Abstract
Immune system (IS) functionality is warranted by inter-dependent processes that balance body defences without exceeding in inflammation. An ideal nutraceutical approach should sustain the protective IS activity while controlling inflammation. The potential immunomodulatory activity of the food supplement (FS) AminoDefence was studied in resting macrophages RAW264.7 and following stimulation of bacterial- and viral-associated inflammation trough LPS and PolyI:C treatments, respectively. In unstimulated macrophages, the formulation exerted a dose-dependent immunostimulant activity by up-regulating NO, IL-6, TNF-α and MCP-1 release, while it dampened the aberrant release of these factors induced by pro-inflammatory stimuli. Exploring the contribution of single components Echinacea purpurea (E. purpurea) extract and quercetin, used at proportional concentrations than in whole formulation, a more pronounced immunostimulant effect was observed for E. purpurea, and an anti-inflammatory activity for quercetin. Hence, AminoDefence exerts an immunomodulatory activity in macrophages by effectively stimulating a protective inflammatory response and limiting it in cases of excessive inflammation.
Introduction
The increasing ageing population, the greater awareness of the causal relationship between disease and nutrition, and the importance of preventive measures to protect individuals health, have helped make the food supplement (FS) industry one of the fastest-growing industries (Brunelli et al. Citation2022). Moreover, the general public has significantly increased the use of FSs since the start of the COVID-19 pandemic (Crawford et al. Citation2022). Indeed, immunity FS sales reported by the Nutrition Business Journal (NBJ) climbed from $3.4 billion US dollars in 2019 to almost $6 billion by the end of 2020, with immune health representing approximately 10% of all US supplement sales (Crawford et al. Citation2022). Focusing on the European context, the market size reached 13.3 billion euros in 2021 (Perelló-Oliver Citation2023), with 52% of consumers that declare to use FSs generically to maintain health status, and 45% of them that use FSs specifically intending to support or boost the immune system (Ipsos Citation2022). The increased use of such FSs specifically aimed at improving the immune system (IS) defense could be related to the need to minimise the negative impact of seasonal infections and their symptoms on health-related quality of life, namely the normal daily activities including swallowing, talking, eating, sleeping, working and performing in school (Addey and Shephard Citation2012). Similarly, influenza patients exhibit upper and/or lower respiratory tract signs and symptoms, frequently accompanied by systemic illness symptoms, such as fever, headache, myalgia, and weakness; often influenza can occur in pandemic form, leading to serious health complications as well as social and economic disruption (Yoshino et al. Citation2021).
Homeostatic mechanisms regulating the IS are essential for the proper body defense (Horwitz et al. Citation2019), despite understanding its composition and function is challenging due to the high number of interdependent cell populations, their complex regulation, as well as the variability among individuals, as a consequence of both heritable (e.g. histocompatibility leukocyte antigen (HLA) genes) and non-heritable factors, with these latter exerting a stronger influence on innate IS (Lakshmikanth et al. Citation2020). In this regard, a systematic review of six separate studies suggests that seasonal immune modulation might be linked to specific seasonal infections (Paynter et al. Citation2015), thus supporting the hypothesis that an intervention aimed at improving immune function is conceivable. In this respect, a growing number of reports suggest that several nutraceutical approaches exert immunomodulatory functions by acting on the IS and particularly by supporting the innate immunity on one side, or balancing the exacerbated pro-inflammatory response (Alba et al. Citation2023) triggered by pathogens on the other side. In particular, in a context of a varied and balanced diet and a healthy lifestyle, FSs might sustain the immune system function by modulating inflammatory response, both supporting the protective pro-inflammatory function and restoring immune homeostasis (Vignesh et al. Citation2021), possibly representing a beneficial strategy to support the body immune defences (Djaoudene et al. Citation2023). Indeed, an ideal and safe nutraceutical approach should counteract infection initiation by stimulating the macrophage protective pro-inflammatory function, but also control and moderate an exaggerated inflammation during infection .
For instance, Echinacea purpurea (E. purpurea) extract, with its array of different bioactive molecules, including alkamides, phenolic compounds and arabinogalactan proteins, is one of the most characterised nutraceuticals with immunomodulatory properties (Kim and Calderón Citation2022). Indeed, E. purpurea extract exerted an immunostimulatory activity in in vitro studies on murine and human peripheral mononuclear cells (PBMCs) and macrophages (Rininger et al. Citation2000, Fu et al. Citation2017). Consistently, in a mouse model of lipopolysaccharides (LPS)-induced lung injury (Zhang et al. Citation2020), E. purpurea upregulated the markers of classically activated M1 macrophages, stimulated the release of pro-inflammatory cytokines and nitric oxide (NO), thus bolstering their anti-microbial effect. Moreover, the anti-inflammatory cytokines release induced by E. purpurea extract has been documented in human macrophages (Zhai et al. Citation2009, Dobrange et al. Citation2019, Vieira et al. Citation2022). As for human evidence, a review considering 14 studies demonstrated that the prophylactic treatment with 2400 mg/day E. purpurea extract for 4 months appeared to be beneficial in common cold treatment and prevention (Rondanelli et al. Citation2018). In addition to E. purpurea extract, a growing number of evidence supports the involvement of quercetin in the modulation of a wide range of biological pathways involved in the immune system, exerting an anti-inflammatory, anti-viral and anti-oxidant properties (Li et al. Citation2016). Besides E. purpurea extract, this strong evidence prompted us to analyse the immunomodulatory contribution of quercetin among the formulation components. Quercetin, indeed, was previously shown to effectively reduce oxidative stress (Cui et al. Citation2019) and to inhibit pro-inflammatory cytokines and NO release from LPS-elicited human and murine macrophages (Cho et al. Citation2016, Tang et al. Citation2019). In addition, an inhibitory activity of quercetin on viral entry and replication has been reported concerning Influenza A (Choi et al. Citation2009), and SARS-CoV (Yi et al. Citation2004). Moreover, in vitro evidence reports a quercetin-derived reduction of the inflammatory response in cultured macrophages exposed to Polyinosinic:polycytidylic acid (PolyI:C), an experimental model of viral-related inflammation (Kim and Park Citation2016).
This study aimed to investigate the immunomodulatory effect of AminoDefence, a FS consisting of a complex of amino acids, proteins, vitamins, minerals, and plant-derived substances, including quercetin and E. purpurea extract, in cultured macrophages. Furthermore, based on the reported activity on IS of both quercetin and E. purpurea extract, their potential ability to stimulate macrophage pro-inflammatory activity, as well as to suppress the macrophage inflammatory response related to bacterial LPS stimulation was assessed. To this aim, PolyI:C was used in a limited experimental setting to evaluate the modulating effect of the nutraceutical formulation on the viral-related inflammation.
Materials and methods
Cell culture and treatments
Murine macrophages RAW264.7 were purchased from ATCC (VA, USA). Cells were cultured in DMEM High Glucose (Euroclone, Italy), supplemented with 10% Fetal Calf Serum (FCS; Euroclone, Italy) at 37 °C, 5% CO2. Twenty-four hours after plating, cells were treated for 48 h with the nutraceutical formulation Endomune® (Laboratoires NHCO Nutrition, Nice, France), commercialised in France with registered trademark Endomune®, in Italy with the tradename AminoDefence and in Spain with the tradename AminoDefense, at the concentration of 0.06 − 0.12 −0.25 − 0.5 − 1 mg/ml, or with the single component quercetin or E. purpurea extract, at the concentrations corresponding to those present in the formulation, to evaluate the immunostimulant activity. A different set of cells were instead treated for 24 h with the nutraceutical formulation, as well as with the single components quercetin or E. purpurea extract, in combination with the inflammatory stimulus Lipopolysaccharides (LPS; Merck, Germany) [1 µg/mL], in order to evaluate the anti-inflammatory activity of the compounds (Kim and Park Citation2016, Aki et al. Citation2020). In these conditions, the nutraceutical formulation AminoDefence, quercetin and E. purpurea extract were tested at the same concentration as indicated above. As explorative test, a limited number of experiments were performed using Polyinosinic:polycytidylic acid (PolyI:C; Merck, Germany) [50 µg/ml] as inflammatory stimulus to specifically mimic the viral infection-related inflammation; the experimental protocol to evaluate the anti-inflammatory effect of the compounds was the same of that used for LPS. Cell viability of the tested compounds was preliminarily investigated in all experimental conditions to exclude possible cytotoxic effects. The whole formulation powder was preliminarily tested for its solubility in cell culture medium supplemented with 0.5%v/v DMSO; in this condition ∼95% of the whole powder was completely solubilised. The composition of the nutraceutical formulation AminoDefence is reported in .
Table 1. Nutraceutical formulation.
Measurement of Nitric Oxide (NO) production
NO production was assessed by measuring nitrite levels in culture media through the Griess assay (Promega, WI, USA), based on the formation of a chromophore after reacting with Griess reagent, which was prepared fresh daily by mixing equal volumes of stock A (1% sulphanilamide, 5% phosphoric acid) and stock B (0,1% N-[naphthyl] ethylenediamine dihydrochloride) (Tang et al. Citation2019). NO production was evaluated for immunostimulant activity, and for the anti-inflammatory activity after stimulation of cells with LPS and PolyI:C.
Measurement of cytokine secretion
Cytokine secretion (TNF-α, IL-6 and MCP-1) in culture medium was assessed through specific enzyme-linked immunosorbent assays (all purchased from Thermofisher, MA, USA) following the manufacturer’s instructions. Cytokine secretion was evaluated in both basal condition for the immunostimulant activity and for the anti-inflammatory activity after stimulation with LPS.
Statistical analyses
Statistical analyses were performed using Prism 8 software (GraphPad Software, San Diego, CA, USA). All data are expressed as mean ± standard deviation (SD) of experimental conditions performed in triplicate. Statistically significant differences among the mean values of each experimental group were investigated using the one-way analysis of variance (ANOVA), followed by the post-hoc Dunnett’s multiple comparison tests. A value of p < 0.05 was considered statistically significant.
Results
Immunostimulant activity
Nitric oxide (NO) release by cultured macrophages
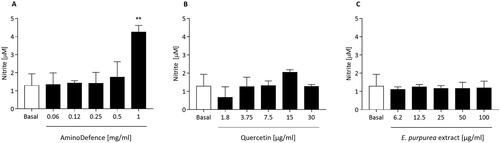
First, the potential modulatory effect of the whole nutraceutical formulation AminoDefence and of its compounds quercetin and E. purpurea extract was evaluated by quantifying NO release by macrophages in resting condition. As reported in , the formulation at the highest concentration tested (1 mg/ml), was able to significantly increase NO secretion (2.3-fold increase as compared to untreated cells (vs. basal; p < 0.01). Differently, in the same experimental conditions, quercetin and E. purpurea extract at all the concentrations tested did not influence the cellular release of NO ().
Figure 1. Effect of the whole formulation AminoDefence, quercetin and E. purpurea extract on NO release by macrophages. RAW264.7 cells were incubated for 48 h with the indicated increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). NO release in the culture media was quantified through Griess test assay. Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. **p < 0.01 vs basal.
Cytokine and chemokine secretion by cultured macrophages
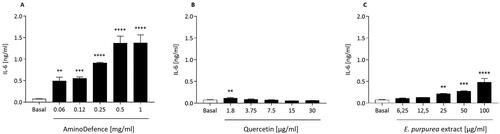
Secondly, in the same cellular model, the impact of the nutraceutical formulation AminoDefence, as well as of quercetin and E. purpurea extract was assessed by investigating the release of pro-inflammatory cytokines. As reported in , AminoDefence significantly and dose-dependently increased the release of IL-6 in cell culture media, reaching at the highest concentration tested a 18-fold increase as compared to basal, p < 0.0001. Differently, quercetin did not show any significant effect on IL-6 release (), except for a slight, significant, induction observed at the concentration of 1.8 µg/ml (p < 0.01). On the other side, similarly to what observed for the whole AminoDefence formulation, E. purpurea extract significantly induced in a dose-dependent manner the macrophage IL-6 secretion (). However this effect occurred at a significant lower extent (5.6-fold increase with E. purpurea extract [100 µg/ml] as compared to untreated macrophages, p < 0.0001, vs 18-fold with AminoDefence [1 mg/ml], p < 0.0001).
Figure 2. Effect of AminoDefence, quercetin and E. purpurea extract on IL-6 release by macrophages. RAW264.7 cells were incubated for 48 h with the indicated increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). IL-6 release was determined through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. **p < 0.01; ***p < 0.001; ****p < 0.0001 vs basal.
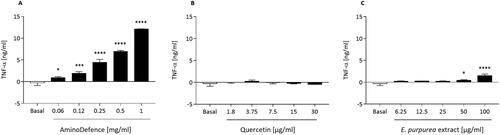
Similarly, the formulation AminoDefence treatment significantly stimulated in a dose-dependent manner TNF-α release from cells, with an increase of 45.5-fold at [1 mg/ml] as compared to untreated macrophages (p < 0.0001) (). No effect was instead observed after quercetin treatment (), while, as reported in , E. purpurea extract induced a slight, significant stimulation of TNF-α release at the two highest concentrations tested (50 µg/ml; p < 0.05 and 100 µg/ml; p < 0.0001 as compared to untreated macrophages). Again, the single component stimulated the cytokine release with a significant lower magnitude with respect to the whole formulation.
Figure 3. Effect of AminoDefence, quercetin and E. purpurea extract on TNF-α release by macrophages. RAW264.7 cells were incubated for 48 h with increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). TNF-α release was determined through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. *p < 0.05; ***p < 0.001; ****p < 0.0001 vs basal.
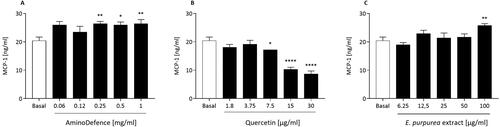
Also, the chemokine monocyte chemoattractant protein-1 (MCP-1) release from macrophages was slightly stimulated by treatment with the AminoDefence formulation, effect that became significant at the three highest concentrations tested (). Quercetin inhibited the basal release of MCP-1 in a dose dependent manner, starting from [7.5 µg/ml] (), while E. purpurea extract stimulated MCP-1 release only at the highest concentration tested (1.3-fold increase vs untreated macrophages; p < 0.01; ).
Figure 4. Effect of the whole formulation AminoDefence, quercetin and E. purpurea extract on MCP-1 release by macrophages. RAW264.7 cells were incubated for 48 h with the indicated increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). MCP-1 release was determined through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. *p < 0.05; **p < 0.01; ****p < 0.0001 vs basal.
Anti-inflammatory activity
LPS-treated macrophages
Nitric Oxide (NO) release
Next, the potential anti-inflammatory effect of the nutraceutical formulation AminoDefence and of its single components quercetin and E. purpurea extract was explored by quantifying NO release by LPS-stimulated murine macrophages, a model of bacterial-related inflammation (Fitzgerald and Kagan Citation2020). As amply previously reported (Aki et al. Citation2020), LPS markedly induced NO secretion by macrophages (28-fold increase as compared to macrophages in basal condition, p < 0.0001; ). The whole formulation AminoDefence significantly suppressed LPS-induced NO secretion in a dose dependent manner, with an almost complete inhibition obtained at the concentrations of 0.5 mg/ml and 1 mg/ml (-97.4% and −94.8% vs basal, respectively; p < 0.0001 both). A similar behaviour was observed for quercetin (), that significantly inhibited the LPS-induced NO release by macrophages in a dose-dependent fashion, albeit with a lower extent as compared to the whole formulation (−81.3% and −82.8% at [15 µg/ml] and [30 µg/ml], respectively, (p < 0.0001 both) vs LPS, concentrations corresponding to the amount of quercetin present in [0.5 mg/ml] and [1 mg/ml] of the whole formulation). Differently, E. purpurea extract did not influence the NO release by LPS-stimulated macrophages ().
Figure 5. Effect of AminoDefence, quercetin and E. purpurea extract on NO release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and the indicated increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). NO release in the culture media was quantified through Griess test assay. Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.
![Figure 5. Effect of AminoDefence, quercetin and E. purpurea extract on NO release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and the indicated increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). NO release in the culture media was quantified through Griess test assay. Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.](/cms/asset/95b7a8f3-f965-47ed-937e-e1a3db4a603d/iijf_a_2283688_f0005_b.jpg)
Cytokine and chemokine secretion
As also previously reported (Zhai et al. Citation2009, Lee et al. Citation2018), LPS incubation induced the release of various inflammatory mediators, such as cytokines and chemokines. Consistently, also under the present experimental setting, LPS treatment induced a marked increase of the pro-inflammatory cytokine IL-6 secretion as compared to macrophages in basal condition. AminoDefence treatment significantly inhibited LPS-induced IL-6 release in a dose-dependent manner, starting from [0.5 mg/ml] (p < 0.001), reaching an inhibition of 69.8% at [1 mg/ml] (vs LPS, p < 0.0001; ). Similarly, quercetin significantly suppressed the IL-6 secretion starting from the concentration of 15 μg/ml, corresponding to that present in [0.5 mg/ml] of formulation, reaching an inhibition of 54.1% at [30 μg/ml] (vs LPS, p < 0.0001; ). Differently, E. purpurea extract showed an overall null effect on IL-6 release induced by LPS ().
Figure 6. Effect of the whole formulation AminoDefence, quercetin and E. purpurea extract on IL-6 release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and the indicated increasing concentrations of AminoDefence (A), quercetin (B) or E. purpurea extract (C). IL-6 release in the culture media was quantified through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. **p < 0.01; ***p < 0.001; ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.
![Figure 6. Effect of the whole formulation AminoDefence, quercetin and E. purpurea extract on IL-6 release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and the indicated increasing concentrations of AminoDefence (A), quercetin (B) or E. purpurea extract (C). IL-6 release in the culture media was quantified through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. **p < 0.01; ***p < 0.001; ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.](/cms/asset/5a6a986f-0cee-47e5-bf95-7da0898a6fce/iijf_a_2283688_f0006_b.jpg)
Consistently to what observed for IL-6, the nutraceutical formulation AminoDefence significantly reduced TNF-α secretion in a dose-dependent fashion, starting from the concentration of 0.25 mg/ml, reaching an inhibition of 21.1% at [1 mg/ml] (vs LPS, p < 0.01; ). Similarly, both quercetin and E. purpurea extract showed to be equally effective in inhibiting the LPS-induced TNF-α secretion, inducing an average inhibition of 26.9% and 22.8% for quercetin and E. purpurea extract, respectively ().
Figure 7. Effect of the whole formulation AminoDefence, quercetin, and E. purpurea extract on TNF-α release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). TNF-α release in the culture media was quantified through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.
![Figure 7. Effect of the whole formulation AminoDefence, quercetin, and E. purpurea extract on TNF-α release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). TNF-α release in the culture media was quantified through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.](/cms/asset/baa0712a-d628-4378-b2b0-8c9b8c2cb009/iijf_a_2283688_f0007_b.jpg)
As reported in , AminoDefence inhibited also the LPS-induced MCP-1 release in a dose-dependent fashion, starting from [0.25 mg/ml], reaching an inhibition of 87.2% at [1 mg/ml] as compared to LPS condition (p < 0.0001). Similarly, quercetin significantly and dose-dependently suppressed the LPS-induced MCP-1 secretion (), however at a lesser extent with respect to the whole formulation (-64.3% at [30 µg/ml], p < 0.0001). No significant changes in MCP-1 were instead observed following E. purpurea extract treatment.
Figure 8. Effect of the whole formulation AminoDefence, quercetin, and E. purpurea extract on MCP-1 release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). MCP-1 release in the culture media was quantified through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.
![Figure 8. Effect of the whole formulation AminoDefence, quercetin, and E. purpurea extract on MCP-1 release by LPS-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Lipopolysaccharides (LPS) [1 µg/ml] and increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). MCP-1 release in the culture media was quantified through immunoenzymatic assay (ELISA). Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs LPS-treated macrophages; °°°°p < 0.0001 vs macrophages in basal conditions.](/cms/asset/00f34975-6cc5-4a2d-9a19-11b7f5a46446/iijf_a_2283688_f0008_b.jpg)
PolyI:C-treated macrophages
Nitric Oxide (NO) release
Finally, the effect of the nutraceutical formulation AminoDefence and of its components quercetin and E. purpurea extract was also assessed in cells treated with the pro-inflammatory stimulus Polyinosinic:polycytidylic Acid (PolyI:C), specifically mimicking the inflammation associated to viral infection (Kim and Park Citation2016).
In these experimental conditions, PolyI:C stimulated NO production by macrophages by about 48-fold as compared to basal (p < 0.0001). As reported in , AminoDefence was able to significantly inhibit the NO release in a dose-dependent manner starting from [0.25 mg/ml], with an almost complete inhibition of PolyI:C-induced NO production at [1 mg/ml]. A similar behaviour was observed for quercetin, that significantly inhibited the NO release by PolyI:C-stimulated macrophages starting from [7.5 µg/ml], the concentration corresponding to that present in the formulation at [0.25 mg/ml] (). However, the inhibitory effect of quercetin occurred at a lower extent as compared to the whole nutraceutical formulation (quercetin [30 µg/ml] − 55.2% and AminoDefence [1 mg/ml] − 94.6% vs PolyI:C; p < 0.0001). Also, E. purpurea extract was able to suppress NO release induced by PolyI:C, however starting from the concentration of 50 µg/ml, corresponding to that present into [0.5 mg/ml] of formulation (). Considering the highest concentration, E. purpurea extract was more effective than quercetin in inhibiting PolyI:C-induced NO release from macrophage (-84.5% E. purpurea extract at [100 µg/ml] and −55.2% quercetin at [30 µg/ml] vs PolyI:C; p < 0.0001).
Figure 9. Effect of AminoDefence, quercetin and E. purpurea extract on NO release by Polyinosinic:polycytidylic acid (PolyI:C)-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Polyinosinic:polycytidylic acid (PolyI:C) [50 µg/ml] and the indicated increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). NO release in the culture media was quantified through Griess test assay. Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. ***p < 0.001; ****p < 0.0001 vs PolyI:C-treated macrophages. °°°°p < 0.0001 vs macrophages in basal conditions.
![Figure 9. Effect of AminoDefence, quercetin and E. purpurea extract on NO release by Polyinosinic:polycytidylic acid (PolyI:C)-stimulated macrophages. RAW264.7 cells were incubated for 24 h at 37 °C with Polyinosinic:polycytidylic acid (PolyI:C) [50 µg/ml] and the indicated increasing concentrations of the formulation AminoDefence (A), quercetin (B) or E. purpurea extract (C). NO release in the culture media was quantified through Griess test assay. Experiments were performed in triplicate and data are expressed as mean ± SD. Statistical analyses were performed using the one-way ANOVA coupled with Dunnett’s multiple comparison test. A value of p < 0.05 was considered statistically significant. ***p < 0.001; ****p < 0.0001 vs PolyI:C-treated macrophages. °°°°p < 0.0001 vs macrophages in basal conditions.](/cms/asset/018a4ed3-ef05-455e-91da-55317d050522/iijf_a_2283688_f0009_b.jpg)
Discussion
Immune system (IS) relies on the balance between body defences and the management of the inflammatory process (Horwitz et al. Citation2019). Food supplements (FSs) are not addressed to substitute a varied and balanced diet, on the contrary their proper use consists in combining them with a balanced and healthy diet. In this context, a FS approach could be addressed to modulate immune activity, by supporting immunity against infections, as well as by helping the recovery of immune homeostasis (Addey and Shephard Citation2012, Vignesh et al. Citation2021). The aim of this in vitro study was to evaluate the immunomodulatory potential of the FS AminoDefence, reporting its ability to increase the activity of unstimulated macrophages, as well as to reduce the secretion of proinflammatory mediators in LPS- or PolyI:C-elicited macrophages, models of bacterial- and viral-associated inflammation, respectively. To this aim, we used cultured RAW264.7 macrophages, a widely used model to measure immunomodulatory activity of several nutraceutical formulations (LaLone et al. Citation2009, Park et al. Citation2016), being macrophage one of the main regulators of the IS (Gauthier and Chen Citation2022). The role of macrophages is indeed to preside over immune surveillance and tissue homeostasis in basal conditions, and, following their activation, to trigger a pro-inflammatory response to remove the cause of tissue damage, eventually recalling other circulating immune cells (Sheu and Hoffmann Citation2022). By using this in vitro model, AminoDefence activity was assessed on NO secretion, due to its critical role in host defence and immune regulation (Swathi Krishna et al. Citation2022). On one hand, NO has a protective activity by exerting an antimicrobial effect; on the other hand, when overproduced, it may lead to tissue damage that may generate relevant pathogenic effects (Xue et al. Citation2018). Interestingly, AminoDefence treatment has shown to have a differential effect on NO release by RAW264.7 culture, depending on the cellular inflammatory state. In unstimulated macrophages, AminoDefence was able to promote NO secretion at the highest concentration tested, with values far below if compared to those obtained in the inflammatory models of LPS and PolyI:C. On the other side, in the presence of pro-inflammatory stimuli, the activation by the TLR4 ligands LPS (Bock and Chang Citation2015) or the synthetic double-strand RNA PolyI:C (Kim and Park Citation2016) led to a strong increase in NO release, that was effectively and dose-dependently dampened by AminoDefence.
Moreover, the activity of AminoDefence to modulate the secretion of the two main pro-inflammatory cytokines IL-6 and TNF-α was evaluated, due to their involvement in acute immune response activation, as they trigger T and B cells activation and differentiation (Arango Duque and Descoteaux Citation2014). The findings reported a dose-dependent increase in both cytokines secretion following AminoDefence treatment in unstimulated macrophages, while a strong and dose-dependent reduction in IL-6 and TNF-α release was detected after LPS stimulation. Consistently, AminoDefence treatment was able to promote a mild secretion of chemokine MCP-1 from unstimulated RAW264.7 cells, as well as to reduce its excessive secretion in LPS-elicited macrophages. Notably, also MCP-1 exerts a pivotal role in the inflammatory process, being responsible for attracting other inflammatory cells and enhancing their activation with the release of pro-inflammatory soluble factors. Moreover, it has been found to be a sensitive biomarker associated with inflammatory diseases such as cardiovascular diseases, rheumatoid arthritis and viral infections, including SARS-CoV2 (Singh et al. Citation2021). Based on the obtained results of this study, AminoDefence as a whole formulation showed to increase the secretory activity of unstimulated macrophages and to reduce the inflammatory response of macrophages when stimulated with LPS.
Besides, this study points to an enhanced effect resulting from the combination of all the components in the formulation, as a lower or null immunomodulatory effect was observed when the activity of the single components E. purpurea or quercetin was compared to that of the whole AminoDefence formulation. This observation may suggest that the AminoDefence immunomodulatory activity might be the result of either a synergistic interaction of the individual components of the formulation, or their cumulative effect. With respect to the specific effects of each tested component, E. purpurea extract exerted a better immunostimulant activity in resting macrophages, while quercetin revealed a stronger effect in LPS- or Polyl:C-stimulated macrophages, confirming its amply described anti-inflammatory activity (LaLone et al. Citation2009, Cho et al. Citation2016, Li et al. Citation2016, Cui et al. Citation2019, Tang et al. Citation2019). Moreover, E. purpurea extract dampened NO release from PolyI:C-elicited RAW264.7 cells, thus suggesting an important anti-viral activity that makes E. purpurea extract a potential candidate as an immunomodulatory agent in viral infections prevention and treatment, as previously reported (Sharma et al. Citation2006, Nagoor Meeran et al. Citation2021).
Nevertheless, this study revealed some limitations: the first one is related to the fact that the in vitro approach does not consider the human metabolism following the intake of most of the AminoDefence components, including E. purpurea extract and quercetin (Shabbir et al. Citation2021, Burlou-Nagy et al. Citation2022), that accounts for different metabotypes which can differently affect physiological functions, producing high inter-individual differences in the biological response. Secondly, IS has an intrinsic complexity, which hardly allows an exhaustive evaluation through an in vitro model. In order to confirm these results, a possible evolution might be to perform an in vitro co-culture experiment, to evaluate interactions between immune cells, and an in vivo study that would allow to evaluate AminoDefence immunomodulatory activity taking into account digestive and metabolic processes and bioavailability, as well as the IS complexity. We decided to focus on macrophages since they play a pivotal role in the cross-road between innate and adaptive immune response (Kumar Citation2019) and represent a solid and well recognised experimental model, as supported by a large amount of studies (LaLone et al. Citation2009, Cui et al. Citation2019, Chen et al. Citation2021). In addition, the study compared the activity of the whole AminoDefence formulation with only 2 of its 31 total active components, i.e. quercetin and E. purpurea extract alone, for which a robust immunomodulatory activity is reported by the literature (Rauš et al. Citation2015, De Rosa et al. Citation2019, Hernáez et al. Citation2019, Vieira et al. Citation2022). Finally, it was only possible to evaluate the activity of the soluble fraction of whole AminoDefence formulation (approximately 95% w/w) in which, of note, quercetin and E. purpurea extract were completely dissolved. We hypothesize that the negligible insoluble fraction of AminoDefence, which appeared as a waxy residue with small fibres inclusion, may be likely related to the presence of Propolis extract from Colla apis, Coenzyme Q10, D-alpha tocopherol and Magnesium salts of fatty acids (E470b), which solubility has been reported to be extremely low in the medium used to dissolve the AminoDefence formulation (Munné-Bosch Citation2007, Kubiliene et al. Citation2018, Choi et al. Citation2019, Zarmpi et al. Citation2020).
The present study could represent a ‘proof of concept’, since the analysed FS AminoDefence is composed of a mixture of several components, all of them with a well-described activity on IS that makes the formulation unique (Fantacone et al. Citation2020, Gombart et al. Citation2020). Indeed, the rationale for this formulation is the complexity of the inflammatory response, which may be sustained by different substances acting, possibly synergistically, on the different mechanisms and factors involved in the IS.
In conclusion, the present study points out a significant in vitro immunomodulatory activity of the FS AminoDefence on both unstimulated and LPS-elicited macrophages. Further studies, using different models, might better clarify its potential role as a support for IS normal function.
Authors’ contribution
All the authors contributed to the design of the study, analysis and interpretation of data.
Experimental data were produced in the laboratories of Department of Food and Drug, University of Parma, Parma, Italy.
All the authors drafted, revised and finally approved the version to be published.
Disclosure statement
Davide Paleari and Laura Rinaldi are employees of the Medical Department of Chiesi Italia S.p.A., Parma, Italy.
Additional information
Funding
References
- Addey D, Shephard A. 2012. Incidence, causes, severity and treatment of throat discomfort: a four-region online questionnaire survey. BMC Ear Nose Throat Disord. 12(1):1. doi: 10.1186/1472-6815-12-9.
- Aki T, Funakoshi T, Noritake K, Unuma K, Uemura K. 2020. Extracellular glucose is crucially involved in the fate decision of LPS-stimulated RAW264.7 murine macrophage cells. Sci Rep. 10(1):10581. doi: 10.1038/s41598-020-67396-6.
- Alba G, Dakhaoui H, Santa-Maria C, Palomares F, Cejudo-Guillen M, Geniz I, Sobrino F, la Paz S, Lopez-Enriquez S. 2023. Nutraceuticals as potential therapeutic modulators in immunometabolism. Nutrients. 15(2):411. doi: 10.3390/nu15020411.
- Arango Duque G, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 5:491. doi: 10.3389/fimmu.2014.00491.
- Bock FJ, Chang P. 2015. Macrophage activation: on par with LPS. Chem Biol. 22(4):432–433. doi: 10.1016/j.chembiol.2015.04.006.
- Brunelli L, Arnoldo L, Mazzilis G, d’Angelo M, Colautti L, Cojutti PG, Parpinel M., 2022. The knowledge and attitudes of pharmacists related to the use of dietary supplements: an observational study in northeastern Italy. Prev Med Rep. 30:101986. doi: 10.1016/j.pmedr.2022.101986.
- Burlou-Nagy C, Bănică F, Jurca T, Vicaș LG, Marian E, Muresan ME, Bácskay I, Kiss R, Fehér P, Pallag A. 2022. Echinacea purpurea (L.) Moench: biological and Pharmacological Properties. A Review. Plants. 11(9):1244. doi: 10.3390/plants11091244.
- Chen C, Xie X, Li X. 2021. Immunomodulatory effects of four polysaccharides purified from Erythronium sibiricum bulb on macrophages. Glycoconj J. 38(4):517–525. doi: 10.1007/s10719-021-10005-z.
- Cho Y-H, Kim N-H, Khan I, Yu JM, Jung HG, Kim HH, Jang JY, Kim HJ, Kim D-I, Kwak J-H, et al. 2016. Anti-inflammatory potential of quercetin-3-O-β-D-(‘2’-galloyl)-glucopyranoside and quercetin isolated from diospyros kaki calyx via suppression of MAP signaling molecules in LPS-induced RAW 264.7 macrophages. J Food Sci. 81(10):C2447–C2456. doi: 10.1111/1750-3841.13497.
- Choi HJ, Song JH, Park KS, Kwon DH. 2009. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci. 37(3-4):329–333. doi: 10.1016/j.ejps.2009.03.002.
- Choi J-S, Park J-W, Park J-S. 2019. Design of coenzyme Q10 solid dispersion for improved solubilization and stability. Int J Pharm. 572:118832. doi: 10.1016/j.ijpharm.2019.118832.
- Crawford C, Avula B, Lindsey AT, Walter A, Katragunta K, Khan IA, Deuster PA. 2022. Analysis of select dietary supplement products marketed to support or boost the immune system. JAMA Netw Open. 5(8):e2226040–e2226040. doi: 10.1001/jamanetworkopen.2022.26040.
- Crawford C, Brown LL, Costello RB, Deuster PA. 2022. Select dietary supplement ingredients for preserving and protecting the immune system in healthy individuals: a systematic review. Nutrients. 14(21):4604. doi: 10.3390/nu14214604.
- Cui S, Wu Q, Wang J, Li M, Qian J, Li S. 2019. Quercetin inhibits LPS-induced macrophage migration by suppressing the iNOS/FAK/paxillin pathway and modulating the cytoskeleton. Cell Adh Migr. 13(1):1–12. doi: 10.1080/19336918.2018.1486142.
- Djaoudene O, Romano A, Bradai YD, Zebiri F, Ouchene A, Yousfi Y, Amrane-Abider M, Sahraoui-Remini Y, Madani K. 2023. A global overview of dietary supplements: regulation, market trends, usage during the COVID-19 pandemic, and health effects. Nutrients. 15(15):3320. doi: 10.3390/nu15153320.
- Dobrange E, Peshev D, Loedolff B, Van den Ende W. 2019. Fructans as immunomodulatory and antiviral agents: the case of Echinacea. Biomolecules. 9(10):615. doi: 10.3390/biom9100615.
- Fantacone ML, Lowry MB, Uesugi SL, Michels AJ, Choi J, Leonard SW, Gombart SK, Gombart JS, Bobe G, Gombart AF. 2020. The effect of a multivitamin and mineral supplement on immune function in healthy older adults: a double-blind, randomized, controlled trial. Nutrients. 12(8):2447. doi: 10.3390/nu12082447.
- Fitzgerald KA, Kagan JC. 2020. Toll-like receptors and the control of immunity. Cell. 180(6):1044–1066. doi: 10.1016/j.cell.2020.02.041.
- Fu A, Wang Y, Wu Y, Chen H, Zheng S, Li Y, Xu X, Li W. 2017. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. J Cell Biochem. 118(9):2664–2671. doi: 10.1002/jcb.25875.
- Gauthier T, Chen W. 2022. Modulation of macrophage immunometabolism: a new approach to fight infections. Front Immunol. 13:780839. doi: 10.3389/fimmu.2022.780839.
- Gombart AF, Pierre A, Maggini S. 2020. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 12(1):236. doi: 10.3390/nu12010236.
- Hernáez Á, Sanllorente A, Castañer O, Martínez-González MÁ, Ros E, Pintó X, Estruch R, Salas-Salvadó J, Corella D, Alonso-Gómez ÁM, et al. 2019. Increased consumption of virgin olive oil, nuts, legumes, whole grains, and fish promotes HDL functions in humans. Mol Nutr Food Res. 63(6):e1800847. doi: 10.1002/mnfr.201800847.
- Horwitz DA, Fahmy TM, Piccirillo CA, La Cava A. 2019. Rebalancing immune homeostasis to treat autoimmune diseases. Trends Immunol. 40(10):888–908. doi: 10.1016/j.it.2019.08.003.
- Ipsos, European Public Affairs. 2022. Consumer survey on food supplements in the EU.
- Kim H, Calderón AI. 2022. Rational and safe use of the top two botanical dietary supplements to enhance the immune system. Comb Chem High Throughput Screen. 25(7):1129–1130. doi: 10.2174/1386207325666220207112937.
- Kim Y-J, Park W. 2016. Anti-inflammatory effect of quercetin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Molecules. 21(4):450. doi: 10.3390/molecules21040450.
- Kubiliene L, Jekabsone A, Zilius M, Trumbeckaite S, Simanaviciute D, Gerbutaviciene R, Majiene D. 2018. Comparison of aqueous, polyethylene glycol-aqueous and ethanolic propolis extracts: antioxidant and mitochondria modulating properties. BMC Complement Altern Med. 18(1):165. doi: 10.1186/s12906-018-2234-5.
- Kumar V. 2019. Macrophages: the potent immunoregulatory innate immune cells. In: K.H. Bhat, ed. Macrophage Activation. Rijeka: IntechOpen.
- Lakshmikanth T, Muhammad SA, Olin A, Chen Y, Mikes J, Fagerberg L, Gummesson A, Bergström G, Uhlen M, Brodin P. 2020. Human immune system variation during 1 year. Cell Rep. 32(3):107923. doi: 10.1016/j.celrep.2020.107923.
- LaLone CA, Rizshsky L, Hammer KDP, Wu L, Solco AKS, Yum M, Nikolau BJ, Wurtele ES, Murphy PA, Kim M, et al. 2009. Endogenous levels of Echinacea alkylamides and ketones are important contributors to the inhibition of prostaglandin E2 and nitric oxide production in cultured macrophages. J Agric Food Chem. 57(19):8820–8830. doi: 10.1021/jf901202y.
- Lee HN, Shin SA, Choo GS, Kim HJ, Park YS, Kim BS, Kim SK, Cho SD, Nam JS, Choi CS, et al. 2018. Anti‑inflammatory effect of quercetin and galangin in LPS‑stimulated RAW264.7 macrophages and DNCB‑induced atopic dermatitis animal models. Int J Mol Med. 41(2):888–898. doi: 10.3892/ijmm.2017.3296.
- Li C, Zhang W-J, Frei B. 2016. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 9:104–113. doi: 10.1016/j.redox.2016.06.006.
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. 2016. Quercetin, Inflammation and Immunity. Nutrients. 8(3):167. doi: 10.3390/nu8030167.
- Munné-Bosch S. 2007. Alpha-tocopherol: a multifaceted molecule in plants. Vitam Horm. 76:375–392. doi: 10.1016/S0083-6729(07)76014-4.
- Nagoor Meeran MF, Javed H, Sharma C, Goyal SN, Kumar S, Jha NK, Ojha S. 2021. Can Echinacea be a potential candidate to target immunity, inflammation, and infection - The trinity of coronavirus disease 2019. Heliyon. 7(2):e05990. doi: 10.1016/j.heliyon.2021.e05990.
- Park DR, Ko R, Kwon SH, Min B, Yun SH, Kim MH, Minatelli J, Hill S, Lee SY. 2016. FlexPro MD, a mixture of krill oil, astaxanthin, and hyaluronic acid, suppresses lipopolysaccharide-induced inflammatory cytokine production through inhibition of NF-κB. J Med Food. 19(12):1196–1203. doi: 10.1089/jmf.2016.3787.
- Paynter S, Ware RS, Sly PD, Williams G, Weinstein P. 2015. Seasonal immune modulation in humans: observed patterns and potential environmental drivers. J Infect. 70(1):1–10. doi: 10.1016/j.jinf.2014.09.006.
- Perelló-Oliver S. 2023. Evidence from irresponsible communication in a health-related industry: presenter(s): Ana García-Arranz, Universidad Rey Juan Carlos, Spain. Patient Education and Counseling. 109:14–15. doi: 10.1016/j.pec.2022.10.043.
- Rauš K, Pleschka S, Klein P, Schoop R, Fisher P. 2015. Effect of an Echinacea-based hot drink versus oseltamivir in influenza treatment: a randomized, double-blind, double-dummy, multicenter, noninferiority clinical trial. Curr Ther Res Clin Exp. 77:66–72. doi: 10.1016/j.curtheres.2015.04.001.
- Rininger JA, Kickner S, Chigurupati P, McLean A, Franck Z. 2000. Immunopharmacological activity of Echinacea preparations following simulated digestion on murine macrophages and human peripheral blood mononuclear cells. J Leukoc Biol. 68(4):503–510.
- Rondanelli M, Miccono A, Lamburghini S, Avanzato I, Riva A, Allegrini P, Faliva MA, Peroni G, Nichetti M, Perna S. 2018. Self-care for common colds: the pivotal role of vitamin d, vitamin c, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds-practical advice on dosages a. Evid Based Complement Alternat Med. 2018:5813095. doi: 10.1155/2018/5813095.
- De Rosa N, Giampaolino P, Lavitola G, Morra I, Formisano C, Nappi C, Bifulco G. 2019. Effect of immunomodulatory supplements based on echinacea angustifolia and echinacea purpurea on the posttreatment relapse incidence of genital condylomatosis: a prospective randomized study. Biomed Res Int. 2019:3548396–3548397. doi: 10.1155/2019/3548396.
- Shabbir U, Rubab M, Daliri EB-M, Chelliah R, Javed A, Oh D-H. 2021. Curcumin, quercetin, catechins and metabolic diseases: the role of gut microbiota. Nutrients. 13(1):206. doi: 10.3390/nu13010206.
- Sharma M, Arnason JT, Hudson JB. 2006. Echinacea extracts modulate the production of multiple transcription factors in uninfected cells and rhinovirus-infected cells. Phytother Res. 20(12):1074–1079. doi: 10.1002/ptr.1998.
- Sheu KM, Hoffmann A. 2022. Functional hallmarks of healthy macrophage responses: their regulatory basis and disease relevance. Annu Rev Immunol. 40(1):295–321. doi: 10.1146/annurev-immunol-101320-031555.
- Singh S, Anshita D, Ravichandiran V. 2021. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol. 101(Pt B):107598. doi: 10.1016/j.intimp.2021.107598.
- Swathi Krishna S, Thennavan A, Kanthlal SK. 2022. Dietary foods containing nitric oxide donors can be early curators of SARS-CoV-2 infection: a possible role in the immune system. J Food Biochem. 46(3):e13884. doi: 10.1111/jfbc.13884.
- Tang J, Diao P, Shu X, Li L, Xiong L. 2019. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264.7 cells: in vitro assessment and a theoretical model. Biomed Res Int. 2019:7039802–7039808. doi: 10.1155/2019/7039802.
- Vieira SF, Gonçalves V, MF, Llaguno CP, Macías F, Tiritan ME, Reis RL, Ferreira H, Neves NM. 2022. On the bioactivity of Echinacea purpurea extracts to modulate the production of inflammatory mediators. Int J Mol Sci. 23(21):13616. doi: 10.3390/ijms232113616.
- Vignesh R, Velu V, Sureban SM. 2021. Could nutraceutical approaches possibly attenuate the cytokine storm in COVID-19 Patients? Front Cell Infect Microbiol. 11:667733. doi: 10.3389/fcimb.2021.667733.
- Xue Q, Yan Y, Zhang R, Xiong H. 2018. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci. 19(12):3805. doi: 10.3390/ijms19123805.
- Yi L, Li Z, Yuan K, Qu X, Chen J, Wang G, Zhang H, Luo H, Zhu L, Jiang P, et al. 2004. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 78(20):11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004.
- Yoshino Y, Wakabayashi Y, Kitazawa T. 2021. The clinical effect of seasonal flu vaccination on health-related quality of life. Int J Gen Med. 14:2095–2099. doi: 10.2147/IJGM.S309920.
- Zarmpi P, Flanagan T, Meehan E, Mann J, Fotaki N. 2020. Impact of magnesium stearate presence and variability on drug apparent solubility based on drug physicochemical properties. Aaps J. 22(4):75. doi: 10.1208/s12248-020-00449-w.
- Zhai Z, Solco A, Wu L, Wurtele ES, Kohut ML, Murphy PA, Cunnick JE. 2009. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. J Ethnopharmacol. 122(1):76–85. doi: 10.1016/j.jep.2008.11.028.
- Zhang H, Lang W, Wang S, Li B, Li G, Shi Q. 2020. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-κB signal pathway. Int Immunopharmacol. 88:106974. doi: 10.1016/j.intimp.2020.106974.

