antibody and the substrate are added in turn (). The number of analytes in the samples is inversely proportional to the generated detectable coloured product.
2.1.1. ELISA detection of furazolidone and its metabolite (AOZ)
Cooper et al. reported the ELISA for furazolidone metabolite (AOZ) residues in prawn tissues for the first time (Cooper et al., Citation2004). AOZ was derivatized with 3-carboxybenzaldehyde (3-CBA) to prepare the hapten 3-CPAOZ, and the corresponding polyclonal antibody that specifically recognized the NPAOZ (a 2-nitrobenzaldehyde derivative of AOZ) was obtained. The limit of detection (LOD) of the assay was 0.1 μg/kg, and the detection capability (CCβ) was below 0.7 μg/kg. Subsequently, the group successfully prepared the specific monoclonal antibody for AOZ, and established a dc-ELISA protocol for the determination of AOZ residues in the muscle tissues of prawns, poultry, pigs, and cows with the CCβ value of 0.4 μg/kg (Diblikova et al., Citation2005). Chang et al. replaced the traditional 2-nitrobenzaldehyde with a less toxic new derivatizing agent, benzaldehyde, and established an ic-ELISA method for the quantification of AOZ residues in the tissues of edible animals such as pork, chicken, and fish (Chang et al., Citation2008). The LOD achieved was 0.3–0.4 μg/kg, and the recovery was in the range of 55.8–96.6%. Ding et al. used 2-CPAOZ, a 2-carboxybenzaldehyde derivative of AOZ, as the hapten to prepare a monoclonal antibody. They obtained a half-maximal inhibitory concentration (IC50) of 0.2 ng/mL. The ic-ELISA established with this antibody achieved a recovery range of 86.2–118.5% in the detection of fish samples, and the coefficient of variation ranged from 4.3% to 9.4% (Ding et al., Citation2017). Chen et al. prepared an anti-FZD monoclonal antibody and obtained an IC50 of 0.06 μg/mL. The ic-ELISA assay based on this antibody achieved a LOD of 6.92 ng/mL (Chen et al., Citation2016). Subsequently, the team successfully prepared the single-chain antibody of FZD with an IC50 of 13.01 ng/mL. The LOD of the established assay was 1.28 ng/mL, showing higher sensitivity and better detection stability compared to the monoclonal antibody (Chen et al., Citation2017). Instead of using a biological antibody, Wen et al. employed molecular imprinting to directly polymerize a molecularly imprinted polymer for AOZ recognition on the surface of a 96-well microtiter plate and performed dc-ELISA detection. Their results showed an IC50 of 2.0 ng/mL and a LOD of 0.008 ng/mL of the prepared molecularly imprinted polymer of AOZ (Wen et al., Citation2013). This artificial antibody overcomes many weaknesses of biological antibodies, such as the long preparation period, susceptibility to deterioration, and difficulty in transportation and storage.
2.1.2. ELISA detection of furaltadone and its metabolite (AMOZ)
Pimpitak et al. established a monoclonal antibody-based ic-ELISA assay for the detection of AMOZ residues in shrimp tissues and obtained a LOD of 0.16 μg/kg for NPAMOZ (Pimpitak et al., Citation2009). Song et al. derivatized AMOZ with 4-formylphenoxy acetic acid (4-FPA) and 3-carboxybenzaldehyde to prepare the hapten 4-FPA-AMOZ and the coating hapten 3-CPAMOZ, respectively, and established an ic-ELISA assay for the detection of AMOZ. The obtained IC50 for NPAMOZ and AMOZ were 0.17 and 1.15 ng/mL, respectively. Owing to the high affinity of the antibody to AMOZ, an ic-ELISA for the direct detection of AMOZ without derivatization was described, with recoveries ranging from 72.6% to 121.2% and coefficients of variation ranging from 6.1% to 17.7%. The proposed ELISA was also confirmed by high-performance liquid chromatography (HPLC) with a good correlation (Song et al., Citation2012). Yan et al. used FTD-BSA as an immunogen to prepare a polyclonal antibody, achieving IC50 of 2.3 and 0.8 ng/mL for AMOZ and FTD, respectively. The ic-ELISA established on the basis of this antibody could perform simultaneous detection of AMOZ and its parent drug FTD without the need for derivatization. The LOD for AMOZ in pork and fish was 0.4 μg/kg (Yan et al., Citation2012). Liu et al. attempted to use poly-lysine dendrimer in place of a traditional carrier protein to prepare the immunogen. The results showed the specific recognition of the antiserum towards free AMOZ. While, the sensitivity of the method was not satisfactory with the inhibition rate of 35% for 1 μg/mL AMOZ (Liu et al., Citation2015).
Shen et al. prepared the hapten 3-FPA-AMOZ by derivatization with 3-formylphenoxy acetic acid (3-FPA). The acquired monoclonal antibody exhibited a high affinity towards NPAMOZ, giving an IC50 value of 0.14 ng/mL and LOD of 0.01 ng/mL. The LOD of the established ic-ELISA assay for AMOZ in fish and shrimp samples was 0.11 μg/kg, and the recovery ranged from 81.0% to 104.0%, making the method an ideal candidate for the monitoring of AMOZ residues at trace level (Shen et al., Citation2012). Xu et al. compared the derivatization reagents 3-FPA and 4-FPA with 3-CBA and 4-CBA, indicating a nearly two-fold improvement in the affinity of antibodies produced with the derivatization reagents 3-FPA and 4-FPA towards NPAMOZ (Xu, Shen et al., Citation2013). This difference was explained by computer-assisted molecular modelling of the lowest energy conformations of NPAMOZ and FPA-AMOZ. It was found that the introduction of phenoxy spacer in FPA induced a conformational change in the hapten of AMOZ, such that the changed hapten conformation resembled NPAMOZ, promoting the recognition of antibodies to NPAMOZ. Wang used a hybrid-hybridoma technique to isolate a monoclonal antibody that was bispecific to NPAMOZ and leucomalachite green (LMG) and established a multi-analyte ic-ELISA for detecting AMOZ and LMG residues. The optimized LOD and IC50 values for NPAMOZ were 0.2 and 1.7 ng/mL, respectively. The recovery ranged from 72.4% to 101.0% with coefficients of variation below 15% when AMOZ were spiked in grass carp and tilapia. The LOD and IC50 for LMG were 4.8 and 45.3 ng/mL, respectively (Wang, Citation2016). This multi-analyte ic-ELISA could be applied in the simultaneous detection for AMOZ and LMG in aquatic products. It is a simpler and faster method compared with the existing single-analyte detection and inspires future research on a novel, multi-analyte detection of different drugs.
2.1.3. ELISA detection of nitrofurantoin and its metabolite (AHD)
Compared with other nitrofurans, few reports are available on the ic-ELISA detection of nitrofurantoin (NFT) or AHD residues. Liu et al. used the conjugation of 3-CBA, AHD, and bovine serum albumin as immunogen to prepare a polyclonal antibody against NFT. Because of the structural similarity between AHD and NFT, the antibody obtained exhibited excellent specificity and sensitivity to the parent drug NFT. Based on this antibody, the first immunoassay for NFT residue detection in water was presented with a LOD of 0.2 ng/mL (Liu et al., Citation2007). Jiang et al. derivatized AHD with 4-CBA to prepare the hapten 4-CPAHD, producing the monoclonal antibody against NPAHD. This antibody has an IC50 of 0.68 ng/mL. The LOD of the established ic-ELISA for the detection of AHD residues in four animal tissues, pork, fish, shrimp, and chicken, were all below 0.2 μg/kg (Jiang et al., Citation2012). Luo et al. developed a kit for the detection of AHD residues, achieving an IC50 of 0.094 ng/mL, and the same LOD of 0.1 μg/kg for chicken, pork, pig liver, and shrimp samples. The recovery and coefficient of variation fell in the range of 78.2–102.7% and 5.1–11.4%, respectively. The results of this kit were in good agreement with those from an established liquid chromatography-tandem mass spectrometry (LC-MS/MS) confirmatory method (Luo et al., Citation2013). In the early days, AHD residue detection mostly relied on ic-ELISA. Li et al. established a dc-ELISA method for AHD by labelling antigens with horseradish peroxidase, achieving an IC50 of 1.291 ng/mL, and LOD values of 0.115, 0.113, and 0.109 μg/kg in pork, chicken, and fish samples, respectively. This method would meet the need of rapid screening for a large number of samples (Li, Wang, et al., Citation2015).
2.1.4. ELISA detection of nitrofurazone and its metabolite (SEM)
Cooper et al. synthesized the hapten 3-CPSEM by derivating SEM with 3-CBA to obtain the first polyclonal antibody towards SEM. The dc-ELISA assay they established for the detection of residual SEM in chicken tissues achieved a LOD of 0.25 μg/kg, which was in line with the minimum required performance limit (MRPL) of the EU (Cooper et al., Citation2007). Since then, Vass et al. and Yao et al. reported ELISA methods for SEM residues detection in eggs (Vass et al., Citation2008) and grass carp (Yao et al., Citation2009), with the LOD values reaching 0.13 and 0.76 μg/kg, respectively. Fang et al. established a more sensitive and facile direct ELISA detection of SEM taking advantage of the amplification effect of the biotin–streptavidin system (Fang et al., Citation2013). In this study, they derivatized sample SEM with biotinylated 4-CBA and then utilized the streptavidin–horseradish peroxidase to amplify the signal. The LOD obtained was as low as 0.07 ng/mL, and the recovery was better than traditional competitive ELISA. What’s more, there was also no need of complicated extraction procedure for real samples. An ic-ELISA assay constructed by Huang et al. used the same principle, in which a biotinylated secondary antibody replaced the enzyme-labelled secondary antibody in conventional ELISA in order to bond with the streptavidin-enzyme conjugate for signal amplification. An IC50 of 0.601 ng/mL and recovery of 88.8–98.5% were obtained in chicken samples under optimal conditions (Huang et al., Citation2017).
Early reports on SEM haptens mostly focused on the 4-CP or 3-CP derivatized structures. These entities did not contain the reactive nitro terminal group of the analyte, nitrobenzaldehyde derivatives (NPSEM). Li et al. obtained a novel hapten with a nitro terminal group in its structure by derivatizing SEM with 4-(3-aldehyde-4-nitro-phenoxy)-butyric acid, thus effectively enhancing the immunogenicity of the antigen and the performance of the antibody generated. The ic-ELISA established attained a LOD of 0.04 ng/mL and an IC50 of 0.23 ng/mL (Li et al., Citation2018). Yu et al. furnished a biomimetic ELISA based on the molecular imprinting technique. The LOD and IC50 of this method were 1.0 and 85.7 ng/mL, respectively. They have successfully applied this assay to the analysis of SEM residues in beef, pork, and shrimp samples, achieving an acceptable recovery of 86.1–90.8% (Yu et al., Citation2020).
2.2 Chemiluminescence immunoassay (CLIA)
According to the different kinds of labelling techniques, the chemiluminescence immunoassay can be categorized into labelled chemiluminescence immunoassay and chemiluminescence enzyme immunoassay. The chemiluminescence enzyme immunoassay is a novel technique that combines the high sensitivity of chemiluminescence with the high specificity of immunoassay (Fang et al., Citation2015). While as rapid and straightforward to operate as ELISA, it offers higher sensitivity than ELISA and has been widely used in the detection of drug residues. Other than using a luminescent agent as the substrate for enzyme reaction, the chemiluminescence enzyme immunoassay has essentially the same as procedure with ELISA.
2.2.1. Direct/indirect competitive chemiluminescence enzyme immunoassay
An et al. synthesized the enzyme-labelled compound CPAOZ-HRP via carbodiimide-mediated coupling of CPAOZ, the reaction product of AOZ and 3-CBA, and horseradish peroxidase (HRP), and established a direct competitive chemiluminescence detection assay for AOZ. Both this essay and HPLC were employed for the detection of AOZ residues in tilapia samples from Xinjiang Province. The results indicated specific detection of AOZ by the established CLIA with no cross-reaction with other drugs. The LOD for the samples was 0.0509 μg/kg, with a recovery of 87.2–95.0%. These results correlated well with those from HPLC, revealing the high accuracy of CLIA (An et al., Citation2019). Wang et al. produced the coating antigen, a conjugate of CPAHD and ovalbumin (OVA), by activated esters method. This coating antigen competed with the NPAHD standard solution for the antigen-binding site on the anti-AHD monoclonal antibody. The HRP-labelled goat anti-mouse secondary antibody was added to establish an indirect competitive CLIA for AHD. This assay gave an IC50 value of 0.753 ng/mL, and a LOD of 0.028 μg/kg for chicken tissues. The recovery was above 80.54%, and the coefficient of variation was below 10% (Wang et al., Citation2015).
2.2.2. Magnetic particle chemiluminescence enzyme-linked immunoassay
Magnetic particle chemiluminescence enzyme-linked immunoassay (MCLIA) combines the enzyme immunoassay with magnetic particles (Tu et al., Citation1999). It is a highly sensitive and highly repeatable method that gives stable colour and does not cause radioactive pollution. At present, this method is not so commonly used in the detection of small molecules such as veterinary drug residues, but the prospect of its application in this aspect is attractive. Liang et al. made use of a fully automated chemiluminescence instrument to develop a magnetic particle chemiluminescence reagent kit for the detection of the four nitrofuran drugs. This rapid detection method took use of magnetic particles as the solid-phase carrier and chemiluminescence as the detection signal. A simultaneous testing to detect the SEM, AOZ, AMOZ, and AHD in 78 samples of chicken and fish from different sources was performed. The LOD achieved was 0.1 ng/mL for the metabolites of all four nitrofuran drugs, and recovery was above 70%. The coincidence rate between this MCLIA method and HPLC in the detection of SEM, AHD, and AMOZ residues in different types of samples was 100%, and the coincidence rate for the detection of AOZ was 98.7% (Liang et al., Citation2019).
2.3. Fluorescence/fluorescence polarization immunoassay (FIA/FPIA)
The fluorescence immunoassay is based on the specific binding between an antigen and an antibody and uses fluorescent molecules as markers. By measuring fluorescence signals of different intensities, it can perform qualitative and quantitative analysis of the drug residues in a sample. Common fluorescent markers include fluorescein, semiconductor quantum dots, complexes of rare-earth ions, and upconversion nanoparticles. This type of assay could be further divided into time-resolved fluoroimmunoassay (TRFIA), fluorescence polarization immunoassay (FPIA), etc. (Fang et al., Citation2019). Compared with the instrumental analysis and conventional enzyme immunoassay, the fluorescence immunoassay offers high sensitivity and high specificity and is simple to operate and low in cost. These strengths ensure a broad application prospect for this method.
2.3.1. Time-resolved fluoroimmunoassay (TRFIA)
Fundamentally, TRFIA uses complexes of rare-earth ions as fluorescent markers. The long fluorescence life of these fluorescent molecules extends the fluorescence measurement time and eliminates the interference from non-specific background fluorescence. The working principle of indirect competitive time-resolved fluoroimmunoassay (ic-TRFIA) could be found in . Zhao et al. used an anti-AOZ monoclonal antibody labelled with chelated rare-earth ion Eu3+ to perform the qualitative and quantitative analysis and develop a time-resolved fluoroimmunoassay for the fast detection of AOZ. This method attained a LOD of 0.5 μg/kg and displayed low cross-reactivity towards AOZ analogs. The test strip fabricated could be stored at room temperature for up to one year (Zhao et al., Citation2018). Deng et al. furnished a Eu3+-labelled ic-TRFIA for the determination of AMOZ in fish samples. Under optimized conditions, the method achieved a LOD of 0.01 ng/mL and an IC50 of 0.26 ng/mL, which was in good correlation with HPLC-MS/MS (Deng et al., Citation2016). Zhao et al. established an ic-TRFIA for the detection of AOZ in crucian. The LOD of the method was 0.015 ng/mL, and the cross-reactivity towards NFT and NFZ was less than 0.1%. The mean value of the intra-assay coefficient of variation, the inter-assay coefficient of variation and the recovery was 6.2%, 9.0%, and 83.0%, respectively (Zhao et al., Citation2014).
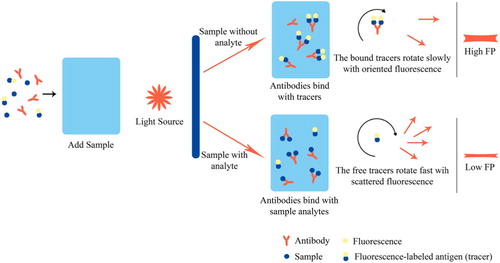
2.3.2. Fluorescence polarization immunoassay (FPIA)
FPIA is a quantitative homologous immunoassay method with the clear superiority in high-throughput performance, fast-speed detection, and simple operation, which is regard as a suitable technique for the rapid screening of a large number of samples. Because of the low molecular weight and fast movement of fluorescence-labelled haptens, these entities have small fluorescence polarization (FP) values. When no metabolite is present in the sample, the fluorescence-labelled hapten binds with the antibody to form an antigen–antibody complex of larger size, which leads to a correspondingly higher FP value. When metabolite concentration in the sample increases, the metabolite will compete with fluorescence-labelled hapten for the limited binding sites on antibodies, and inhibit hapten-antibody binding. The fluorescent hapten molecules are thus present as free entities in the system, and their FP value decreases. This is the principle that quantitative analysis with FPIA is based upon (). Shen et al. designed and synthesized a fluorescence tracer of CEPSEM-HDF and established a highly specific and stable FPIA method for the detection of SEM. The IC50 of the method was 47.9 ng/mL, and the LOD was 8.3 ng/mL. The calibration curve showed good linearity in the range of 15.8–145.7 ng/mL (Shen et al., Citation2009). Zhang et al. successfully prepared seven novel fluorescence tracers for FZD and applied FPIA to detect FZD residues in livestock feed. These tracers significantly improved the sensitivity of FPIA. The IC50 of the optimized FPIA was 5.5 ng/mL, and the cross-reactivity toward NFZ and ciprofloxacin was less than 0.1%. This method gave a LOD of 0.5–0.9 ng/mL on feed samples. The recovery was in the range of 79.0–85.0% with the coefficient of variation below 12% when FZD was spiked in the samples at concentrations of 5, 20, and 50 ng/mL (Zhang et al., Citation2010). Xu et al. produced a monoclonal antibody with high cross-reactivity to FTD and NPAMOZ and studied the effect of several synthetic tracers on the sensitivity of the hapten. The optimum pair of antibody and tracer was used to construct an FPIA assay, resulting in an IC50 of 4.3 ng/mL and a LOD of 0.6 ng/mL for FTD, and an IC50 of 2.7 ng/mL and a LOD of 0.3 ng/mL for NPAMOZ. A good correlation (R2>0.99) was obtained between the results of this FPIA and a standard analytical method (Xu, Zhang, et al., Citation2013).
2.4. Immunochromatographic assay (ICA)
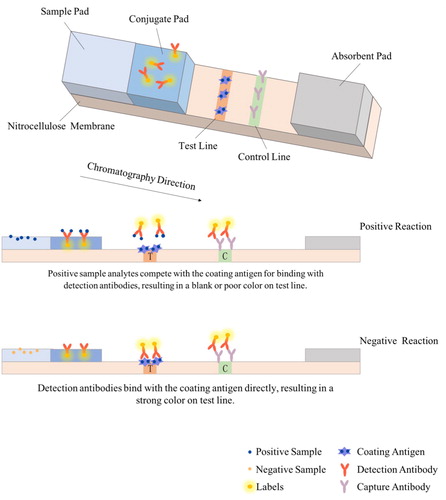
Immunochromatography is a convenient, fast, low-cost, safe, and reliable method widely used in instant detection, and plays an essential role in the large-scale screening of samples (Wang, Citation2019). The working principle of this method is shown in . The signal reading mode of immunochromatography is determined by the performance of the labelling agent, while its sensitivity is determined by the signal strength and stability. At present, ICA could be sub-divided into the following categories by the used labelling agent.
2.4.1. Colloidal gold immunochromatographic assay
As the colloidal gold is biocompatible, brightly coloured, easy and cost-effective to synthesize, it is considered to be a good candidate for biomarkers (Sperling et al., Citation2008). Colloidal gold immunochromatographic assay (CG-ICA) is a solid-phase labelled immunoassay technique that integrates gold labelling technique, immunoassay method, and chromatography assay (Dykman & Bogatyrev, Citation2007). Tang et al. established a CG-ICA for the detection of AHD in pork. In this method, the qualitative analysis could be completed in one minute, with a cut-off value of 1.40 ng/mL (Tang et al., Citation2011). Li et al. constructed a CG-ICA for the detection of AMOZ in meat and livestock feed, utilizing a monoclonal antibody that could specifically recognize the metabolite AMOZ (Li et al., Citation2014). Instead of the complicated derivatization in sample pretreatment, directly extraction can meet the requirement of detection. The visual detection limit (vLOD) achieved was 10.00 ng/mL. Xie et al. fabricated a CG-ICA for the detection of AOZ in chicken, pork, fish, and shrimp tissues. The cut-off value of this method was 10.00 ng/mL, and the IC50, given by colour intensity measurements of a strip reader, was 1.30 ng/mL. The LOD and the limit of quantification (LOQ), based on the results of 20 different blank animal tissue samples (chicken, pork, fish, and shrimp) were 0.15 and 0.31 μg/kg, respectively. The recovery was in the range of 76.3–98.4%, and the coefficient of variation was below 15.0% (Xie et al., Citation2017).
Single colloidal gold labelling has a limited effect on improving the sensitivity of an immunoassay. The effective combination of other techniques or substances with this method is the current trend in the development of CG-ICA. Dou et al. designed an innovative and simple colloidal-gold signal amplification system in which a pair of probes were fabricated by separately coupling colloidal gold to a primary and a secondary antibody, and dispensing them onto different positions of the same conjugate pad. As the test solution migrated along the test strip, the two probes self-assembled into a composite of a colloidal gold network, thus effectively amplifying the signal as the composite was captured by the antigen immobilized on the test line (Dou et al., Citation2018). This strategy guaranteed the colour intensity of CG-ICA for the detection of small molecules and ensured the sensitivity of the immunochromatographic assay. When applied to the detection of AOZ in animal source foods, the cut-off value of the method was 1.00 ng/mL (CPAOZ), and the LOD and LOQ were 0.13 and 0.42 ng/mL (CPAOZ), respectively. The recovery was in the range of 87.0–120.1%. Li et al. successfully combined the CG-ICA with surface-enhanced Raman scattering (SERS). The surface of colloidal gold was modified with 4-mercaptobenzoic acid for enhanced Raman activity. The modified gold particles were then labelled with a specific antibody to form the signaling probe and used for the competitive immunochromatography detection of AMOZ in animal tissues and urine. Using portable Raman spectrometers, the Raman intensity obtained on 4-mercaptobenzoic acid was measured for the quantitative determination of AMOZ. The IC50 of the assay was 0.04 ng/mL, and the LOD was 0.28 pg/mL. The recovery was between 87.8% and 105.9% (Li, Yang, et al., Citation2015).
In a practical situation, traditional immunochromatography could no longer meet detection needs and market requirements. High-throughput detection is an inevitable current and future trend. Currently, many research groups have developed multiplex immunochromatographic tests incorporating a variety of probes. Wang et al. coated nitrocellulose (NC) membrane with four different types of antigens to form four test lines. Four different types of specific antibodies were labelled with colloidal gold to make signaling probes. The same group subsequently established a competitive, multiplex CG-ICA for the simultaneous detection of four nitrofuran metabolites in fish samples. The cut-off values of this test were 0.50 ng/mL (AOZ) and 0.75 ng/ml (SEM, AHD, and AMOZ) (Wang et al., Citation2018).
2.4.2. Magnetic bead-based immunochromatographic assay
Magnetic beads are micrometer-sized particles composed of magnetic metal oxides with a macromolecular organic polymer shell modified by specific functional groups to bound to reactive groups. The modified magnetic beads are then conjugated with biological molecules such as antibodies for use as signaling probe in reactions (Chen, Citation2018). Lu et al. developed a multiplex immunochromatographic assay based on immuno-functionalized magnetic beads. The magnetic beads conjugated with metabolite-specific antibodies were used for sample pre-processing and also used as the signaling probe to detect one or several metabolites among SEM, AHD, AMOZ, and AOZ at the same time. This method achieved a LOD of 0.10 ng/mL (Lu et al., Citation2016). Yan et al. established a signal amplified magnetic bead-based immunochromatographic assay for the monitoring of AOZ in milk. Network complexes of magnetic beads were prepared via the specific binding between primary antibody and secondary antibody to realize signal amplification and enhance the response sensitivity. The cut-off value of the method was 0.88 ng/mL (Yan et al., Citation2018).
2.4.3. Latex bead immunochromatographic assay
Latex beads are labelling agents with coloured dye in a polystyrene polymer shell. They are more uniform, stable, and easier to observe than colloidal gold. These beads can be prepared in different colours by embedding different coloured dyes in the polystyrene shell, giving them broad application prospects in the multi-component analysis (Gao et al., Citation2019). Wang established a latex bead immunochromatographic assay (LB-ICA) for the simultaneous detection of SEM, AHD, AMOZ, and AOZ residues in eggs and chicken, fish, and shrimp tissues. A multi-channel quantitative reader for the immunochromatographic assay was used to allow a rapid and quantitative measurement. This LODs achieved in the four different types of animal tissues mentioned above for SEM determination were 0.02, 0.02, 0.04, and 0.05 μg/kg, for AHD test were 0.04, 0.05, 0.08, and 0.07 μg/kg, for AMOZ detection were 0.10, 0.10, 0.15, and 0.12 μg/kg, and for AOZ quantitation were 0.02, 0.02, 0.02, and 0.03 μg/kg. When blank animal samples were fortified with metabolites of LOD, 2LOD, 4LOD, and 1 μg/kg concentration, the intra-assay recovery and coefficient of variation were in the range of 73.5–109.2% and 3.5–12.4%, respectively. The inter-assay recovery and coefficient of variation were in the range of 75.8–105.5% and 2.3–12.9%, respectively (Wang, Citation2019).
2.4.4. Quantum dot immunochromatographic assay
Quantum dots, also known as semiconductor nanoparticles, are zero-dimensional nanomaterials with a size of 1–10 nm. They stand out from the traditional organic dyes with the ability to effectively distinguish among different tracers, good optical stability, and long shelf-life (Mu, Citation2014). Le et al. established a quantum dot immunochromatographic assay (QD-ICA) for the rapid detection of AOZ in chicken, pork, fish, and shrimp samples. The linear range of this method was in the range of 0.10–100.00 ng/mL, and the IC50 was 1.06 ng/mL. The recovery fell in the range of 76.3–98.4%, and the coefficient of variation was less than 15.0% (Le et al., Citation2016). The same group subsequently applied the QD-ICA technique to the detection of AHD (Le et al., Citation2018), and achieved a LOD of 0.14 μg/kg, which was below the MRPL of 1 μg/kg for AHD set by the European Commission. This method offered a fast and simple on-site detection of the desired analyte. Xie et al. developed an immunochromatographic test strip (immunostrip) based on quantum dots for the rapid detection of the furaltadone metabolite, AMOZ. They harvested four monoclonal antibodies to 2-NPAMOZ from hybridoma cell lines. With 2-NPAMOZ as the target, the immunostrip showed a linear range of 1–30 ng/mL, with an IC50 of 5.22 ng/mL and a LOD of 0.07 ng/mL. The recovery of this method ranged from 78.6% to 108.9%, and the coefficient of variation was below 12%. There was a good correlation (R2=0.9901) between the results given by the immunostrip and LC-MS/MS. Hence, the method could be used for the rapid and quantitative detection of AMOZ residues in edible animal tissues (Xie et al., Citation2019).
Guo et al. prepared a monoclonal antibody to SEM and fabricated a fluorescence immunochromatographic test strip for SEM by conjugating SEM to quantum dot beads (QBs) and generating QBs probe. The LOD of this test strip was 0.247 ng/mL for fish tissues. The strip showed good stability and was stable at 2–8°C for up to 6 months (Guo et al., Citation2019). Following this, the authors fabricated a multiplex fluorescence immunochromatographic strip for the simultaneous detection of AOZ, AHD, SEM, and AMOZ in fish tissues (Guo, Citation2019), and achieved LOD values of 0.4, 0.4, 0.5, and 0.5 ng/mL, respectively. Spike-and-recovery assessment on the intra-assay and inter-assay repeatability and accuracy of the test strip showed an intra-assay recovery of 80–110% and an inter-assay recovery of 80–100%, while the coefficient of variation was below 15% for both cases. The cross-reactivity among AOZ, AHD, SEM, and AMOZ was found to be less than 0.1%.
2.5. Immunosensor assay
Immunosensors comprise a novel type of biosensors that integrate the specificity of immune response with the high sensitivity of a biosensor. They offer high speed, high sensitivity, strong selectivity, and ease for automation. Immunosensors could be either labelled or label-free, but are all based on solid-phase immunoassay, that is, to detect antibodies or antigens in a sample by immobilizing antigens or antibodies on the surface of the solid support. Label-free immunosensors convert changes in the immune response to photoelectric signals through a transducer, while labelled immunosensors use enzymes, fluorescent reagents, or other tracers that generate a signal in the immune response for detection (Zhu et al., Citation2019). Thompson et al. combined a polyclonal antibody with a SPR sensor and successfully developed an immunobiosensor screening for multiple residues of a range of nitrofuran compounds in avian eyes. A detection capability (CCβ) of less than 1 ng/eye was realized for nitrofurazone (Thompson et al., Citation2010). Jin et al. reported on a novel label-free electrochemical impedimetric immunosensor based on the anti-AMOZ monoclonal antibody. Signals were triggered by the immune response between AMOZ and the antibody. Under optimized conditions, the relative change in impedance was proportional to the logarithmic value of AMOZ concentration with a LOD of 1.0 ng/mL. This immunosensor exhibited high sensitivity, a wide linear range, and good stability (Jin et al., Citation2011). Subsequent studies (Jin et al., Citation2013; Jin et al., Citation2014; Yang et al., Citation2011) employed the impedimetric immunosensor for the detection of AOZ, SEM, and AHD residues, and the LOD values reached were 20.0, 1.0, and 2.0 ng/mL, respectively.
3. Conclusions and prospects
The various types of immunoassays for nitrofuran drugs have their unique characteristics, and their strengths and weaknesses are compared in . A rational selection of one or multiple methods should be made during analysis based on actual needs.
Table 1. Comparison of immunoassay of nitrofurans in animal food.
The immunoassay is based on the specificity of the antigen–antibody reaction. It is able to detect trace amounts of the target substance and is sensitive, fast, and efficient, making it the most important high-speed method for the screening of nitrofuran residues. Current modes of immunoassay deployment enable efficient and rapid detection of nitrofuran drugs, but drawbacks are still present, such as high false-positive rate, the ability to detect only one component, and the requirement for analyte derivatization before detection. The complex and varied effects of matrices in the samples also restrict the adaptation of the immunoassay to different scenarios (Wang et al., Citation2013). A more in-depth and systematic study on immunoassay methodology is needed to solve these problems. The antibody is the key reagent in an immunoassay. The fabrication of specific and sensitive antibodies to the metabolites of the four nitrofuran drugs and the subsequent realization of derivatization-free testing are the focuses of future research. The establishment of direct and multi-residue detection, and the reduction of pre-processing time and testing cost are still the focal points for nitrofuran immunoassay development. With the rapid progress in immunology, molecular biology, and analytical chemistry, new immunoassay techniques that improve the immunoassay of nitrofurans will emerge.
Acknowledgements
This project received funding from Chinese Ministry of Science and Technology for the National Key R & D Program of China (2017YFE0110800) and H2020 EU–China-Safe (727864).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- An, J., Gu, L. S. T., Song, B., Jiang, N., Fu, X. C., & Zhou, X. L. (2019). Determination of furazolidone metabolite (AOZ) by direct competitive chemiluminescence immunoassay (CLIA). Analytical Instrumentation, 05, 65–70. https://doi.org/10.3969/j.issn.1001-232x.2019.05.015.
- Announcement No.1077-2-2008 of the Ministry of Agriculture Determination of nitrofuran metabolites residues in fishery products by high performance liquid chromatography[S].
- Announcement No.783-1-2006 of the Ministry of Agriculture Determination of nitrofuran metabolic residues in aquatic products by LC-MS/MS method[S].
- Chang, C., Peng, D. P., Wu, J. E., Wang, Y. L., & Yuan, Z. H. (2008). Development of an indirect competitive ELISA for the detection of furazolidone marker residue in animal edible tissues. Journal of Agricultural and Food Chemistry, 56(5), 1525–1531. https://doi.org/10.1021/jf0726684
- Chen, Q. (2018). Immunomagnetic separation and enzymetic reaction-based biosensors for detection of foodborne bacteria. China Agriculture University.
- Chen, Q., Chen, Y. N., Chen, D. H., Lin, H. H., & Shi, X. A. (2017). Establishment of enzyme-linked immunosorbent assay method for detecting furazolidone based on single chain fragment antibody. Food Science, 38(20), 242–247. https://doi.org/10.7506/spkx1002-6630-201720035.
- Chen, Y. N., Chen, H., Shi, X. A., Ye, X. J., & Fan, H. P. (2016). Preparation and application of monoclonal antibody against furazolidone. Food Science, 37(03|3), 151–157. https://doi.org/10.7506/spkx1002-6630-201603029.
- Cooper, K. M., Elliott, C. T., & Kennedy, D. G. (2004). Detection of 3-amino-2-oxazolidinone (AOZ), a tissue-bound metabolite of the nitrofuran furazolidone, in prawn tissue by enzyme immunoassay. Food Additives and Contaminants: Part A, 21(9), 841–848. https://doi.org/10.1080/02652030412331272476
- Cooper, K. M., Samsonova, J. V., Plumpton, L., Elliott, C. T., & Kennedy, D. G. (2007). Enzyme immunoassay for semicarbazide-the nitrofuran metabolite and food contaminant. Analytica Chimica Acta, 592(1), 64–71. https://doi.org/10.1016/j.aca.2007.04.013
- Deng, L.-H., Dai, J.-B., Xu, Z.-L., Yang, J.-Y., Wang, H., Xiao, Z.-L., Lei, H.-T., Sun, Y.-M., & Shen, Y.-D. (2016). Application of time-resolved fluroimmunoassay for determination of furaltadone metabolite 3-amino-5-morpholinomethyl-2-oxazolidinone. Chinese Journal of Analytical Chemistry, 44(08), 1286–1290. https://doi.org/10.1016/S1872-2040(16)60951-9
- Diblikova, I., Cooper, K. M., Kennedy, D. G., & Franek, M. (2005). Monoclonal antibody-based ELISA for the quantification of nitrofuran metabolite 3-amino-2-oxazolidinone in tissues using a simplified sample preparation. Analytica Chimica Acta, 540(2), 285–292. https://doi.org/10.1016/j.aca.2005.03.039
- Ding, X., Liu, L. Q., Song, S. S., Kuang, H., & Xu, C. L. (2017). Rapid and ultrasensitive detection of 3-amino-2-oxazolidinone in catfish muscle with indirect competitive enzyme-linked immunosorbent and immunochromatographic assays. Food and Agricultural Immunology, 28(3), 463–475. https://doi.org/10.1080/09540105.2017.1297778
- Dou, L., Zhao, B., Bu, T., Zhang, W., Huang, Q., Yan, L., Huang, L., Wang, Y., Wang, J., & Zhang, D. (2018). Highly sensitive detection of a small molecule by a paired labels recognition system based lateral flow assay. Analytical and Bioanalytical Chemistry, 410(13), 3161–3170. https://doi.org/10.1007/s00216-018-1003-0
- Dykman, L. A., & Bogatyrev, V. A. (2007). Gold nanoparticles: Preparation, functionalisation and applications in biochemistry and immunochemistry. Russian Chemical Reviews, 76(2), 181–194. https://doi.org/10.1070/RC2007v076n02ABEH003673
- Fang, Z., Jiang, B., Wu, W., Xiang, Z., Ouyang, C., Huang, T., Chen, J., & Zeng, L. (2013). ELISA detection of semicarbazide based on a fast sample pretreatment method. Chemical Communications, 49(55), 6164–6166. https://doi.org/10.1039/c3cc42790k
- Fang, Q., Wang, L., Hua, X., Wang, Y., Wang, S., Cheng, Q., Cai, J., & Liu, F. (2015). An enzyme-linked chemiluminescent immunoassay developed for detection of butocarboxim from agricultural products based on monoclonal antibody. Food Chemistry, 166(1), 372–379. https://doi.org/10.1016/j.foodchem.2014.06.060
- Fang, F., Zheng, J. J., Sun, Z. W., Li, R. R., Liu, W., & Yang, L. (2019). Application progress of fluorescence detection technology for veterinary drug residues in dairy products. China Food Safety Magazine, 0(1), 31–33. https://doi.org/10.3969/j.issn.1674-0270.2019.01.020.
- Gao, J. X., Zang, Y. X., Du, X. J., Li, P., & Wang, S. (2019). A rapid detection method of Staphylococcus aureus by combining the recombinant polymerase isothermal amplification and latex microsphere test strips. Food Research and Development, 40(1), 168–172.
- Guo, H. C. (2019). Preparation of a fluorescent immunochromatographic strip for simultaneous detection of four nitrofuran metabolites. Meat Research, 33(04), 29–35. https://doi.org/10.7506/rlyj1001-8123-20190322-065.
- Guo, H. C., Cui, H. B., & Xu, D. M. (2019). Preparation of monoclonal antibody and fluorescence immunochromatographic test strip for detecting furacilin metabolites. Meat Research, 33(03), 46–51. https://doi.org/10.7506/rlyj1001-8123-20181211-229.
- Huang, D. Y., Feng, M., Li, Y. N., Gao, L. X., Gao, W. J., & Yang, X. S. (2017). Determination of nitrofurazone metabolite by biotin-avidin enzyme-linked immunosorbent assay. Journal of Food Safety and Quality, 8((02|2)), 394–401.
- Jiang, W., Luo, P., Wang, X., Chen, X., Zhao, Y., Shi, W., Wu, X., Wu, Y., & Shen, J. (2012). Development of an enzyme-linked immunosorbent assay for the detection of nitrofurantoin metabolite, 1-amino-hydantoin, in animal tissues. Food Control, 23(1), 20–25. https://doi.org/10.1016/j.foodcont.2011.05.014
- Jin, W. J., Yang, G. J., Shao, H. X., & Qin, A. J. (2013). A novel label-free impedimetric immunosensor for detection of semicarbazide residue based on gold nanoparticles-functional chitosan composite membrane. Sensors and Actuators B-Chemical, 188, 271–279. https://doi.org/10.1016/j.snb.2013.07.031
- Jin, W. J., Yang, G. J., Shao, H. X., & Qin, A. J. (2014). A label-free impedimetric immunosensor for detection of 1-aminohydantoin residue in food samples based on sol–gel embedding antibody. Food Control, 39, 185–191. https://doi.org/10.1016/j.foodcont.2013.11.001
- Jin, W., Yang, G., Wu, L., Wang, Q., Shao, H., Qin, A., Yu, B., Li, D., & Cai, B. (2011). Detecting 5-morpholino-3-amino-2-oxazolidone residue in food with label-free electrochemical impedimetric immunosensor. Food Control, 22(10), 1609–1616. https://doi.org/10.1016/j.foodcont.2011.03.017
- Le, T., Xie, Y., Zhu, L. Q., & Zhang, L. (2016). Rapid and sensitive detection of 3-amino-2-oxazolidinone using a quantum dot-based immunochromatographic fluorescent biosensor. Journal of Agricultural and Food Chemistry, 64(45), 8678–8683. https://doi.org/10.1021/acs.jafc.6b03732
- Le, T., Zhang, Z. H., Wu, J., Shi, H. X., & Cao, X. D. (2018). A fluorescent immunochromatographic strip test using a quantum dot-antibody probe for rapid and quantitative detection of 1-aminohydantoin in edible animal tissues. Analytical and Bioanalytical Chemistry, 410(2), 565–572. https://doi.org/10.1007/s00216-017-0756-1
- Li, F., Chen, Y., Li, X. G., Li, X. M., & Yu, F. J. (2016). Progress on determination of nitrofuran and their metabolites in 1animal-derived food. Journal of Food Safety and Quality, 7(6), 2320–2327.
- Li, S. Q., Song, J., Yang, H., Cao, B. Y., Chang, H. F., & Deng, A. P. (2014). An immunochromatographic assay for rapid and direct detection of 3-amino-5-morpholino-2-oxazolidone (AMOZ) in meat and feed samples. Journal of the Science of Food and Agriculture, 94(4), 760–767. https://doi.org/10.1002/jsfa.6423
- Li, Y. N., Wang, R., Sun, Y. Y., Yang, D. Y., Huang, Z. Q., Wang, Y. G., … Huang, D. Y. (2015). Determination of nitrofurantion metabolite by direct competitive enzyme-linked immunosorbent assay. Journal of Food Safety and Quality, 6((09|9)), 3723–3729.
- Li, M., Yang, H., Li, S., Liu, C., Zhao, K., Li, J., Jiang, D., Sun, L., Wang, H., & Deng, A. (2015). An ultrasensitive competitive immunochromatographic assay (ICA) based on surface-enhanced Raman scattering (SERS) for direct detection of 3-amino-5-methylmorpholino-2-oxazolidinone (AMOZ) in tissue and urine samples. Sensors and Actuators: B Chemical, 211, 551–558. https://doi.org/10.1016/j.snb.2014.12.135
- Li, B., Yu, S. M., Hu, R., & Yu, S. J. (2018). Preparation of nitrofurazone metabolite hapten and its applications in immunoassay. Modern Food Science and Technology, 34(03|3), 90–94. https://doi.org/10.13982/j.mfst.1673-9078.2018.03.013.
- Liang, G. D., Mao, X., Huang, C. G., Zhao, S. S., Jin, W., Zhang, D. J., & Ji, M. Q. (2019). Full-automatic magnetic particle chemiluminescence enzyme-linked immunoassay for the detection of nitrofurans residues in animal derived food. Environmental Science Technology, 42(01|1), 178–183. https://doi.org/10.19672/j.cnki.1003-6504.2019.01.026.
- Liu, H., Liang, D. P., Hua, T. G., She, Z. Y., Deng, X. F., Wu, S. Z., & Wu, B. (2013). Progress in analytical methods of nitrofuran antibiotics and their metabolites in food: A review. Journal of Food Safety and Quality, 4(2), 383–386.
- Liu, F. Y., Shen, Y. D., Xiao, Z. L., Yang, J. Y., Sun, Y. M., Wang, H., & Xu, Z. L. (2015). Enhancing the immunogenicity of hapten 3-amino-5-morpholinomethyl-2-oxazolidone (AMOZ) using dendrimer as a carrier. Modern Food Science and Technology, 31(08|8), 31–36. https://doi.org/10.13982/j.mfst.1673-9078.2015.8.006.
- Liu, W., Zhao, C. B., Zhang, Y. L., Lu, S. X., Liu, J. T., & Xi, R. (2007). Preparation of polyclonal antibodies to a derivative of 1-aminohydantoin (AHD) and development of an indirect competitive ELISA for the detection of nitrofurantoin residue in water. Journal of Agricultural and Food Chemistry, 55(17), 6829–6834. https://doi.org/10.1021/jf070620k
- Lu, X. W., Liang, X. L., Dong, J. H., Fang, Z. Y., & Zeng, L. W. (2016). Lateral flow biosensor for multiplex detection of nitrofuran metabolites based on functionalized magnetic beads. Analytical and Bioanalytical Chemistry, 408(24), 6703–6709. https://doi.org/10.1007/s00216-016-9787-2
- Luo, X. Q., Fang, H. L., Zhang, D. H., Sun, Z., Zhao, Z. M., & Cui, T. T. (2013). Development of an enzyme linked immunosorbent assay kit for furantoin metabolite residues determination in animal tissue. Science and Technology of Food Industry, 34((04|4)), 59–62.
- Mu, Q. (2014). Preparation of quantum dots based fluorescence probes and their application in detection. East China University of Science and Technology.
- Pimpitak, U., Putong, S., Komolpis, K., Petsom, A., & Palaga, T. (2009). Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for detection of the furaltadone metabolite, AMOZ, in fortified shrimp samples. Food Chemistry, 116(3), 785–791. https://doi.org/10.1016/j.foodchem.2009.03.028
- Shen, Y.-D., Xu, Z.-L., Zhang, S.-W., Wang, H., Yang, J.-Y., Lei, H.-T., Xiao, Z.-L., & Sun, Y.-M. (2012). Development of a monoclonal antibody-based competitive indirect enzyme-linked immunosorbent assay for furaltadone metabolite AMOZ in fish and shrimp samples. Journal of Agricultural and Food Chemistry, 60(44), 10991–10997. https://doi.org/10.1021/jf302913h
- Shen, Y. D., Zhang, S. W., Cai, Z. T., Lei, H. T., Xiao, Z. L., Wang, H., & Sun, Y. M. (2009). Determination of nitrofurazone metabolite using fluorescence polarisation immunoassay. Journal of Instrumental Analysis, 28(01|1), 27–31. +36. https://doi.org/10.3969/j.issn.1004-4957.2009.01.006.
- Song, J., Yang, H., Wang, Y. Z., Si, W. H., & Deng, A. P. (2012). Direct detection of 3-amino-5-methylmorpholino-2-oxazolidinone (AMOZ) in food samples without derivatisation step by a sensitive and specific monoclonal antibody-based ELISA. Food Chemistry, 135(3), 1330–1336. https://doi.org/10.1016/j.foodchem.2012.05.107
- Sperling, R. A., Rivera gil, P., Zhang, F., Zanella, M., & Parak, W. J. (2008). Biological applications of gold nanoparticles. Chemical Society Reviews, 37(9), 1896–1908. https://doi.org/10.1039/b712170a
- Tang, Y., Xu, X. L., Liu, X., Huang, X. M., Chen, Y. Q., Wang, W. Z., & Xiang, J. J. (2011). Development of a lateral flow immunoassay (LFA) strip for the rapid detection of 1-aminohydantoin in meat samples. Journal of Food Science, 76(6), T138–T143. https://doi.org/10.1111/j.1750-3841.2011.02217.x
- Tang, H. M., Zeng, F., & Li, C. H. (2016). Progress on the detection of nitrofurans drugs residues and their metabolites in food. Journal of Food Safety and Quality, 7(10), 3952–3959.
- Thompson, C. S., Traynor, I. M., Fodey, T. L., Crooks, S. R. H., & Kennedy, D. G. (2010). Screening method for the detection of a range of nitrofurans in avian eyes by optical biosensor. Analytica Chimica Acta, 700(1), 177–182. https://doi.org/10.1016/j.aca.2010.10.048.
- Tu, Y., Chen, Y. Q., & Zhao, Y. Q. (1999). Determination of progesterone, testosterone and pituitary prolactin by magnetic separation enzyme-labeled immunoassay. Labeled Immunoassay and Clinical Practice, 2, 132–133.
- Vass, M., Diblikova, I., Kok, E., Stastny, K., Frgalova, K., Hruska, K., & Franek, M. (2008). In-house validation of an ELISA method for screening of semicarbazide in eggs. Food Additives and Contaminants: Part A, 25(8), 930–936. https://doi.org/10.1080/02652030701883203
- Wang, F. (2016). Study on preparation of bispecific monoclonal antibody against furaltadone metabolite (AMOZ) derivative and LMG[D]. South China Agricultural University.
- Wang, J. (2019). Immunochromatographic assays for quantitative determination of nitrofuran metabolite residues in animal-derived food[D]. Southwest University.
- Wang, Y. T. (2017). Prohibited fishery drugs-toxicity and harm of nitrofurans. China Fish, 4, 85–86.
- Wang, X. D., Lin, H., Sui, J. X., & Cao, L. M. (2013). The effect of fish matrix on the enzyme-linked immunosorbent assay of antibiotics. Journal of the Science of Food and Agriculture, 93(7), 1603–1609. https://doi.org/10.1002/jsfa.5931
- Wang, Q., Liu, Y. C., Wang, M. Y., Chen, Y. J., & Jiang, W. (2018). A multiplex immunochromatographic test using gold nanoparticles for the rapid and simultaneous detection of four nitrofuran metabolites in fish samples. Analytical and Bioanalytical Chemistry, 410(1), 223–233. https://doi.org/10.1007/s00216-017-0714-y
- Wang, R., Lv, Y. X., Huang, D. Y., Wang, Y. G., & Li, T. (2015). Study of chemiluminescent enzyme immunoassay method for nitrofurantoin metabolite. China Journal of Veterinary Medicine, 49(4), 35–41.
- Wen, Y. J., Zhang, F., Du, X. J., & Zhang, H. X. (2013). Synthesis of furazolidone marker molecularly imprinted polymer and the establishment of the detection method. Applied Chemical Industry, 42((07|7)), 1347–1350.
- Xie, Y., Wu, J., Shi, H. X., & Le, T. (2019). A fluorescent immunochromatographic strip using quantum dots for 3-amino-5-methylmorpholino-2-oxazolidinone (AMOZ) detection in edible animal tissues. Food and Agricultural Immunology, 30(1), 208–221. https://doi.org/10.1080/09540105.2019.1566301
- Xie, Y., Zhang, L., & Le, T. (2017). An immunochromatography test strip for rapid, quantitative and sensitive detection of furazolidone metabolite, 3-amino-2-oxazolidinone, in animal tissues. Food and Agricultural Immunology, 28(3), 403–413. https://doi.org/10.1080/09540105.2017.1293013
- Xu, Z.-L., Shen, Y.-D., Sun, Y.-M., Campbell, K., Tian, Y.-X., Zhang, S.-W., Lei, H.-T., & Jiang, Y.-M. (2013). Novel hapten synthesis for antibody production and development of an enzyme-linked immunosorbent assay for determination of furaltadone metabolite 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). Talanta, 103, 306–313. https://doi.org/10.1016/j.talanta.2012.10.059
- Xu, Z.-L., Zhang, S.-W., Sun, Y.-M., Shen, Y.-D., Lei, H.-T., Jiang, Y.-M., A. Eremin, S., Yang, J.-Y., & Wang, H. (2013). Monoclonal antibody-based fluorescence polarization immunoassay for high throughput screening of furaltadone and its metabolite AMOZ in animal feeds and tissues. Combinatorial Chemistry & High Throughput Screening, 16(6), 494–502. https://doi.org/10.2174/1386207311316060010
- Yan, L., Dou, L., Bu, T., Huang, Q., Wang, R., Yang, Q., Huang, L., Wang, J., & Zhang, D. (2018). Highly sensitive furazolidone monitoring in milk by a signal amplified lateral flow assay based on magnetite nanoparticles labeled dual-probe. Food Chemistry, 261, 131–138. https://doi.org/10.1016/j.foodchem.2018.04.016
- Yan, X. D., Hu, X. Z., Zhang, H. C., Liu, J., & Wang, J. P. (2012). Direct determination of furaltadone metabolite, 3-amino-5-morpholinomethyl-2-oxazolidinone, in meats by a simple immunoassay. Food and Agricultural Immunology, 23(3), 203–215. https://doi.org/10.1080/09540105.2011.615060
- Yang, G., Jin, W., Wu, L., Wang, Q., Shao, H., Qin, A., Yu, B., Li, D., & Cai, B. (2011). Development of an impedimetric immunosensor for the determination of 3-amino-2-oxazolidone residue in food samples. Analytica Chimica Acta, 706(1), 120–127. https://doi.org/10.1016/j.aca.2011.08.018
- Yao, J. Y., Shen, J. Y., Hao, G. J., Xu, Y., Pan, X. Y., & Yin, W. L. (2009). Preparation of anti-semicarbazide monoclonal antibodies and ELISA for detection of semicarbazide residues. Chinese Journal of Animal Health Inspection, 26(9), 40–43. https://doi.org/10.3969/j.issn.1005-944X.2009.09.021.
- Yu, W. L., Liu, M. X., Liu, R. B., Sang, Y. X., Wang, S., & Wang, X. H. (2020). Development of biomimetic enzyme-linked immunosorbent assay based on molecular imprinting technique for semicarbazide detection. Food and Agricultural Immunology, 31(1), 17–32. https://doi.org/10.1080/09540105.2019.1692789
- Zhang, S. W., Shen, Y. D., & Sun, Y. M. (2010). Monoclonal antibody-based fluorescence polarization immunoassay for furazolidone in feed. Analytical Letters, 43(17), 2716–2729. https://doi.org/10.1080/00032711003731449
- Zhao, Y. L., Li, Y., Sang, L. Y., Wang, Z. G., Xiao, M., Wang, W. L., & Chen, X. X. (2018). Development and application of time-resolved fluoroimmunoassay rapid test strip of furazolidone metabolites. Journal of Food Safety and Quality, 9(19), 5187–5194.
- Zhao, C. H., Zhang, Y., Wang, X. L., Zong, C., Li, Y., & Gao, Y. Y. (2014). Indirect competitive time-resolved fluoroimmunoassay for detecting the metabolite residues of furazolidone in crucian (Carassius auratus). Chinese Fishery Quality and Standards, 4(3), 23–28.
- Zhou, Q., Gao, Y. S., Tang, M. J., Zhang, J., Zhang, X. Y., Gu, R., … Wan, Y. (2016). Progress on determination methods of nitrofuran metabolites in foods. Journal of Food Safety and Quality, 7(9), 3285–3290.
- Zhu, X. T., Zhang, Y., Peng, H. W., & Feng, G. (2019). Application of immunosensors in food safety testing. Journal of Food Safety and Quality, 10(03), 626–632.