Abstract
Contaminated sediments, as a secondary pollution source in rivers and lakes, are of critical importance to water quality. More and more attention thus has been paid to understand the release mechanisms of nutrients from river sediments, especially in estuary and water transfer areas. In this work, flume experiments were conducted to measure the release characteristics of total dissolved phosphorus (TDP) and nitrogen (TDN) from sediments collected from a river bed near Lake Tai under various flow conditions. The release of TDP and TDN was the most dramatic in the initial 30 min, then slowed down from 30 to 60 min, and finally achieved equilibria. Total amount of TDP and TDN released and their equilibrium concentrations were all significantly increased with the increase of flow rate, but slow down after a critical velocity was reached, which could be described as a Logarithmic relationship. A process-based mathematical model was established to describe the distribution of nutrients in the water columns and model simulations matched experimental data well. The re-suspension of sediments induced by flow rate higher than the threshold, is the dominant process affecting nutrient release from sediments.
1. Introduction
The mass transfer processes of particulates or dissolved substances like nutrients, heavy metals, and other potentially harmful substances between contaminated sediments and overlying water columns in rivers, lakes, reservoirs, and estuaries are of crucial importance to the water environment [Citation1–3]. The fate and transport of nutrients/sediments in water columns are controlled by hydrodynamics, especially at the sediment-water interface [Citation4]. Nutrients are often rich in sediments and pore water, change of dynamic conditions thus has a significant impact on nutrient release from sediments into water column [Citation5].
Because the dynamics release of nutrients from sediments is still not well-understood, the effects of hydrodynamic conditions on nutrient release from sediments become a growing area of focus. Laboratory experiments with oscillating grid, annular tank, and open water channel have been used to study the contaminated sediments release regularity under different flow conditions [Citation6–9]. The relationship between nutrient concentrations in water column and flow velocity was determined from the experimental data. With improved understanding of phosphorus in sediments, it is generally accepted that the interaction between the sediments and overlying water take place only within the thin top layer of sediments with a thickness of no more than 10 cm [Citation10,11]. It is also recognized that release of phosphorus in dynamic condition is much higher than that in static condition [Citation12]. The effect of flow velocity in the overlying water column on the transport across the sediment-water interface has been found to be significant because the thickness of the diffusive boundary layer is determined or at lease influenced by the mean velocity or velocity profile above the sediment bed [Citation13–15]. The velocity profile thus could influence the characteristics of nutrient release from sediments.
Several theoretical or empirical models have been developed to accurately quantify the exchange fluxes at the sediment-water interface. The majority of the models have been developed in terms of diffusive flux under quasi-steady state molecular diffusion process using Fick’s first law [Citation16–19]. However, the quasi-steady conditions are not always applicable under many circumstances, particularly with the presence of several combined transport mechanisms acting on both overlying water column and permeable sediment bed. Unlike the static release process controlled by molecular diffusion alone, the release process due to the re-suspension/settling of the sediments is more dynamic and significant in rivers, shallow lakes and estuarine environments [Citation20,21]. Dynamic release from the disturbed sediments to the overlying water is often time dependent and to a great extent controlled by the frequent suspension/settling of sediments [Citation22]. The flow velocity profile above the sediments could impact the suspension/settling process remarkedly. This study investigated the release and transport of nutrients from sediments under different dynamic conditions. Laboratory experiments and model simulations were applied to determine the release characteristics of nutrients from sediments into water column under different flow rate conditions.
2. Materials and methods
2.1. Sediment sampling
Sediment samples were collected with the Petersen sampler from a river bed behind Yubu bridge in Jiaxing City (N 30°43′48.74″, E 120°52′7.51″) near Lake Tai in June 2014. Soil within 15 cm bellow the sediments surface was collected and saved in ice box and then sent back to laboratory. After removal of weeds or other impurities in sampled sediments, all samples were saved in a freezer at 4 °C.
2.2. Experimental device
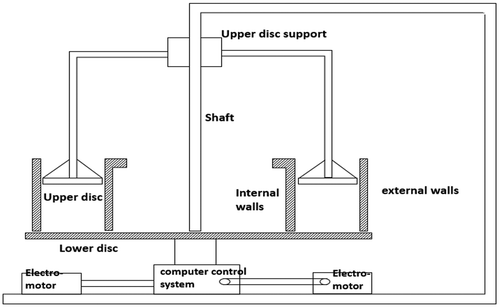
The experiment was carried out in a circular flume (Figure ) in the hydrodynamic laboratory in Nanjing Institute of Geography and Limnology, Chinese academy of Science. The circular flume is composed of upper/lower disc, variable speed drive system and controller, and measurement system. The lower disk is a plexi – glass circular groove with inner diameter 100 cm and outer diameter 120 cm. The upper disc is a ring covering the groove. The upper and lower disc can move in an opposite way driven by speed regulating motor automatically controlled by computer. During the experiment, flow was generated under the action of shear stress. The speeds of upper disc and lower disc are rationally allocated, so that the lateral secondary flow disappeared and the flow in the tank is basically uniform [Citation23]. The volume of water tank is small so that the amount of sediments required for the experiment is low, about 5 cm thick. Flow velocity and water depth can be easily controlled, and change of hydrodynamic conditions can be easily achieved so that the results of the experiment can represent the actual situation in the river.
2.3. Experimental procedures
Experiments were conducted in the hydrodynamic laboratory in Nanjing Institute of Geography and Limnology, Chinese academy of Science. The air temperature was 25 °C. Sediments was laid evenly in the bottom of the tank, and gently pressed to make it in a relatively flat state. The thickness of sediments layer was 0.05 m. Tap water was then slowly poured in till the depth of water was 0.25 m. Then the water column and sediments system was let sit for 24 h before experiment started. By adjusting the speed of the device, the flow velocity was set to 0.05, 0.10, 0.15, 0.20, 0.25 and 0.30 m/s, respectively. The flow rate of the experiment started from zero, and accelerated to the required flow rate for 3 h. Samples were collected at 0.25, 0.12, and 0.01 m above from the surface of sediments with three cross sections uniformly distributed in the annular flume. Water samples were taken at 0, 10, 20, 30, 40, 60, 90, 120, and 180 min at every flow velocity using 50 mL plastic bottles.
2.4. Analytical methods
The water samples were all filtered through 0.45 μm filter before testing. Total dissolved phosphorus (TDP) and total dissolved nitrogen (TDN) were analyzed by flow injection apparatus (Skalar San++). Sediments diameter were analyzed by Laser particle size analyzer (Saturn DigiSizer II). Other properties of sediments were analyzed in Laboratory of Nanjing Institute of Geography and Limnology.
3. Theory
3.1. Model description
The movement of water and sediments across the sediment-water interface can transport and redistribute chemicals in sediments and water column lying on sediments. Chemicals in sediment-water system typically exist in two phases: (1) dissolved phase in water and (2) particle-associated phase. The process that affect chemical movements and interactions in the environment depending on the phase in which the chemicals were present. The main processes controlling chemical fate and transport include: (1) advection-diffusion; (2) chemical partitioning and phase distribution; (3) erosion; (4) deposition; and (5) transformation processes [Citation24]. For the nutrients transport process in water column in this study, transformation processes were neglected. To model the nutrient release from sediments, it was assumed: (1) chemicals were well mixed in lateral directions, i.e., vertical transport was only considered; (2) chemical concentrations in the sediments layer were constant; and (3) no flow velocity in vertical direction so that advection process in vertical direction was neglected. Chemical fluxes involved in each process could be calculated as follows:(1)
(2)
where: Jn = The net nutrient flux across sediment water interface (g/(m2s)); C = Average nutrient concentrations in water column (g/m3); z = The height of the water column (m); Jd = The dispersive and diffusive flux (g/(m2s)); Jr = The nutrient flux caused by erosion (re-suspension) sediments (g/(m2s)); and Jw = The nutrient flux caused by deposited sediments (g/(m2s)). Each part can be described as follows:
3.1.1. Dispersion/diffusion
(3)
where: Jd = Chemical diffusion flux in vertical direction (g/(m2s)); D = Comprehensive diffusion coefficient (m2/s); C = Average nutrient concentrations in water column (g/m3); C1 = Nutrient concentrations in bottom boundary of column (g/m3); z = Thickness of water column (m).
3.1.2. Re-suspension (erosion)
Re-suspension was the entrainment of sediments from a bottom boundary into a flow by the action of water [Citation25]. Many chemicals were hydrophobic, readily partition between dissolved and particle-associated (particulate) phases. Partitioning was a function of the equilibrium rate at which chemicals sorb (move out of the dissolved phase) and desorb (move back into the dissolved phase) [Citation26,27]. Nutrients adsorbed on particulate matter suspended in the water column can release to water. Processes can be described as follow:
(4)
(5)
where: Js = Sediments re-suspension flux (g/(m3s)); Jr = Nutrient flux caused by sediments re-suspension (g/(m3s)); vr = Sediments re-suspension (erosion) velocity (m/s); Cs = Concentrations of sediments at the bottom boundary (in the bed) (g/m3); Csv = Concentrations of nutrient in sediments at the bottom boundary (g/g); k1 = Kinetic coefficients of particle release (dimensionless).
Method to get entrainment rates including site-specific erosion rate studies or, from the difference between sediments transport capacity and advective fluxes:(6)
where: vr = Re-suspension (erosion) velocity (m/s); Jc = Sediments transport capacity areal flux (g/(m2s)); va = Flow velocity (in the x- or y- direction) (m/s); Cs = Concentrations of sediments entrained in the flow (g/m3); ρb = Bulk density of bed sediments (g/m3).
Summaries of numerous total load transport relationships were provided by Yang (1996) and Julien (1998) [Citation28,29]. A reasonable method to estimate total sediments load was provided by Engelund and Hansen (1967) relationship [Citation30]:
(7)
(8)
where: Cw = Concentrations of entrained sediment particles by weight at the transport capacity (dimensionless); G = Particle specific gravity (dimensionless); va = Advective (flow) velocity (in the down-gradient direction)(m/s); Sf = Friction slope (dimensionless); Rh = Hydraulic radius of flow (m); g = Gravitation acceleration (m/s); dp = Particle diameter (m); Ac = Cross sectional area of flow (m2); Ct = Concentrations of entrained sediment particles at the transport capacity = 106GCw / [G + (1−G)Cw] (g/m3)
3.1.3. Deposition
Nutrients could be deposited with particles from water column to bed. The deposition flux may be expressed as follow:
(9)
where: Jw = Nutrient deposition flux (g/(m2s)); vd = Effective settling (deposition) velocity (m/s); Cw = Concentrations of nutrient in the flow (g/m3); Cs = Concentrations of nutrient in particles (g/m3); distribution coefficient between particles and water phase (dimensionless).
In flowing water, effective settling velocity of a particle could be described as a reduction in the quiescent settling velocity by the probability of deposition [Citation31,32]. For non-cohesive particles, the probability of deposition had been described as a function of bottom shear stress [Citation33–35]. For coarse particles, the critical shear stress for deposition could be computed from a force balance as summarized by QEA (1999), with the particle diameter equal to the mean diameter for a range of particle size in a class (i.e. dp = d50) [Citation36]. For cohesive particles, the probability of deposition had also been described as a function of bottom shear stress [Citation37]. The hydrodynamic condition of the flow had impact on the deposition process through the shear stress.
3.2. Deterministic coefficients
Deterministic coefficients of the model were calculated by Equation (Equation10(11) ),
(10)
where: C(t) = Simulated nutrient concentrations in the overlying water (g/m3); Cw(i) = Measured nutrient concentrations in the overlying water (g/m3); = The average of measured nutrient concentrations in the overlying water (g/m3).
4. Results and discussion
4.1. Release of TDP and TDN from sediments at different flow velocities
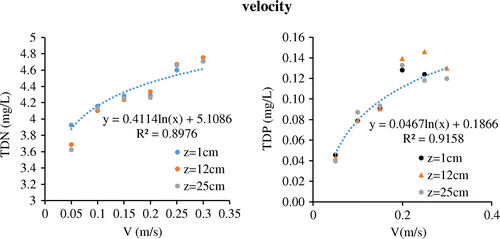
Samples were taken from water column at 1, 12, 25 cm from sediments layer. There were 3 samples in each layer, average concentrations in those 3 samples represented TDP and TDN concentrations in each layer. Average concentrations in water column were represented by mean average concentrations in water layer 1, 12 and 25 cm respectively (mg/L).
Experiments were conducted in five velocities: 0.05, 0.10, 0.15, 0.20, 0.25 and 0.30 m/s, which are commonly found in the rivers around Taihu Lake in estuary and water transfer areas. At the flow rate of 0.05 m/s, almost all the sediments stayed in river bed, contents of TDP in overlying water were low and increased little when achieved equilibrium concentrations (Figure ). Compared with other flow velocities, the equilibrium concentrations of TDN at flow rate of 0.05 m/s was relatively low (Figure ), since nutrients in sediments and pore water were transported into overlying water mainly through dispersion and diffusion processes. When flow rate increased to 0.10, 0.15 and 0.20 m/s, TDP concentrations in overlying water increased from around 0.03 to about 0.08 mg/L within 30 min, then slowly achieved equilibrium after 60 min (Figure ). For TDN, in the early 30 min, the slope of the concentrations at the velocities between 0.10 and 0.20 m/s was larger and the equilibrium concentrations significantly increased compared with that at the velocities of 0.05 m/s (Figure ). It might be due to disturbance (re-suspension) of sediment particles in the bed at relatively high flow velocity, which caused nutrients in pore water to mixing with overlying water. In addition, TDN and TDP on re-suspended sediment particles would also be released into the water column, which lead to increased nutrients concentrations in overlying water. The release curves of TDP and TDN at 0.25 and 0.30 m/s were similar to that at 0.10 to 0.20 m/s, but with higher equilibrium concentrations (Figure ).
Figure 2. TDP concentration variation with time under different flow velocity. (A) flow velocities of 0.05 and 0.1 m/s; (B) flow velocities of 0.15 and 02 m/s; (C) flow velocities of 0.25 and 0.3 m/s.
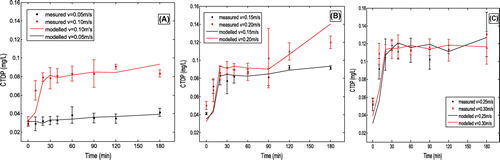
Figure 3. TDN concentration variation with time under different flow velocity. (A) flow velocities of 0.05 and 0.1 m/s; (B) flow velocities of 0.15 and 02 m/s; (C) flow velocities of 0.25 and 0.3 m/s.
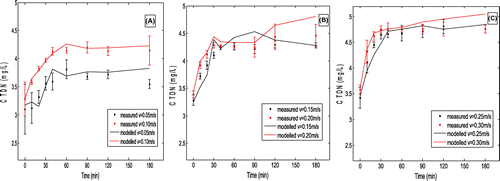
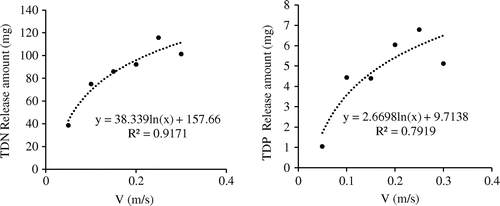
Based on the results in Figures and , nutrient release from the sediments was divided into 3 steps: (1) quickly release stage, (2) mildly release, and (3) equilibrium. From 0.041 to 0.131 mg/L, TDP equilibrium concentrations under flow rate of 0.25 m/s were 6 times of that of 0.05 m/s (Figure ), which were similar to the findings of Zhang et al. (2012). In general, the total amount of TDP and TDN released into the water column increased with the flow velocity (Figure ). Further, the TDP and TDN equilibrium concentrations also increased with flow velocity (Figure ). For both cases, the curves followed a Logarithmic relationship.
4.2. Model simulation
As described above, nutrients concentrations in water column can be described by following mass conservation equation:(11)
After simplification:(12)
The solution of the equation is:(13)
TDP variation under different flow velocity were simulated by equation (Equation13(13) ). In this studies, Jr got by equation listed in Section 3.1.2, vr were calculated by Equation (Equation6
(6) ), Cs and Csv were measured during experiment. k1 and k2 were fitting parameters in the modelling process. Values of parameters for TDN and TDP were listed in Table . Value of parameter k1 under different flow velocity and time for TDN and TDP were showed in Tables and . It was notable that k1 is a variable of both flow velocity and time. Simulation results were in Figures and . Values of k1 for TDN were much less than those for TDP. It was probably due to TDN concentrations in overlying water were much higher than TDP. Comprehensive diffusion coefficients (D) were increased remarkably with flow rates (Table ), which showed the static nutrients releasing process was enhanced by increasing flow velocity. Another important process was sediment re-suspension. Both re-suspension (erosion) velocity (vr) and concentrations of sediments at the bottom boundary (in the bed) (Cs) increased significantly with the increased of flow velocity. With flow rate of 0.05 m/s, no much sediments was suspended in the water column, and vr was 1.08 × 10−4 m/s and Cs was 10 g/m3. While flow rate increased to 0.30 m/s, vr was 3.9 × 10−3 m/s, which was nearly 30 times larger than that with flow velocity of 0.05 m/s; while Cs was 130 g/m3, 13 times larger than that with flow rate of 0.05 m/s. Sediments re-suspension process was also remarkably impacted by the flow velocity, It could be explained by the growing shear stresses with increased velocity. For the deposition process, since values of particles deposition velocity (vd) and distribution coefficient between particles and water (k2) changed very little with flow velocity, suggesting that the effect of deposition process on nutrients in the overlying water was ignorable. The re-suspended process was the dominant nutrients releasing process here.
Table 1. Parameters of the model.
Table 2. Value of parameter k1 under different flow velocity and time (TDP).
Table 3. Value of parameter k1 under different flow velocity and time (TDN).
As showed in Table , value of DC all exceed 0.7 for all the simulations. Suggesting that simulated data were well matched with the measured value.
Table 4. Deterministic coefficient of the modeling result.
4.3. Nutrients transport processes influenced by flow condition
As indicated, nutrients in overlying water were governed by several processes including: dispersive and diffusion processes, release from re-suspension (erosion) and deposition processes. When flow condition changed, all processes mentioned above were influenced. Dispersion/diffusion flux were stronger in high flow velocity than low flow velocity or quiescent conditions. As this process driven by the concentration gradient, so that dispersion/diffusion flux decreased as nutrients concentrations in overlying water increased. However, sediments re-suspension release flux was several orders of magnitude larger than diffusive fluxes under high flow velocity conditions. From the modelling results, nutrient release from re-suspension (erosion) particles was the main factor that accounted for nutrients concentrations variation under different flow rates. Nutrient released from increased re-suspended particles significantly increased with flow velocity, as more particles re-suspended under higher flow velocity. Further, nutrient release from re-suspended particles was a kinematic processes governed by kinetic coefficients of particle release (k1) [Citation38]. Kinetic coefficient (k1) means the distribution of particulate and dissolved form of nutrients. It changed over time till nutrients get balanced between particulate and dissolved form, following the desorption process of Langmuir Adsorption Isotherms. From the modelling results, kinetic coefficients of particle release (k1) were the largest at the beginning of disturbing, however, gradually decreased as time elapsed (Tables and ). This is consistent with the experimental results that more nutrient released from sediments to water at the initial 30 min. Further, the kinetic coefficients of particle release (k1) with lower flow rates were higher than those with higher flow rates. For example, when the flow rate was 0.10 m/s, k1 was 0.9 at the beginning of the experiment, which was almost 18 times larger than when flow rate of 0.30 m/s.
Deposition is the sedimentation (loss) of material entrained in a flow to a bottom boundary by gravity. The deposition process is influenced by many factors including particle density, diameter, shape, and fluid turbulence. In low flow rate or quiescent conditions, deposition velocity is larger than high flow rate conditions. As a result of turbulence and other factors, not all particles settling through a column of flowing water will necessarily reach the sediment-water interface or be incorporated into the sediment bed [Citation31,39]. The probability of deposition varies with shear stress near the sediment bed and particle size. As particle size decreases or shear stress increases, the probability of deposition decreases. As a result, effective settling velocities in flowing water can be much less than quiescent settling velocities. With increase of flow rate, re-suspension release flux increased, however, nutrients deposition flux decreased, induced nutrient release flux significantly increased.
5. Conclusion
Nutrients (TDP and TDN) release from sediments into overlying water column under different flow velocity were measured with laboratory experiments and simulated with mathematical model. On static conditions, nutrients were released to water column through diffusion. However, when flow rate increased, large amount of nutrients in sediments and pore water were released into overlying water. Experimental study showed that release process can be divided into 3 stages: quickly release in initial 30 min, mildly release from 30 to 60 min, and finally equilibrium. In addition, total release amount of TDP and TDN and their equilibrium concentrations increased with the increase of flow rate, but slowed down after reaching the critical velocity. Mathematic Model were used to describe the transport of nutrients in water column under different flow rate conditions. The modeling results indicated that nutrient release from re-suspension particles was the main factor controlling nutrient release under different flow velocity conditions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Major science and technology projects of National water pollution control and Governance [grant number 2014ZX07101-011]; Chinese Academy of Sciences (CAS) Key Research Program [grant number KZZD-EW-13]; the National Natural Science Foundation of China [grant number 51509069, 41323001, 51539003]; the Fundamental Research Funds for the Central Universities [grant number B14020167]; the Special Fund of State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering [grant number 20145027312].
References
- Huettel M, Røy H, Precht E, et al. Hydrodynamical impact on biogeochemical processes in aquatic sediments. Hydrobiologia. 2003;494:231–236.10.1023/A:1025426601773
- Havens KE, Fukushima T, Xie P, et al. Nutrient dynamics and the eutrophication of shallow lakes Kasumigaura (Japan), Donghu (PR China), and Okeechobee (USA). Environ Pollut. 2001;111(2):263–272.10.1016/S0269-7491(00)00074-9
- Huang Q, Wang Z, Wang C, et al. Phosphorus release in response to pH variation in the lake sedimentswith different ratios of iron-bound P to calcium-bound P. Chem Speciation Bioavailability. 2005;17(2):55–61.10.3184/095422905782774937
- Huang J, Xu Q, Xi B, et al. Impacts of hydrodynamic disturbance on sediment resuspension, phosphorus and phosphatase release, and cyanobacterial growth in Lake Tai. Environ Earth Sci. 2015;74(5):3945–3954.10.1007/s12665-015-4083-6
- You B, Zhong J, Fan C, et al. Effects of hydrodynamics process on phosphorus fluxes from sediment in large, shallow Taihu Lake. J Environ Sci. 2007;19:1055–1060.10.1016/S1001-0742(07)60172-7
- Zhang L. Phosphorus release and absorption of surficial sediments in taihu lake under simulative disturbing conditions. J Lake Sci. 2001;13(1):35–42.
- Bin Li, Zhang K, Zhong BC, et al. An experimental study on release of pollutants from sediment under hydrodynamic conditions. J Hydrodyn. 2008;23(2):126–133 . (in Chinese).
- Zhang L. Kinetics of pollutants release from sediments. Techniques & Equipment for Environmental Pollution Control. 2003;4(2):22–26.(in Chinese)
- Pang Y, Han T, Li YP, et al. Simulation and model computation on dynamic release of nutrition factors of bottom mud in taihu lake. Environ Sci. 2007;28(9):1960–1964.
- Pratihary AK, Naqvi SWA, Narvenkar G, et al. Benthic mineralization and nutrient exchange over the inner continental shelf of western India. Biogeosci Discuss. 2013;10(6):9603–9659.10.5194/bgd-10-9603-2013
- Wang JY, Pant HK. Phosphorus sorption characteristics of the Bronx River bed sediments. Chem Speciation Bioavailability. 2010;22(3):171–181.10.3184/095422910X12827492153851
- Sun X, Zhu G, Luo L, et al. Experimental study on phosphorus release from sediments of shallow lake in wave flume. Sci Chin. 2006;49(S1):92–101.10.1007/s11430-006-8109-5
- Dade WB. Near-bed turbulence and hydrodynamic control of diffusional mass transfer at the sea floor. Limnol Oceanogr. 1993;38(1):52–69.10.4319/lo.1993.38.1.0052
- Inoue T, Nakamura Y. Effects of hydrodynamic conditions on DO transfer at a rough sediment surface. J Environ Eng. 2011;137(1):28–37.10.1061/(ASCE)EE.1943-7870.0000293
- Fries JS. Predicting interfacial diffusion coefficients for fluxes across the sediment-water interface. J Hydraulic Eng. 2007;133(3):267–272.10.1061/(ASCE)0733-9429(2007)133:3(267)
- Thouvenot M, Billen G, Garnier J. Modeling nutrient exchange at the sediment–water interface of river systems. J Hydrol. 2007;341(1–2):55–78.10.1016/j.jhydrol.2007.05.001
- Chen XJ, Sheng YP. Three-dimensional modeling of sediment and phosphorus dynamics in lake okeechobee, florida: spring 1989 simulation. J Environ Eng. 2005;131(3):359–374.10.1061/(ASCE)0733-9372(2005)131:3(359)
- Wang H, Appan A, Gulliver JS. Modeling of phosphorus dynamics in aquatic sediments: I – model development. Water Res. 2003;37(16):3928–3938.10.1016/S0043-1354(03)00304-X
- Kelderman P, Ang’Weya RO, Rozari PD, et al. Sediment characteristics and wind-induced sediment dynamics in shallow lake markermeer, the netherlands. Aquat Sci. 2012;74(2):1–13.
- Wilson RF, Fennel K, Mattern JP. Simulating sediment–water exchange of nutrients and oxygen: a comparative assessment of models against mesocosm observations. Cont Shelf Res. 2013;63(4):69–84.10.1016/j.csr.2013.05.003
- Brady DC, Testa JM, Toro DM, et al. Sediment flux modeling: calibration and application for coastal systems. Estuarine Coastal Shelf Sci. 2013;117(1):107–124.10.1016/j.ecss.2012.11.003
- Hu K, Pang Y, Wang H, et al. Simulation study on water quality based on sediment release flume experiment in Lake Taihu, China. Ecol Eng. 2011;37(4):607–615.10.1016/j.ecoleng.2010.12.022
- Li Y, Pang Y, Jun LU, et al. On the relation between the release rate of TN, TP from sediment and water velocity. J Lake Sci. 2004;16(4):318–324.
- Dudley LM, Grismer ME, Suarez DL, et al. Hydrodynamics and chemical transport in the root zone and shallow ground water system: modeling. Groundwater Management ASCE. San Antonio, Texas, United States: Randall J. Charbeneau; 2014. p. 385–389.
- Shi JZ. Tidal resuspension and transport processes of fine sediment within the river plume in the partially-mixed Changjiang River estuary, China: a personal perspective. Geomorphology. 2010;121(3–4):133–151.10.1016/j.geomorph.2010.04.021
- Thomann RV. Principles of surface water quality modeling and control. New York (NY): Harper & Row; 1987.
- Chapra SC. Surface water-quality modeling. New York (NY): McGraw-Hill; 1997. p. 56–64.
- Yang CT. Sediment transport: theory and practice. Int J Sediment Res. 1996;2:87–88.
- Julien PY. Erosion and sedimentation. Eos Trans Am Geophys Union. 1995;23(1):312–313.
- Engelund FA, Hansen E. A monograph on sediment transport in alluvial sreams. Hydrotech Constr. 1967;33(7):699–703.
- Krone RB. Flume studies of the transport of sediments in estuarial shoaling processes. Final report. Berkeley: Hydraulic Engineering Laboratory and Sanitary Engineering Research Laboratory, University of California; 1962.
- Mehta A, McAnally W, Hayter E, et al. Cohesive sediment transport. II: application. J Hydraul Eng. 1989;115–8:1094–1112.
- Gessler J. The beginning of bedload movement of mixtures investigated as natural armouring in channels. Technical report no. 69. The Laboratory of Hydraulic Research and Soil Mechanics, Swiss Federal Institute of Technology, Zurich (translation by W. M. Keck Laboratory of Hydraulics and Water Resources, California Institute of Technology, Pasadena, California); 1965.
- Gessler J. The beginning of bed load movement of mixtures investigated as natural armoring in channels. Pasadena: California Institute of Technology; 1967. p. 89.
- Gessler J. Beginning and ceasing of sediment motion. Phys Lett B. 1971;251(2):279–283.
- Farley kJ, Thomann RV. An integrated model for the fate and bioaccumulation of PCBs in the Hudson River estuary. Pensacola (FL): Society of Environmental Toxicology and Chemistry; 1995.
- Partheniades E. Estuarine sediment dynamics and shoaling processes. In: Herbich JB, editor. Handbook of coastal and ocean engineering, volume 3: harbours, navigation channels, estuaries, and environmental effects. Houston (TX): Gulf Publishing Company; 1992. p. 985–1071.
- Valverde F, Costas M, Pena F, et al. Determination of total silver and silver species in coastal seawater by inductively-coupled plasma mass spectrometry after batch sorption experiments with Chelex-100 resin. Chem Speciation Bioavailability. 2008;20(20):217–226.10.3184/095422908X381306
- Beuselinck L, Govers G, Steegen A, et al. Sediment transport by overland flow over an area of net deposition. Hydrol Processes. 1999;13(17):2769–2782.10.1002/(ISSN)1099-1085